Abstract
Cyclooxygenase-2 (COX-2) is a cellular enzyme in the eicosanoid synthetic pathway that mediates the synthesis of prostaglandins from arachidonic acid. The eicosanoids function as critical regulators of a number of cellular processes, including the acute and chronic inflammatory response, hemostasis, and the innate immune response. Human cytomegalovirus (HCMV), which does not encode a viral COX-2 isoform, has been shown to induce cellular COX-2 expression. Importantly, although the precise role of COX-2 in CMV replication is unknown, COX-2 induction was shown to be critical for normal HCMV replication. In an earlier study, we identified an open reading frame (Rh10) within the rhesus cytomegalovirus (RhCMV) genome that encoded a putative protein (designated vCOX-2) with high homology to cellular COX-2. In the current study, we show that vCOX-2 is expressed with early-gene kinetics during RhCMV infection, resulting in production of a 70-kDa protein. Consistent with the expression of a viral COX-2 isoform, cellular COX-2 expression was not induced during RhCMV infection. Finally, analysis of growth of recombinant RhCMV with vCOX-2 deleted identified vCOX-2 as a critical determinant for replication in endothelial cells.
Cytomegaloviruses (CMVs) are a family of ubiquitous betaherpesviruses that establish a lifelong infection within the host. Although generally benign in individuals with a normal immune system, CMV infection can be devastating in the immune-compromised host, such as AIDS patients and neonates (31). Rhesus CMV (RhCMV) and human CMV (HCMV) are closely related viruses with comparable genomic organization and genome sequence (12, 28). Both viruses encode approximately 200 open reading frames (ORFs), with 60% of ORFs sharing significant sequence homology. RhCMV and HCMV have also been shown to infect comparable cell types, and both viruses result in similar disease pathology, with the establishment of lifelong asymptomatic infections in healthy adults (19, 25, 31).
HCMV has been shown to establish a long-term noncytopathic infection in endothelial cells (ECs) (9), which together with the observation of infected ECs in asymptomatic HCMV-infected individuals, suggests that ECs may represent a site of CMV persistence in vivo (14, 30, 44, 46). The presence of HCMV-infected ECs in the circulation of individuals during active CMV disease (37), combined with the ability of ECs to mediate infection of monocytes (21, 52), also suggests a role for ECs in virus dissemination. Consequently, an understanding of CMV replication in ECs is critical for elucidating mechanisms of CMV persistence and dissemination.
Recently, we identified a novel ORF in the RhCMV genome (Rh10) that is predicted to encode a homologue of cellular cyclooxygenase-2 (cCOX-2) (12). In contrast to sequence analysis of RhCMV, analysis of all other CMVs for which the genomic sequence is known does not identify any ORF with homology to cCOX-2. cCOX-2 is a critical enzyme in the eicosanoid synthetic pathway, a pathway that results in the synthesis of the eicosanoids prostaglandin (PG), prostacyclin, and thromboxane A2 from arachidonic acid (29, 45). Specifically, cCOX-2 converts arachidonic acid to PGH2, through a PGG2 intermediate. Various tissue-specific isomerases then convert PGH2 to other PG isoforms: PGD2, PGE2, PGF2, and PGI2. The presence of a virally encoded COX-2 homologue is a unique characteristic of RhCMV. However, recent studies have shown that other CMVs, as well as other DNA and RNA viruses, upregulate the eicosanoid pathway during infection (13, 15, 22, 26, 27, 47, 48, 55). HCMV infection induces cCOX-2 and phospholipase A2 (cPLA2), another enzyme involved in this pathway, while downregulating lipocortin, a negative inhibitor of cPLA2 activation (55). Inhibitors of cCOX-2 prevent normal HCMV replication in vitro (47, 50, 55), demonstrating the importance of the eicosanoid pathway for CMV replication. This effect of COX-2 inhibition on HCMV replication is rescued by treatment with PGE2, indicating a critical role of PGs in HCMV replication.
In the current study, we examined the role of the Rh10 ORF in RhCMV replication. We show that a viral COX-2 homologue (designated vCOX-2) is expressed from the Rh10 ORF during RhCMV infection. Drug inhibition studies showed that the vCOX-2 gene was expressed with early (E) gene kinetics; and, in contrast to HCMV, RhCMV did not induce cCOX-2 expression. Interestingly, comparison of growth of a RhCMV recombinant with vCOX-2 deleted in different cell types identified vCOX-2 as a critical determinant for CMV replication in ECs.
MATERIALS AND METHODS
Cells and virus.
Wild-type (WT) RhCMV (strain 68-1) and RhCMV recombinants were propagated and titers were determined on telomerase life-extended fetal rhesus macaque fibroblasts (Telo-RFs) as previously described (6). Rhesus macaque microvascular ECs were isolated from the brain of pathogen-free juvenile macaques. More than 95% of ECs examined at various passages stained positive for von Willebrand factor, and cells were used at low passage (eight or less). ECs were cultured in endothelial cell basal medium (Clonetics, San Diego, Calif.) supplemented with 10% human serum, 35 μg of endothelial cell growth serum (BD Biosciences, Bedford, Mass.) per ml, penicillin/streptomycin, and glutamine.
Cloning of vCOX-2.
Total cellular RNA from RhCMV-infected Telo-RFs was primed with oligo(dT) and used to generate cDNA, using Superscript II reverse transcriptase (Invitrogen, Carlsbad, Calif.). vCOX-2 was amplified from cDNA by PCR using vCOX-2-specific primers vCOX-2 for (5′-GGCTATGAGTAAAAACATCATCGTACTG-3′) and vCOX-2.rev (5′-TGCTCTAGATCATAACTCAGCATGCTCTCTT-3′). The vCOX-2 PCR product was cloned into the pGEM-T Easy vector (Promega, Madison, Wis.) and confirmed by DNA sequence analysis.
Generation of RhCMV recombinants.
Recombinant viruses were constructed by E/T recombination (1) using a bacterial artificial chromosome (BAC) containing the entire RhCMV 68-1 genome (pRhCMV/BAC-Cre) (5). For construction of RhCMV containing a deletion of the vCOX-2 gene (RhCMVΔvCOX-2), a vector (pBSKSII+ΔNaeI/SspI/MCS) derived from pBSKSII+ (Stratagene, La Jolla, Calif.) was used as a template for generation of the necessary PCR product for E/T recombination. PCR was performed using primers ΔCOX-2AmpLacZ for (5′-AAATCATGCCGGTACGTATTAAAGACACTACATTCTGGCTGCTGGCACGACAGGTTTCCCGACT-3′) and ΔCOX-2AmpLacZ.rev (5′-ACCAACAAAAAAGACACACGGGAGTTCGATTGTTTTGTGAGAGTTGGTAGCTCTTGATCCGGCA-3′), resulting in amplification of the ampicillin resistance (Ampr) marker and lacZ gene of pBSKSII+ΔNaeI/SspI/MCS. The 5′ end of primers contained 40 nucleotides with homology to RhCMV sequence flanking the site of vCOX-2 gene deletion. E/T recombination was performed essentially as described previously (1) by transformation of recombinogenic bacteria (EL250) containing pRhCMV/BAC-Cre with the PCR product. Blue colonies were selected with ampicillin (50 μg/ml), chloramphenicol (15 μg/ml), 5-bromo-3-indolyl-β-d-galactopyranoside (80 μg/ml), and isopropyl-β-d-thiogalactopyranoside (20 mM). Restriction enzyme digestion combined with Southern analysis using 32P-labeled DNA probes against the vCOX-2 gene or the Ampr-lacZ cassette and DNA sequence analysis confirmed deletion of vCOX-2 from the pRhCMVΔvCOX-2/BAC-Cre genome. To reconstitute RhCMVΔvCOX-2 virus, Telo-RFs were transfected with pRhCMVΔvCOX-2/BAC-Cre DNA, virus plaques were expanded, and stocks were prepared as described above.
An RhCMVΔvCOX-2 revertant (RhCMVΔvCOX-2Revt) was constructed by using a two-step procedure. First, an Flp recombinase target (FRT)-flanked kanamycin-resistance (Kanr) marker was inserted immediately downstream from the vCOX-2 ORF in pRhCMV/BAC-Cre by E/T recombination. Plasmid pcp015 (7) was used as a template for generation of the necessary PCR product, using primers COX-2Kan for (5′-TCCCGGGGTTGTAATAAAAAGAGAGCATGCTGAGTTATGAGTAAAACGACGGCCAGT-3′) and COX-2Kan.rev (5′-ACCAACAAAAAAGACACACGGGAGTTCGATTGTTTTGTGACAGGAAACAGCTATGAC-3′). The resultant pRhCMVCOX-2Kanr/BAC-Cre was selected with chloramphenicol and kanamycin (25 μg/ml). In the second step, pRhCMVCOX-2Kanr/BAC-Cre DNA was used as a template to generate a PCR product that contained the entire vCOX-2 gene, extending from the 5′ noncoding region to the 3′ noncoding region and included the FRT-flanked Kanr positioned immediately after the vCOX-2 gene. Primers used for PCR were COX-2Revt for (5′-GCGTGTAGTGTTTGTTCGGT-3′) and COX-2Revt.rev (5′-GGGATCTAGCATACGCGTTACGCACCAAT-3′). The PCR product was used to replace the deleted region of vCOX-2 in pRhCMVΔvCOX-2/BAC-Cre followed by selection with chloramphenicol and kanamycin. The FRT-flanked Kanr marker was then removed by arabinose induction of Flp recombinase (24) and screening for kanamycin sensitivity. BAC recombinants were characterized, including DNA sequence analysis of the entire vCOX-2 gene, and virus stocks were prepared as described above. RhCMVΔvCOX-2Revt contains the entire vCOX-2 gene and is WT in sequence except for a single FRT site positioned in the 3′ noncoding region of the vCOX-2 gene.
A recombinant RhCMV containing a hemagglutinin A (HA) epitope fused to the carboxyl terminus of vCOX-2 was constructed essentially as described above. Plasmid pcp015 served as a template for amplification of the necessary PCR product, using primers COX-2HA for (5′-TCGATCCCGGGGTTGTAATAAAAAGAGAGCATGCTGAGTTATACCCATACGATGTTCCAGATTACGCTTGAGTAAAACGACGGCCAGT-3′) and COX-2Kan.rev. The PCR product contains the FRT-flanked Kanr marker immediately downstream of the site of the HA tag insertion. E/T recombination was performed in EL250 bacteria containing pRhCMV/BAC-Cre, and recombinants were selected with chloramphenicol and kanamycin followed by Flp-mediated removal of the FRT-flanked Kanr. Following BAC characterization, virus was reconstituted by electroporation of Telo-RFs with RhCMVvCOX-2HA/BAC DNA as described above.
Northern analysis.
Telo-RFs were infected with either WT RhCMV or RhCMVΔvCOX-2 at a multiplicity of infection (MOI) of 3. At 2 h postinfection (p.i.), virus inocula were removed and replaced with fresh medium. Cells were then cultured for the times indicated. Foscarnet (200 μg/ml) (Sigma, St. Louis, Mo.) was added to the designated cultures to inhibit viral DNA synthesis. Total cellular RNA was harvested by using Trizol (Invitrogen, Carlsbad, Calif.). RNA (10 μg) was then separated by electrophoresis on a 1% agarose-formaldehyde gel, followed by transfer to GeneScreen Plus nylon membranes (NEN, Boston, Mass.) and UV cross-linking using a Stratagene Stratalinker. Hybridization with [32P]DNA probes was performed using ExpressHyb (Clontech, Palo Alto, Calif.) and visualized by autoradiography. [32P]DNA probes directed against vCOX-2 (exon 1) and GAPDH were labeled with the Random Primed Nucleotide kit (Roche, Mannheim, Germany).
Western analysis.
Telo-RFs were infected at an MOI of 10 with either RhCMVWT or RhCMVvCOX-2HA and cultured for the times indicated. Cell lysates were harvested in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer, and proteins were separated by SDS-7% PAGE. Following electrophoretic transfer to polyvinylidene difluoride membranes (Millipore, Bedford, Mass.) and blocking, HA-tagged vCOX-2 was identified by using a rabbit anti-HA antibody (Becton Dickinson, Clontech, Palo Alto, Calif.) (used at a 1:400 dilution) and goat anti-rabbit horseradish-peroxidase-conjugated secondary antibody (Amersham, Piscataway, N.J.) (used at a 1:20,000 dilution). Horseradish peroxidase activity was visualized by using the West Pico chemiluminescence substrate (Pierce, Rockford, Ill.).
RhCMV growth analysis.
Telo-RFs or ECs were infected with RhCMVWT or RhCMV recombinants at an MOI of 0.01. Inocula were removed after 2 h, and cells were washed three times with Dulbecco's phosphate-buffered saline prior to addition of fresh medium. At indicated times p.i., cells and supernatants were harvested and titers were determined by plaque assay as previously described (16). Experiments were performed three times, and titers were determined in duplicate.
Quantitative RT-PCR analysis of cCOX-2.
Telo-RFs were infected with RhCMVWT or RhCMV recombinants at an MOI of 3. At times indicated p.i., total cellular RNA was harvested and used to prepare cDNA, using Omniscript RT (QIAGEN, Valencia, Calif.) according to the manufacturer's procedure. Quantitative real-time PCR (RT-PCR) was performed using SYBR green and primers specific for rhesus macaque cellular COX-2: Forward primer (5′-TCCACCCGCAGTACAAAAAGT-3′) and Reverse primer (5′-GCTTCAGCATAAAGCGTTTGC-3′). RT-PCR was performed with an ABI Prism 7700 machine (Applied Biosystems, Foster City, Calif.) and the following conditions: 50°C for 2 min and 95°C for 10 min, followed by 40 cycles at 95°C for 15 s and 60°C for 1 min. β-Actin was used as an internal control, and cycle threshold values were determined by automated threshold analysis with ABI Prism version 1.0 software.
RESULTS
The RhCMV vCOX-2 gene produces a multiply spliced transcript that encodes a predicted protein (vCOX-2) with high homology to human cCOX-2.
The recent DNA sequence analysis of the complete RhCMV genome reveals a high level of sequence conservation between the RhCMV and HCMV genomes. However, regions of significant sequence divergence between the two viruses also exist (12). The Rh10 ORF is unique to RhCMV and is predicted to give rise to a multiply spliced transcript that encodes a protein with high homology to human cCOX-2. To confirm expression of Rh10 (designated vCOX-2), as well as to determine the splicing pattern of the vCOX-2 transcript, cDNA was synthesized from total cellular RNA derived from RhCMV-infected Telo-RFs at 48 h p.i. The vCOX-2 gene was then amplified from the cDNA by using primers upstream of the predicted translational start site at nucleotide (nt) 10933 and downstream from the predicted stop codon at nt 8521. With these PCR primers, amplification of the cDNA resulted in a PCR product of 2.0 kb. Sequence comparison of the vCOX-2 cDNA with the genomic RhCMV DNA sequence indicated that the vCOX-2 ORF is composed of seven exons. A schematic showing the genetic structure of the vCOX-2 gene is shown in Fig. 1A. Although devoid of a recognizable Kozak sequence, the ATG positioned at nt 10933 is the most probable translational start site of the vCOX-2 transcript based on comparison with the human cCOX-2 sequence. Several consensus TATA-box elements are located at positions −124, −154, and −256 (relative to the ATG at nt 10933 to 10935). A stop codon (UGA) is positioned at nt 8521, and a consensus polyadenylation site (AATAAA) is located at nt 8544 to 8549.
FIG. 1.
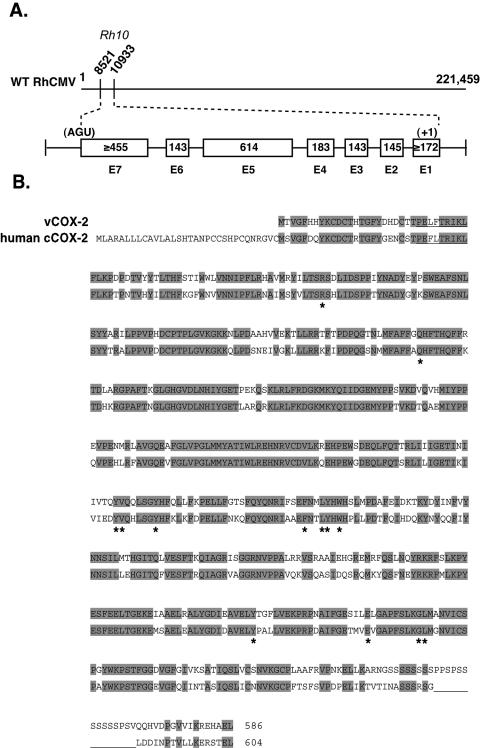
Genomic localization and structure of the RhCMV vCOX-2 gene. (A) Schematic showing position of the vCOX-2 gene within the RhCMV genome. The vCOX-2 exons are shown below, with the predicted translational start (+1) at nt 10933 and stop (UGA) site at nt 8521 to 8523. (B) Alignment of the amino acid sequences of vCOX-2 cloned from cDNA with the human cCOX-2 protein. Identical residues are shown in grey. Active-site residues of human cCOX-2 are marked by asterisks.
The complete rhesus macaque cCOX-2 sequence is not known. However, comparison of the predicted vCOX-2 protein (585 amino acids) with human cCOX-2 (604 amino acids) shows a high level of amino acid sequence conservation (67%) throughout the coding region (Fig. 1B). Importantly, 13 residues identified by site-directed mutagenesis or crystal structure as critical for cCOX-2 enzymatic activity are conserved between vCOX-2 and human cCOX-2 (18, 20, 32, 36, 38, 41, 51). Two significant regions of divergence between the two proteins are the absence of a hydrophobic N-terminal signal peptide in vCOX-2 and the presence of a 14-amino-acid serine/proline-rich insertion near the C terminus of vCOX-2. Together, these results indicate that the vCOX-2 gene is expressed during RhCMV infection as a multiply spliced transcript that is predicted to encode a vCOX-2 protein with high homology to human cCOX-2. Conservation of residues known to be critical for human cCOX-2 function indicate that vCOX-2 is likely to be an enzymatically active COX-2 isoform.
RhCMV vCOX-2 is expressed with E gene kinetics during RhCMV infection of Telo-RFs.
Northern analysis of total cellular RNA from RhCMV-infected Telo-RFs was performed to determine the kinetics of vCOX-2 gene expression during RhCMV infection. RNA from RhCMV-infected Telo-RFs was harvested at 4, 8, 12, 24, and 48 h p.i. Foscarnet was added as indicated to inhibit viral DNA synthesis. Following RNA electrophoresis and transfer, Northern analysis was performed using a probe specific for the predicted exon 1 of vCOX-2. As shown in Fig. 2, a transcript (2.4 kb) was observed as early as 24 h p.i. which corresponds to the predicted size of the vCOX-2 gene with an additional 400 bp of 5′ and 3′ untranslated regions. Expression of the 2.4-kb transcript was still observed in the presence of the viral DNA polymerase inhibitor Foscarnet. The absence of any signal when probes directed against ORFs adjacent (rh9, rh12, and rh13) or overlapping (rh11) the vCOX-2 gene were used indicate that the vCOX-2-containing transcript is the major transcript expressed from this region of the RhCMV genome during RhCMV infection of Telo-RFs. Together, these results show that the vCOX-2 gene is expressed during RhCMV infection of Telo-RFs and is expressed with E gene kinetics.
FIG. 2.
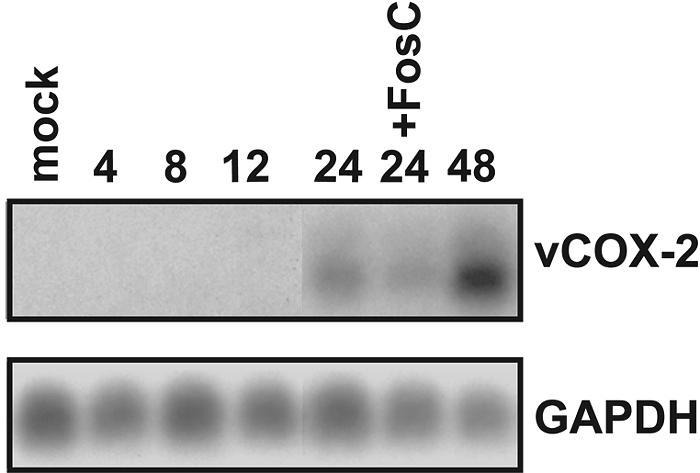
Northern analysis of vCOX-2 gene expression during RhCMVWT infection of Telo-RFs. Telo-RFs were infected with RhCMVWT at an MOI of 3, and total cellular RNA was isolated at the indicated times p.i. (in hours). Foscarnet (FosC) was added to cells as indicated. RNA (10 μg/lane) was separated by electrophoresis and transferred prior to Northern analysis using 32P-labeled DNA probes corresponding to vCOX-2 (exon 1) and GAPDH.
The endogenous RhCMV vCOX-2 gene encodes a 70-kDa vCOX-2 protein that is produced during RhCMV infection of Telo-RFs.
To confirm that the vCOX-2 protein was expressed during RhCMV infection, an HA tag was fused in frame with the predicted C terminus of the endogenous RhCMV vCOX-2 gene, using E/T linear recombination as described previously (3), resulting in the RhCMV recombinant RhCMVvCOX-2HA (Fig. 3A). Given the high level of homology between cellular and vCOX-2 proteins, the HA tag was critical to enable discrimination of the viral from the cellular form of the protein in RhCMV-infected cells. Western analysis of cellular lysates from Telo-RFs infected with RhCMVvCOX-2HA identified an HA-reactive doublet migrating at approximately 70 kDa that was not observed in lysates from RhCMVWT cells (Fig. 3B). The identification of the HA-tagged protein also confirms that the vCOX-2 protein terminates at the predicted stop codon (UGA) positioned at nt 8521 to 8523 within the RhCMV genome. Appearance of the vCOX-2HA protein within infected lysates was consistent with E gene kinetics observed by Northern analysis (Fig. 2). The vCOX-2HA protein was detected in lysates as early as 24 h p.i. and was expressed through 48 h p.i., even in the presence of an inhibitor of viral DNA polymerase. These studies show that the RhCMV vCOX-2 gene encodes a protein which is expressed with E gene kinetics during in vitro infection of Telo-RFs.
FIG. 3.
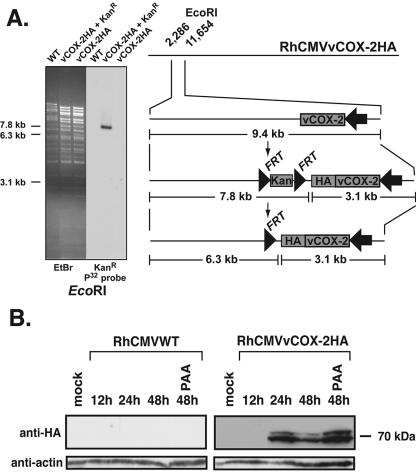
Construction and characterization of a RhCMVvCOX-2HA recombinant expressing an HA-tagged endogenous vCOX-2 protein. (A) Schematic showing construction of RhCMVvCOX-2HA/BAC-Cre. To generate RhCMVvCOX-2HA/BAC-Cre, an HA epitope was fused in frame with the 3′ end of the vCOX-2 ORF by E/T recombination. Following EcoRI digestion, insertion of the HA tag results in loss of a 9.4-kb vCOX-2-containing fragment and production of new 7.8- and 3.1-kb fragments. The FRT-flanked Kanr marker was then removed by Flp recombinase-mediated excision, resulting in a 7.8- to 6.3-kb band shift. Also shown is DNA electrophoresis of EcoRI-digested RhCMVvCOX-2HA/BAC-Cre, showing predicted DNA band shifts. Southern blotting using a Kanr-specific probe shows insertion of a single Kanr marker in the appropriate 7.8-kb EcoRI fragment and Kanr removal following Flp recombinase induction. (B) Western analysis of RhCMVvCOX-2HA-infected Telo-RFs. Rhesus macaque Telo-RFs were infected with either RhCMVWT or RhCMVvCOX-2HA at an MOI of 10, and protein was harvested at the times indicated p.i. Viral DNA polymerase was inhibited by the addition of phosphonoacetic acid (PAA) as indicated. Proteins were separated by SDS-PAGE, transferred, and assayed by Western immunoblotting for the presence of vCOX-2HA (using anti-HA antibody) or actin (loading control).
RhCMV does not induce rhesus cCOX-2 expression during infection of Telo-RFs.
In a previous study, HCMV-mediated induction of cCOX-2 expression was shown to be critical for normal HCMV replication in fibroblasts (55). We hypothesized that the expression of a virally encoded COX-2 would circumvent the requirement of RhCMV to induce the rhesus cCOX-2 molecule during infection. To determine the effect of RhCMVWT and RhCMVΔvCOX-2 on cCOX-2 expression, Telo-RFs were infected with the viruses at an MOI of 3 and the level of cCOX-2 expression was measured by quantitative RT-PCR at various times p.i. As shown in Fig. 4, although a transient increase in cCOX-2 expression was observed at early times p.i. (4 and 8 h), infection with either RhCMVWT or RhCMVΔvCOX-2 did not result in a significant induction of cCOX-2 expression at later times during the replication cycle.
FIG. 4.
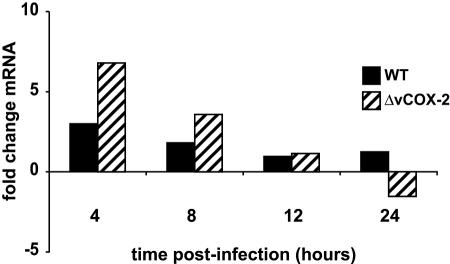
Quantitative RT-PCR showing absence of rhesus cCOX-2 gene induction during RhCMV infection of Telo-RFs. Telo-RFs were infected with RhCMVWT (black bars) and RhCMVΔvCOX-2 (hatched bars) at an MOI of 3, and the level of cCOX-2 expression was measured by quantitative RT-PCR at increasing times p.i. Primers specific to endogenous cCOX-2 were used, and amplified products were detected with SYBR green. The results shown are representative of two independent experiments.
RhCMV vCOX-2 is a tropism determinant for RhCMV replication in rhesus ECs.
The ability of CMV to replicate in a diverse number of specific cell types is believed to be critical for persistence and dissemination of the virus within the host (for a review, see reference 17). Consequently, a large component of the CMV genome is thought to encode genes required for replication in these different cell types. To investigate the role of vCOX-2 in cellular tropism, the growth of an RhCMV recombinant containing a deletion of the vCOX-2 gene (RhCMVΔCOX-2) was analyzed in Telo-RFs and ECs. RhCMVΔCOX-2 was constructed by replacement of the entire vCOX-2 ORF with a lacZ-Ampr cassette (Fig. 5A), resulting in complete ablation of the vCOX-2 coding region, as shown by Northern analysis (Fig. 5B). Analysis was performed of viral growth in Telo-RFs and ECs. As shown in Fig. 6A, RhCMVΔvCOX-2 grew at comparable levels (peak titer, approximately 1 × 106 PFU/ml) and with similar kinetics to those of RhCMVWT in Telo-RFs. However, comparison of virus replication in ECs showed that RhCMVΔvCOX-2 growth was dramatically impaired in this cell type compared to either RhCMVWT or a revertant of RhCMVΔvCOX-2 containing a repaired vCOX-2 gene (RhCMVΔvCOX-2Revt) (Fig. 6B). At the peak of their growth curves, RhCMVΔvCOX-2 grew to titers that were 4 log units lower than those of either RhCMVWT or RhCMVΔvCOX-2Revt. Growth of RhCMVWT and RhCMVΔvCOX-2Revt in either Telo-RF or ECs was comparable, both in level (peak titer, approximately 1 × 106 PFU/ml) and kinetics. The ability of RhCMVΔvCOX-2Revt to rescue the EC growth defect of RhCMVΔvCOX-2 definitively establishes that the EC growth defect is due to a lack of the vCOX-2 gene. Together, these results identify the RhCMV-encoded vCOX-2 as a novel determinant of EC tropism.
FIG. 5.
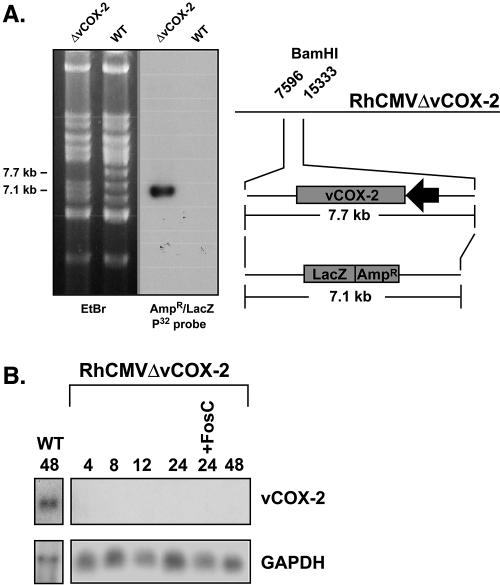
Construction and characterization of an RhCMVΔvCOX-2 recombinant. (A) Schematic showing construction of RhCMVΔvCOX-2/BAC-Cre. RhCMVΔvCOX-2/BAC-Cre was constructed by using E/T recombination to replace the entire vCOX-2 ORF with an Ampr-lacZ cassette. Following BamHI digestion, deletion of the vCOX-2 ORF results in the shift of a 7.7- to 7.1-kb fragment that is reactive with an Ampr-lacZ probe in Southern blotting. (B) Northern analysis showing absence of vCOX-2- reactive transcripts from RhCMVΔvCOX-2-infected Telo-RFs.
FIG. 6.
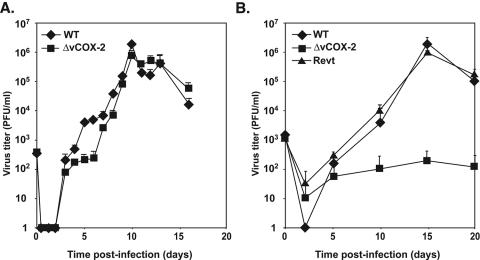
Growth analysis of RhCMVΔvCOX-2, RhCMVvCOX-2Revt, and RhCMVWT. Telo-RFs (A) or ECs (B) were infected at an MOI of 0.01 with either RhCMVΔvCOX-2, RhCMVvCOX-2Revt, or RhCMVWT. Samples were collected at the indicated days p.i., and titers were determined by plaque assay on Telo-RFs. Results are the averages of three experiments, and standard errors are shown.
DISCUSSION
In the current study, we have shown that the recently identified RhCMV Rh10 ORF (12) encodes a cCOX-2 homologue that functions as a critical determinant of EC tropism. The product of Rh10 (designated viral COX-2; vCOX-2) has 67% amino acid identity with human cCOX-2, and conservation of critical residues required for cCOX-2 activity suggests that vCOX-2 is enzymatically active. Northern analysis showed that vCOX-2 is expressed in infected rhesus fibroblasts with E gene kinetics. Previously, HCMV infection was shown to induce high levels of cCOX-2 throughout the course of infection (55). Although rhesus macaque cCOX-2 expression was increased immediately following RhCMV infection, cCOX-2 expression was decreased at later times. Finally, deletion of the vCOX-2 gene from the RhCMV genome resulted in a dramatic 4-log-unit reduction in the ability of RhCMV to replicate in ECs compared to fibroblasts, identifying vCOX-2 as a novel determinant of EC tropism.
ECs are an important cell type in CMV pathogenesis and have been implicated as sites for virus persistence and dissemination. In murine CMV, M45, a ribonucleotide reductase homologue, was recently shown to be required for replication in ECs by mediating inhibition of apoptosis (2). However, the identification of viral determinants of EC tropism in the primate CMVs (HCMV, RhCMV, and chimpanzee CMV) has been elusive. Earlier studies using HCMV showed a marked difference in the abilities of different virus strains to grow in ECs, and a possible block in growth of several non-EC tropic strains was identified at the level of nuclear translocation of the viral genome (42, 43). However, variation in the ability of even the same strain of virus to replicate to a comparable level in ECs, as well as an inability to map the genetic determinants by mutagenesis, prevented identification of tropic determinants. More recently, deletion of the M45 homologue (UL45) from a stable BAC-cloned endothelial tropic strain showed that UL45 was not involved in EC tropism (11). In our study, deletional analysis of the RhCMV genome, using BAC-based technology, has enabled definitive identification of RhCMV ORF Rh10 (vCOX-2) as a critical determinant of EC tropism. This observation represents identification of the first tropic determinant of a primate CMV.
The presence of a cCOX-2 homologue within the viral genome appears to be unique to RhCMV. However, COX-2 has been shown to be critical for the replication of many DNA and RNA viruses (22, 34, 35, 53, 55). For HCMV, infection of fibroblasts was shown to upregulate cCOX-2 expression and cCOX-2 activity was shown to be essential for normal virus replication (55). In our studies, the existence of a virally encoded COX-2 homologue further emphasizes the importance of COX-2 for the CMV life cycle. However, in contrast to HCMV, RhCMV has apparently adopted a strategy of expressing a virally encoded form of the enzyme, and we show that RhCMV infection induces only a transient induction of cCOX-2 expression, contrary to the prolonged induction observed with HCMV (wherein, cCOX-2 was still upregulated sevenfold at 24 h following HCMV infection of fibroblasts [54]). The mechanism of cCOX-2 involvement in HCMV replication is not clear, although cCOX-2 inhibitors were shown to decrease expression of IE2 (an HCMV immediate-early transcriptional regulator), and PGE2 treatment completely reversed the inhibitory effects of the COX-2-specific inhibitors on HCMV growth (55). The mechanism by which vCOX-2 enables replication in ECs has not been determined. However, the conservation of residues critical for enzymatic activity between the cellular and viral COX-2 suggests that the vCOX-2 may be enzymatically active, indicating a potential importance of PGs in mediating EC tropism.
CMV has been implicated in long-term chronic diseases, including atherosclerosis, chronic rejection, and colorectal cancer (13, 49). It is possible that the effect of CMV on the PG biosynthetic pathway may, at least in part, be involved in these processes. PGs are generally proinflammatory molecules that affect vascular permeability, which allows infiltration of proinflammatory cells and effector molecules. Many studies show a positive correlation between cCOX-2 activity and angiogenesis (10), and this effect of cCOX-2 has specifically been shown in ECs (23). The proangiogenic properties of cCOX-2 are also believed to lead to the involvement of this molecule in tumorigenesis (8, 39, 40) and atherosclerosis (4, 33). Consequently, the eicosanoid pathway may be a potential target for pharmaceutical intervention to prevent these CMV-meditated pathogenic processes.
In summary, we have identified a cCOX-2 homologue as a novel determinant of EC tropism, which represents the first EC tropic determinant in a primate CMV. RhCMV is the virus most closely related to HCMV with a working animal model. Consequently, the identification of RhCMV vCOX-2 as a critical determinant of cellular tropism provides an excellent opportunity to study the role of tropism in pathogenesis in a CMV model closely related to HCMV.
Acknowledgments
We thank D. L. Court (National Cancer Institute, Frederick, Md.) and W. Wackernagel (Genetik, Fachbereich Biologie, Universität Oldenburg, Oldenberg, Germany) for their kind gifts of the EL250 recombinogenic bacteria and the pcp015 plasmid, respectively, used in this study.
The study was supported by National Institutes of Health grants RR15094 (D.G.A. and S.W.W.), RR00163 (S.W.W. and J.A.N.), AI21640 (J.A.N. and M.A.J.), and 5F32AI057218-02 (S.G.H.), a Long-Term Fellowship of the Human Frontiers Science Program (M.W.), and an OHSU Department of Hematology and Medical Oncology NIH Training Grant (C.A.R.).
REFERENCES
- 1.Britt, W. J., M. A. Jarvis, J.-Y. Seo, D. D. Drummond, and J. A. Nelson. 2004. Rapid genetic engineering of human cytomegalovirus using a lamda phage linear recombination system: demonstration that pp28 (UL99) is essential for production of infectious virus. J. Virol. 78:539-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brune, W., C. Menard, J. Heesemann, and U. H. Koszinowski. 2001. A ribonucleotide reductase homolog of cytomegalovirus and endothelial cell tropism. Science 291:303-305. [DOI] [PubMed] [Google Scholar]
- 3.Bubeck, A., U. Reusch, M. Wagner, T. Ruppert, W. Muranyi, P. M. Kloetzel, and U. H. Koszinowski. 2002. The glycoprotein gp48 of murine cytomegalovirus L proteasome-dependent cytosolic dislocation and degradation. J. Biol. Chem. 277:2216-2224. [DOI] [PubMed] [Google Scholar]
- 4.Burleigh, M. E., V. R. Babaev, J. A. Oates, R. C. Harris, S. Gautam, D. Riendeau, L. J. Marnett, J. D. Morrow, S. Fazio, and M. F. Linton. 2002. Cyclooxygenase-2 promotes early atherosclerotic lesion formation in LDL receptor-deficient mice. Circulation 105:1816-1823. [DOI] [PubMed] [Google Scholar]
- 5.Chang, W. L., and P. A. Barry. 2003. Cloning of the full-length rhesus cytomegalovirus genome as an infectious and self-excisable bacterial artificial chromosome for analysis of viral pathogenesis. J. Virol. 77:5073-5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang, W. L., V. Kirchoff, G. S. Pari, and P. A. Barry. 2002. Replication of rhesus cytomegalovirus in life-expanded rhesus fibroblasts expressing human telomerase. J. Virol. Methods 104:135-146. [DOI] [PubMed] [Google Scholar]
- 7.Cherepanov, P. P., and W. Wackernagel. 1995. Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene 158:9-14. [DOI] [PubMed] [Google Scholar]
- 8.Eberhart, C. E., R. J. Coffey, A. Radhika, F. M. Giardiello, S. Ferrenbach, and R. N. DuBois. 1994. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology 107:1183-1188. [DOI] [PubMed] [Google Scholar]
- 9.Fish, K. N., C. Soderberg-Naucler, L. K. Mills, S. Stenglein, and J. A. Nelson. 1998. Human cytomegalovirus persistently infects aortic endothelial cells. J. Virol. 72:5661-5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghosh, A. K., N. Hirasawa, H. Niki, and K. Ohuchi. 2000. Cyclooxygenase-2-mediated angiogenesis in carrageenin-induced granulation tissue in rats. J. Pharmacol. Exp. Ther. 295:802-809. [PubMed] [Google Scholar]
- 11.Hahn, G., H. Khan, F. Baldanti, U. H. Koszinowski, M. G. Revello, and G. Gerna. 2002. The human cytomegalovirus ribonucleotide reductase homolog UL45 is dispensable for growth in endothelial cells, as determined by a BAC-cloned clinical isolate of human cytomegalovirus with preserved wild-type characteristics. J. Virol. 76:9551-9555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansen, S. G., L. I. Strelow, D. C. Franchi, D. G. Anders, and S. W. Wong. 2003. Complete sequence and genomic analysis of rhesus cytomegalovirus. J. Virol. 77:6620-6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harkins, L., A. L. Volk, M. Samanta, I. Mikolaenko, W. J. Britt, K. I. Bland, and C. S. Cobbs. 2002. Specific localisation of human cytomegalovirus nucleic acids and proteins in human colorectal cancer. Lancet 360:1557-1563. [DOI] [PubMed] [Google Scholar]
- 14.Hendrix, M., P. H. J. Dormans, P. Kitseelar, F. Bosman, and C. A. Bruggeman. 1989. The presence of CMV nucleic acids arterial walls of atherosclerotic and non-atherosclerotic patients. Am. J. Pathol. 134:1151-1157. [PMC free article] [PubMed] [Google Scholar]
- 15.Janelle, M. E., A. Gravel, J. Gosselin, M. J. Tremblay, and L. Flamand. 2002. Activation of monocyte cyclooxygenase-2 gene expression by human herpesvirus 6. Role for cyclic AMP-responsive element-binding protein and activator protein-1. J. Biol. Chem. 277:30665-30674. [DOI] [PubMed] [Google Scholar]
- 16.Jarvis, M. A., T. R. Jones, D. D. Drummond, P. P. Smith, W. J. Britt, J. A. Nelson, and C. J. Baldick. 2004. Phosphorylation of human cytomegalovirus glycoprotein B (gB) at the acidic cluster casein kinase 2 site (Ser900) is required for localization of gB to the trans-Golgi network and efficient virus replication. J. Virol. 78:285-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jarvis, M. A., and J. A. Nelson. 2002. Human cytomegalovirus persistence and latency in endothelial cells and macrophages. Curr. Opin. Microbiol. 5:403-407. [DOI] [PubMed] [Google Scholar]
- 18.Karthein, R., R. Dietz, W. Nastainczyk, and H. H. Ruf. 1988. Higher oxidation states of prostaglandin H synthase. EPR study of a transient tyrosyl radical in the enzyme during the peroxidase reaction. Eur. J. Biochem. 171:313-320. [DOI] [PubMed] [Google Scholar]
- 19.Kaur, A., N. Kassis, C. L. Hale, M. Simon, M. Elliott, A. Gomez-Yafal, J. D. Lifson, R. C. Desrosiers, F. Wang, P. Barry, M. Mach, and R. P. Johnson. 2003. Direct relationship between suppression of virus-specific immunity and emergence of cytomegalovirus disease in simian AIDS. J. Virol. 77:5749-5758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kiefer, J. R., J. L. Pawlitz, K. T. Moreland, R. A. Stegeman, W. F. Hood, J. K. Gierse, A. M. Stevens, D. C. Goodwin, S. W. Rowlinson, L. J. Marnett, W. C. Stallings, and R. G. Kurumbail. 2000. Structural insights into the stereochemistry of the cyclooxygenase reaction. Nature 405:97-101. [DOI] [PubMed] [Google Scholar]
- 21.Knight, D. A., W. J. Waldman, and D. D. Sedmak. 1999. Cytomegalovirus-mediated modulation of adhesion molecule expression by human arterial and microvascular endothelial cells. Transplantation 68:1814-1818. [DOI] [PubMed] [Google Scholar]
- 22.Lara-Pezzi, E., M. V. Gomez-Gaviro, B. G. Galvez, E. Mira, M. A. Iniguez, M. Fresno, A. C. Martinez, A. G. Arroyo, and M. Lopez-Cabrera. 2002. The hepatitis B virus X protein promotes tumor cell invasion by inducing membrane-type matrix metalloproteinase-1 and cyclooxygenase-2 expression. J. Clin. Investig. 110:1831-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leahy, K. M., R. L. Ornberg, Y. Wang, B. S. Zweifel, A. T. Koki, and J. L. Masferrer. 2002. Cyclooxygenase-2 inhibition by celecoxib reduces proliferation and induces apoptosis in angiogenic endothelial cells in vivo. Cancer Res. 62:625-631. [PubMed] [Google Scholar]
- 24.Lee, E. C., D. Yu, J. Martinez de Velasco, L. Tessarollo, D. A. Swing, D. L. Court, N. A. Jenkins, and N. G. Copeland. 2001. A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics 73:56-65. [DOI] [PubMed] [Google Scholar]
- 25.Lockridge, K. M., G. Sequar, S. S. Zhou, Y. Yue, C. P. Mandell, and P. A. Barry. 1999. Pathogenesis of experimental rhesus cytomegalovirus infection. J. Virol. 73:9576-9583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molina-Holgado, E., A. Arevalo-Martin, S. Ortiz, J. M. Vela, and C. Guaza. 2002. Theiler's virus infection induces the expression of cyclooxygenase-2 in murine astrocytes: inhibition by the anti-inflammatory cytokines interleukin-4 and interleukin-10. Neurosci. Lett. 324:237-241. [DOI] [PubMed] [Google Scholar]
- 27.Murono, S., H. Inoue, T. Tanabe, I. Joab, T. Yoshizaki, M. Furukawa, and J. S. Pagano. 2001. Induction of cyclooxygenase-2 by Epstein-Barr virus latent membrane protein 1 is involved in vascular endothelial growth factor production in nasopharyngeal carcinoma cells. Proc. Natl. Acad. Sci. USA 98:6905-6910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy, E., D. Yu, J. Grimwood, J. Schmutz, M. Dickson, M. A. Jarvis, G. Hahn, J. A. Nelson, R. M. Myers, and T. E. Shenk. 2003. Coding potential of laboratory and clinical strains of human cytomegalovirus. Proc. Natl. Acad. Sci. USA 100:14976-14981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Narumiya, S., Y. Sugimoto, and F. Ushikubi. 1999. Prostanoid receptors: structures, properties, and functions. Physiol. Rev. 79:1193-1226. [DOI] [PubMed] [Google Scholar]
- 30.Pampou, S., S. N. Gnedoy, V. B. Bystrevskaya, V. N. Smirnov, E. I. Chazov, J. L. Melnick, and M. E. DeBakey. 2000. Cytomegalovirus genome and the immediate-early antigen in cells of different layers of human aorta. Virchows Arch. 436:539-552. [DOI] [PubMed] [Google Scholar]
- 31.Pass, R. F. 2001. Cytomegalovirus, p. 2675-2705. In P. M. H. D. M. Knipe, D. E. Griffin, R. A. Lamb M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 32.Picot, D., P. J. Loll, and R. M. Garavito. 1994. The X-ray crystal structure of the membrane protein prostaglandin H2 synthase-1. Nature 367:243-249. [DOI] [PubMed] [Google Scholar]
- 33.Pratico, D., C. Tillmann, Z. B. Zhang, H. Li, and G. A. FitzGerald. 2001. Acceleration of atherogenesis by COX-1-dependent prostanoid formation in low density lipoprotein receptor knockout mice. Proc. Natl. Acad. Sci. USA 98:3358-3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rahman, M. A., D. K. Dhar, E. Yamaguchi, S. Maruyama, T. Sato, H. Hayashi, T. Ono, A. Yamanoi, H. Kohno, and N. Nagasue. 2001. Coexpression of inducible nitric oxide synthase and COX-2 in hepatocellular carcinoma and surrounding liver: possible involvement of COX-2 in the angiogenesis of hepatitis C virus-positive cases. Clin. Cancer Res. 7:1325-1332. [PubMed] [Google Scholar]
- 35.Rohrenbeck, A. M., M. Bette, D. C. Hooper, F. Nyberg, L. E. Eiden, B. Dietzschold, and E. Weihe. 1999. Upregulation of COX-2 and CGRP expression in resident cells of the Borna disease virus-infected brain is dependent upon inflammation. Neurobiol. Dis. 6:15-34. [DOI] [PubMed] [Google Scholar]
- 36.Rowlinson, S. W., B. C. Crews, C. A. Lanzo, and L. J. Marnett. 1999. The binding of arachidonic acid in the cyclooxygenase active site of mouse prostaglandin endoperoxide synthase-2 (COX-2). A putative L-shaped binding conformation utilizing the top channel region. J. Biol. Chem. 274:23305-23310. [DOI] [PubMed] [Google Scholar]
- 37.Salzberger, B., D. Myerson, and M. Boeckh. 1997. Circulating cytomegalovirus (CMV)-infected endothelial cells in marrow transplant patients with CMV disease and CMV infection. J. Infect. Dis. 176:778-781. [DOI] [PubMed] [Google Scholar]
- 38.Seibold, S. A., J. F. Cerda, A. M. Mulichak, I. Song, R. M. Garavito, T. Arakawa, W. L. Smith, and G. T. Babcock. 2000. Peroxidase activity in prostaglandin endoperoxide H synthase-1 occurs with a neutral histidine proximal heme ligand. Biochemistry 39:6616-6624. [DOI] [PubMed] [Google Scholar]
- 39.Sheng, G. G., J. Shao, H. Sheng, E. B. Hooton, P. C. Isakson, J. D. Morrow, R. J. Coffey, Jr., R. N. DuBois, and R. D. Beauchamp. 1997. A selective cyclooxygenase 2 inhibitor suppresses the growth of H-ras-transformed rat intestinal epithelial cells. Gastroenterology 113:1883-1891. [DOI] [PubMed] [Google Scholar]
- 40.Sheng, H., J. Shao, S. C. Kirkland, P. Isakson, R. J. Coffey, J. Morrow, R. D. Beauchamp, and R. N. DuBois. 1997. Inhibition of human colon cancer cell growth by selective inhibition of cyclooxygenase-2. J. Clin. Invest. 99:2254-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shimokawa, T., R. J. Kulmacz, D. L. DeWitt, and W. L. Smith. 1990. Tyrosine 385 of prostaglandin endoperoxide synthase is required for cyclooxygenase catalysis. J. Biol. Chem. 265:20073-20076. [PubMed] [Google Scholar]
- 42.Sinzger, C., M. Kahl, K. Laib, K. Klingel, P. Rieger, B. Plachter, and G. Jahn. 2000. Tropism of human cytomegalovirus for endothelial cells is determined by a post-entry step dependent on efficient translocation to the nucleus. J. Gen. Virol. 81:3021-3035. [DOI] [PubMed] [Google Scholar]
- 43.Slobbe-van Drunen, M. E., A. T. Hendrickx, R. C. Vossen, E. J. Speel, M. C. van Dam-Mieras, and C. A. Bruggeman. 1998. Nuclear import as a barrier to infection of human umbilical vein endothelial cells by human cytomegalovirus strain AD169. Virus Res. 56:149-156. [DOI] [PubMed] [Google Scholar]
- 44.Smiley, M. L., E. C. Mar, and E. S. Huang. 1988. Cytomegalovirus infection and viral-induced transformation of human endothelial cells. J. Med. Virol. 25:213-226. [DOI] [PubMed] [Google Scholar]
- 45.Smith, W. L., D. L. DeWitt, and R. M. Garavito. 2000. Cyclooxygenases: structural, cellular, and molecular biology. Annu. Rev. Biochem. 69:145-182. [DOI] [PubMed] [Google Scholar]
- 46.Speir, E., R. Modali, E. S. Huang, M. B. Leon, F. Shawl, T. Finkel, and S. E. Epstein. 1994. Potential role of human cytomegalovirus and p53 interaction in coronary restenosis. Science 265:391-394. [DOI] [PubMed] [Google Scholar]
- 47.Speir, E., Z. X. Yu, V. J. Ferrans, E. S. Huang, and S. E. Epstein. 1998. Aspirin attenuates cytomegalovirus infectivity and gene expression mediated by cyclooxygenase-2 in coronary artery smooth muscle cells. Circ. Res. 83:210-216. [DOI] [PubMed] [Google Scholar]
- 48.Steer, S. A., J. M. Moran, L. B. Maggi, Jr., R. M. Buller, H. Perlman, and J. A. Corbett. 2003. Regulation of cyclooxygenase-2 expression by macrophages in response to double-stranded RNA and viral infection. J. Immunol. 170:1070-1076. [DOI] [PubMed] [Google Scholar]
- 49.Streblow, D. N., S. L. Orloff, and J. A. Nelson. 2001. Do pathogens accelerate atherosclerosis? J. Nutr. 131:2798S-2804S. [DOI] [PubMed] [Google Scholar]
- 50.Tanaka, J., T. Ogura, H. Iida, H. Sato, and M. Hatano. 1988. Inhibitors of prostaglandin synthesis inhibit growth of human cytomegalovirus and reactivation of latent virus in a productively and latently infected human cell line. Virology 163:205-208. [DOI] [PubMed] [Google Scholar]
- 51.Thuresson, E. D., K. M. Lakkides, C. J. Rieke, Y. Sun, B. A. Wingerd, R. Micielli, A. M. Mulichak, M. G. Malkowski, R. M. Garavito, and W. L. Smith. 2001. Prostaglandin endoperoxide H synthase-1: the functions of cyclooxygenase active site residues in the binding, positioning, and oxygenation of arachidonic acid. J. Biol. Chem. 276:10347-10357. [DOI] [PubMed] [Google Scholar]
- 52.Waldman, W. J., D. A. Knight, E. H. Huang, and D. D. Sedmak. 1995. Bidirectional transmission of infectious cytomegalovirus between monocytes and vascular endothelial cells: an in vitro model. J. Infect. Dis. 171:263-272. [DOI] [PubMed] [Google Scholar]
- 53.Zavagno, G., B. Jaffe, and M. Esteban. 1987. Role of prostaglandins and non-steroid anti-inflammatory drugs in the pathogenicity of vaccinia virus. J. Gen. Virol. 68:593-600. [DOI] [PubMed] [Google Scholar]
- 54.Zhu, H., J. P. Cong, G. Mamtora, T. Gingeras, and T. Shenk. 1998. Cellular gene expression altered by human cytomegalovirus: global monitoring with oligonucleotide arrays. Proc. Natl. Acad. Sci. USA 95:14470-14475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu, H., J. P. Cong, D. Yu, W. A. Bresnahan, and T. E. Shenk. 2002. Inhibition of cyclooxygenase 2 blocks human cytomegalovirus replication. Proc. Natl. Acad. Sci. USA 99:3932-3937. [DOI] [PMC free article] [PubMed] [Google Scholar]


