Abstract
By electron microscopy and image analysis, we find that baculovirus-expressed UL6 is polymorphic, consisting of rings of 11-, 12-, 13-, and 14-fold symmetry. The 12-mer is likely to be the oligomer incorporated into procapsids: at a resolution of 16 Å, it has an axial channel, peripheral flanges, and fits snugly into a vacant vertex site. Its architecture resembles those of bacteriophage portal/connector proteins.
The herpes simplex virus type 1 (HSV-1) capsid initially assembles as a precursor particle or procapsid that transforms into the mature capsid (15, 19). During maturation, the internal scaffolding protein is expelled after processing by the viral protease, and the procapsid shell undergoes a stabilizing structural transition (10). In these properties, HSV-1 follows the paradigm previously determined in studies of double-stranded DNA bacteriophages (2, 3, 9, 11): as with the phages, it is likely that HSV-1 capsid maturation accompanies DNA packaging in vivo. Since assembly precedes packaging, how does DNA enter the capsid? Bacteriophages solve this problem by installing, at one of the capsid's 12 icosahedral vertices, a dodecameric ring that serves as the portal channel via which DNA is packaged (20). This protein is also called the connector, because it serves as the attachment site for the phage tail. In 2001, Newcomb et al. (16) reported several lines of evidence strongly suggesting that UL6 is the portal of HSV-1. In immunogold electron microscopy (EM) experiments, they observed that an anti-UL6 antibody labeled one vertex site per capsid. They also expressed UL6 in a recombinant baculovirus system and examined the product by EM. Negative staining revealed ring-like molecules with peripheral petals, and scanning transmission EM mass measurements yielded a broad distribution that peaked at a mass corresponding to ∼12 subunits. In the present study, we have extended the structural investigation of UL6.
Although the evidence so far supports the view that phage portals in capsids are 12-mers (6, 13, 14), 13-mers have also been detected when the portals of T7 (12), T3 (21), and SPP1 (7) have been overexpressed in bacteria, and polymorphism has also been observed with the P22 portal (5). The width of the distribution of UL6 mass measurements (700 to 1,200 kDa) (16) suggested that this (baculovirus) expression product is also polymorphic, but background noise in the micrographs could not be ruled out as an explanation for the observed width. We addressed this question by applying a symmetry detection algorithm to electron micrographs of negatively stained UL6 molecules. The predominant appearance is of round particles about 150 Å across, with stain accumulating in the center (Fig. 1a). It follows that either the molecules are rings that preferentially present axial projections (top views) or they are hollow spheres that may present a variety of views. We analyzed the rotational power spectra of 725 particles with the Rotastat algorithm (12), which assays whether any orders of symmetry are more strongly represented in the data set than in a reference set of background images that is assumed to be nonsymmetric. Statistical significance is expressed in terms of P values by a t test. At the stringent confidence level of P < 10−6, we found that 11-, 12-, and 13-fold symmetries were present. At the lower but also significant confidence level of P < 10−4, 14-fold symmetry was detected.
FIG. 1.
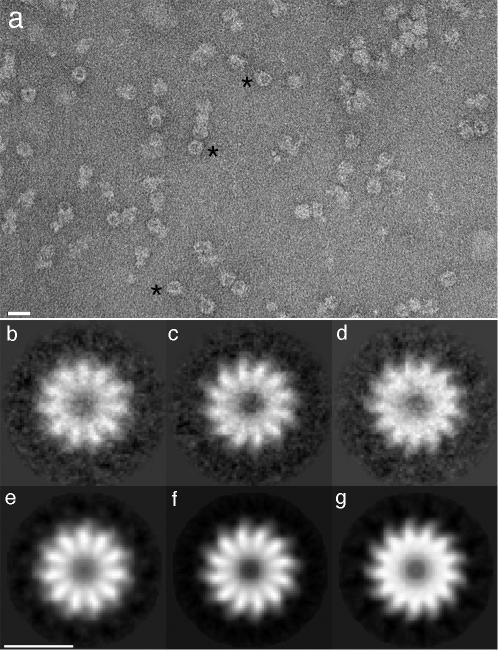
Negative staining EM of the HSV-1 UL6 portal protein. (a) Field of UL6 molecules, prepared as described previously (16), and negatively stained with uranyl acetate. Bar, 200 Å. Round particles likely to represent axial views (a few examples are marked with asterisks) were analyzed with the symmetry detection algorithm, Rotastat (12), which detected 11-fold, 12-fold, 13-fold, and less strongly, 14-fold molecules (see Results). When the images were classified in terms of their strongest harmonics, they were estimated to be 23% 11-mers, 27% 12-mers, 22% 13-mers, and 16% 14-mers. Correlation-averaged images of molecules presenting the first three symmetries are shown in panels b, c, and d. Their resolutions were 28 Å for panel b and 26 Å for both panels c and d. These images were further enhanced by rotational symmetrization in panels e, f, and g. The 14-mers were not only fewer but appeared less regular, to judge by the symmetry detection analysis and markedly lower resolution achieved on averaging (data not shown). Bar, 100 Å.
These particles were classified on the basis of the relative strengths of these harmonics in their rotational power spectra. Within each rotamer class, the images were aligned and averaged. The results, shown in Fig. 1b to g for the three prevalent classes, reveal 11-mers, 12-mers, and 13-mers, each with a strongly staining central feature ∼40 Å across, surrounded by petals. These images have resolutions of ∼26 Å. We conclude that UL6 is indeed polymorphic, with a wider range of polymorphism than has hitherto been detected among overexpressed phage portal proteins. Because the symmetry detection algorithm yielded clear-cut answers, we infer that UL6 tends to present top views.
3D structure.
Our goal was to calculate the structure of UL6 from cryoelectron micrographs, using an iterative projection-matching method (1). However, certain complications had to be addressed. First, for three-dimensional (3D) analysis of ring-like oligomers, side views are more conducive than top views, because a preponderance of top views limits the resolution in the axial dimension. However, UL6 preferentially presents top views when negatively stained on carbon films. Such also turned out to be the case for vitrified specimens on holey carbon films. We addressed this problem by collecting data on tilted specimens. Second, we had to generalize the projection-matching scheme to include multiple models corresponding to the various rotamers. Third, we had to acquire an appropriate starting model and then demonstrate that the final result was not biased by that choice. Although UL6 is fairly large (889 kDa for the 12-mer), there is little differential contrast in cryomicrographs to discriminate between different views and different rotamers (Fig. 2a). Accordingly, we used a two-step approach, first calculating 3D models from images of negatively stained specimens which have stronger contrast despite poorer structural preservation and then using these models as the starting point for cryo-EM reconstruction.
FIG. 2.

Cryo-EM of the HSV-1 UL6 portal protein. (a) A field of ice-embedded molecules (protein is dark). Bar, 200 Å. (b to i) Various views of a 3D reconstruction of the 12-mer at 16-Å resolution. Bar, 50 Å. A movie clip of the reconstruction may be seen at http://www.niams.nih.gov/rcn/labbranch/lsbr/UL6_movie.html.
For an initial model, we needed a barrel-like particle of the correct symmetry and about the right size, anticipating that the structural information implicit in the micrographs would gradually impose the correct structural details as the calculation proceeded. For this purpose, we chose to use the φ29 portal/ connector which has been resolved at high resolution (8, 18). We band limited the PDB 1FOU crystal structure (18) to a resolution of 35 Å and expanded it isotropically to compensate for the UL6 subunit being larger (74 versus 36 kDa) (Fig. 3a). Using models with 11-, 12-, and 13-fold symmetry, 5,352 negatively stained images were analyzed. To minimize the risk of incorrectly identified orientations, a conservative threshold was imposed on their correlation coefficients. This procedure led to a 12-fold model with a resolution of 29 Å (Fig. 3c): the 11-fold and 13-fold density maps were similar but contained more noise (data not shown).
FIG. 3.

Robustness of the 3D reconstruction of the UL6 dodecamer with respect to the choice of starting model. (a) A resolution-limited, appropriately swollen, rendition of the bacteriophage φ29 portal led to (c), a model at 29-Å resolution, calculated from images of negatively stained molecules, which in turn led to (d) a model at 16-Å resolution calculated from cryoelectron micrographs. Starting the analysis with an entirely different, computer-generated, model (b) led to essentially the same result.
These models were fed into the cryo-EM analysis. Roughly half of the 48 micrographs represented specimens that had been tilted by 10 to 45 degrees. These data eventually yielded the dodecamer shown in Fig. 2b to i and Fig. 3d, which is based on 3,477 particles and has a resolution of 16 Å. We believe that this structure is not biased by the choice of starting model for the following reasons. (i) The final model is markedly and stably different from the starting model in terms of its internal cavity and the position and shape of the peripheral flanges. (ii) The results for the 11-mer and 13-mer are similar except for the order of rotational symmetry, albeit of lower resolution (data not shown). (iii) We performed a control experiment with an entirely different starting model, in this case, a computer-generated construction based on the cartoon in Fig. 5D of reference 16 (Fig. 3b). It led to essentially the same structure.
The UL6 dodecamer is ∼130 Å long with a circumscribing diameter of ∼155 Å. Its two ends are distinct: at one end (Fig. 2d), a hollow cylinder about 65 Å across protrudes, which is plugged into a wider cylinder about 90 Å across at the other end, with a two-tiered outer ring in the central region. The connections between the two cylinders are rather tenuous. In overall shape, the UL6 portal resembles the SPP1 connector (17) more closely than that of φ29 (8, 18). We have not yet been able to determine the handedness of the portal, nor is it clear which end is exposed on the outer surface of the capsid. However, docking experiments with a capsid model (4) from which one penton had been computationally excised showed that the wider end fits snugly into a vacant vertex (Fig. 4), whereas the other end is too narrow to make extensive contact with the surrounding hexons. This result favors the former orientation, provided that insertion of UL6 at a vertex site does not induce major conformational changes in the surrounding VP5 molecules.
FIG. 4.
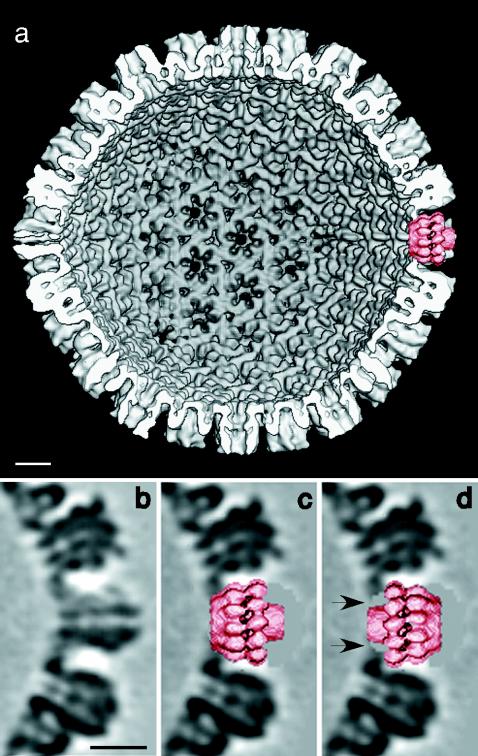
Fitting of the UL6 portal protein into a vacant vertex in the HSV-1 capsid. (a) Interior view of a capsid model at 18-Å resolution (4) from which a penton of VP5, the major capsid protein, was computationally excised and replaced by the portal (right-hand side at 3 o'clock). Panel b shows a section through a vertex site occupied by a VP5 pentamer and is compared in panels c and d with the same site with an implanted portal in both orientations. In one orientation (c), the portal fits snugly into this cavity. In the other orientation (d), the portal also fits but makes only tenuous contact with the surrounding VP5 molecules, leaving gaps between the molecules and the capsid (arrows), suggesting a less stable interaction. Bars, 100 Å.
In conclusion, the architecture of UL6, including its continuous axial channel and set of peripheral flange-like domains reproduces those of bacteriophage portal or connectors; moreover, its dimensions are compatible with those of the vertex site. These observations firmly support the proposition that UL6 is also a portal protein.
Acknowledgments
We thank D. Belnap, B. Heymann, E. Kocsis, T. Ishikawa, and M. McAuliffe, for help with programming and for providing software, L. You for scanning micrographs, and D. Thomsen for assistance with baculovirus expression.
This work was supported in part by the NIH IATAP program (to A.C.S.) and by NIH grant 41644 (to J.C.B.).
REFERENCES
- 1.Baker, T. S., and R. H. Cheng. 1996. A model-based approach for determining orientations of biological macromolecules imaged by cryoelectron microscopy. J. Struct. Biol. 116:120-130. [DOI] [PubMed] [Google Scholar]
- 2.Black, L. W., M. K. Showe, and A. C. Steven. 1994. Morphogenesis of the T4 head, p. 218-258. In J. Karam (ed.), Molecular biology of bacteriophage T4. American Society for Microbiology, Washington, D.C.
- 3.Cerritelli, M. E., J. F. Conway, N. Cheng, B. L. Trus, and A. C. Steven. 2002. Molecular mechanisms in bacteriophage T7 assembly, maturation and DNA containment. Adv. Protein Chem. 64:301-323. [DOI] [PubMed] [Google Scholar]
- 4.Cheng, N., B. L. Trus, D. M. Belnap, W. W. Newcomb, J. C. Brown, and A. C. Steven. 2002. Handedness of the herpes simplex virus capsid and procapsid. J. Virol. 76:7855-7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cingolani, G., S. D. Moore, P. E. J. Prevelige, and J. E. Johnson. 2002. Preliminary crystallographic analysis of the bacteriophage P22 portal protein. J. Struct. Biol. 277:20794-20803. [DOI] [PubMed] [Google Scholar]
- 6.Donate, L. E., L. Herranz, J. P. Secilla, J. M. Carazo, H. Fujisawa, and J. L. Carrascosa. 1988. Bacteriophage T3 connector: three-dimensional structure and comparison with other viral head-tail connecting regions. J. Mol. Biol. 201:91-100. [DOI] [PubMed] [Google Scholar]
- 7.Dube, P., P. Tavares, R. Lurz, and M. van Heel. 1993. The portal protein of bacteriophage SPP1: a DNA pump with 13-fold symmetry. EMBO J. 12:1303-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guasch, A., J. Pous, B. Ibarra, F. X. Gomis-Ruth, J. M. Valpuesta, N. Sousa, J. L. Carrascosa, and M. Coll. 2002. Detailed architecture of a DNA translocating machine: the high-resolution structure of the bacteriophage φ29 connector particle. J. Mol. Biol. 315:663-673. [DOI] [PubMed] [Google Scholar]
- 9.Hendrix, R. W., and R. L. Duda. 1998. Bacteriophage HK97 head assembly: a protein ballet. Adv. Virus Res. 50:235-288. [DOI] [PubMed] [Google Scholar]
- 10.Heymann, J. B., N. Cheng, W. W. Newcomb, B. L. Trus, J. C. Brown, and A. C. Steven. 2003. Dynamics of herpes simplex virus capsid maturation visualized by time-lapsed cryo-EM. Nat. Struct. Biol. 10:334-341. [DOI] [PubMed] [Google Scholar]
- 11.King, J., and W. Chiu. 1997. The procapsid-to-capsid transition in double-stranded DNA bacteriophages, p. 288-311. In W. Chiu, R. M. Burnett, and R. Garcea (ed.), Structural biology of viruses. Oxford University Press, New York, N.Y.
- 12.Kocsis, E., M. E. Cerritelli, B. L. Trus, N. Cheng, and A. C. Steven. 1995. Improved methods for determination of rotational symmetries in macromolecules. Ultramicroscopy 60:219-228. [DOI] [PubMed] [Google Scholar]
- 13.Lurz, R., E. V. Orlova, G. Gunther, P. Dube, A. Droge, F. Weise, M. van Heel, and P. Tavares. 2001. Structural organisation of the head-to-tail interface of a bacterial virus. J. Mol. Biol. 310:1027-1037. [DOI] [PubMed] [Google Scholar]
- 14.Muller, D. J., A. Engel, J. L. Carrascosa, and M. Velez. 1997. The bacteriophage φ29 head-tail connector imaged at high resolution with the atomic force microscope in buffer solution. EMBO J. 16:2547-2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newcomb, W. W., F. L. Homa, F. P. Booy, D. R. Thomsen, B. L. Trus, A. C. Steven, J. V. Spencer, and J. C. Brown. 1996. Assembly of the herpes simplex virus capsid: characterization of intermediates observed during cell-free capsid formation. J. Mol. Biol. 263:432-446. [DOI] [PubMed] [Google Scholar]
- 16.Newcomb, W. W., R. M. Juhas, D. R. Thomsen, F. L. Homa, A. D. Burch, S. K. Weller, and J. C. Brown. 2001. The UL6 gene product forms the portal for entry of DNA into the herpes simplex virus capsid. J. Virol. 75:10923-10932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orlova, E. V., B. Gowen, A. Droge, A. Stiege, F. Weise, R. Lurz, M. van Heel, and P. Tavares. 2003. Structure of a viral DNA gatekeeper at 10Å resolution by cryo-electron microscopy. EMBO J. 22:1255-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simpson, A. A., Y. Z. Tao, P. G. Leiman, M. O. Badasso, Y. N. He, P. J. Jardine, N. H. Olson, M. C. Morais, S. Grimes, D. L. Anderson, T. S. Baker, and M. G. Rossmann. 2000. Structure of the bacteriophage φ29 DNA packaging motor. Nature 408:745-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trus, B. L., F. P. Booy, W. W. Newcomb, J. C. Brown, F. L. Homa, D. R. Thomsen, and A. C. Steven. 1996. The herpes simplex virus procapsid: structure, conformational changes upon maturation, and roles of the triplex proteins VP19c and VP23 in assembly. J. Mol. Biol. 263:447-462. [DOI] [PubMed] [Google Scholar]
- 20.Valpuesta, J. M., and J. L. Carrascosa. 1994. Structure of viral connectors and their function in bacteriophage assembly and DNA packaging. Q. Rev. Biophys. 27:107-155. [DOI] [PubMed] [Google Scholar]
- 21.Valpuesta, J. M., N. Sousa, I. Barthelemy, J. J. Fernandez, H. Fujisawa, B. Ibarra, and J. L. Carrascosa. 2000. Structural analysis of the bacteriophage T3 head-to-tail connector. J. Struct. Biol. 131:146-155. [DOI] [PubMed] [Google Scholar]


