Abstract
By using an isothermal multiply primed rolling-circle amplification protocol, the complete genomic DNA of a novel papillomavirus was amplified from a skin lesion biopsy of a Florida manatee (Trichechus manatus latirostris), one of the most endangered marine mammals in United States coastal waters. The nucleotide sequence, genome organization, and phylogenetic position of the Trichechus manatus latirostris papillomavirus type 1 (TmPV-1) were determined. TmPV-1 is the first virus isolated from the order of Sirenia. A phylogenetic analysis shows that TmPV-1 is only distantly related to other papillomavirus sequences, and it appears in our phylogenetic tree as a novel close-to-root papillomavirus genus.
Papillomaviruses (PVs) are a large group of epitheliotropic pathogens that cause proliferations of the stratified squamous epithelium in a wide variety of host species. Ninety-three different types of human PV (HPV), causing a wide spectrum of genotype-specific lesions (22), have been completely genomically characterized (9). Although only a limited number of nonhuman PVs have been fully characterized, they cover a broad range of host species (9, 14, 16). Considering the numerous partial sequences of putative novel animal PVs published recently (2) and the large number of suspected PV infections in animals, based on clinical, histopathological, electron microscopic, and immunohistochemical findings (14), the PV genotype variety in nonhuman vertebrates might exceed that of the HPVs, and every vertebrate species could have its own set of PVs.
The Florida manatee (Trichechus manatus latirostris) belongs to the order of Sirenia and is presently listed as an endangered species. Natural disease in manatees is uncommon (3, 7, 8), and the majority of manatee deaths are directly or indirectly attributable to human activities, as is the case for collisions with watercrafts and habitat degradation (3). In 1997, seven captive manatees from the Homosassa Springs State Wildlife Park, Homosassa, Florida, developed multiple pedunculated cutaneous papillomas, distributed over the leading contact regions of the anterior body. Four of these animals subsequently developed topically and clinically distinct sessile papillomas which were more diffuse and numerous and frequently showed a linear distribution along lines of scratch marks or trauma. In both lesion types, PVs were suspected as a causative agent, based on histological, ultrastructural, and immunohistochemical data (5). Papillomatous skin lesions have since been observed in free-ranging Florida manatees, and fragmental PV DNA sequences were obtained from these lesions by degenerate primer PCR (R. A. Woodruff, R. K. Bonde, and C. H. Romero, Abstr. 34th Annu. Conf. Int. Assoc. Aquat. Anim. Med., 2003. [Online.] http://cars.er.usgs.gov/posters/Manatee/Manatee_Papilloma_Virus/manatee_papilloma_virus.html).
A major impediment to the discovery and genomic characterization of novel PVs in animal lesion biopsy material, which is often only available in minute quantities, is the absence of a conventional cell culture system for in vitro viral propagation. Recently, we optimized a multiply primed rolling-circle amplification (RCA) method with random hexamer primers and the φ29 DNA polymerase for sequence-independent amplification of PV complete genomic DNA (13). Using this method, we were able to detect and genetically characterize a novel close-to-root PV from a sessile papillomatous skin lesion of a Florida manatee, representing the first characterized virus isolated from a species of the Sirenia order.
Biopsy material was obtained from the female Florida manatee Willoughby, which is maintained at Homosassa Springs State Wildlife Park. The tissue was finely minced, digested overnight at 56°C in 500 μl of digestion buffer (10 mM Tris and 0.5% sodium dodecyl sulfate [pH 7.4]) with 500 μg of PCR-grade proteinase K (Roche Diagnostics Belgium), and genomic DNA was extracted as described previously (17). Multiply primed RCA was performed with a TempliPhi 100 amplification kit (Amersham Biosciences), following our optimized protocol (13). One microliter of manatee-extracted DNA (2.1 μg of total DNA) or water (negative control) was transferred into a 0.5-ml tube with 5 μl of TempliPhi sample buffer, denatured at 95°C for 3 min, and afterwards placed on ice. A premix was prepared on ice, by mixing for each sample 5 μl of TempliPhi reaction buffer, 0.2 μl of TempliPhi enzyme mix containing the φ29 DNA polymerase, and 450 μM extra deoxynucleoside triphosphates. After vortexing, 5 μl of the premix was added to the cooled samples. The reactions were incubated overnight at 30°C and were then heated to 65°C for 10 min to inactivate the φ29 DNA polymerase, and stored at −20°C. To investigate whether PV DNA was amplified, 2 μl of the RCA product was digested with 10 U of BamHI, EcoRI, KpnI, SalI, and XbaI and run on a 0.8% agarose gel to look for a band consistent with full-length PV DNA or for multiple bands with sizes adding up to this length. Digestion with SalI revealed a single DNA band of approximately 8 kb, consistent with a full-length PV genome. To confirm that this band was indeed PV DNA, PCR with degenerate primers specific for cutaneous PVs was performed on the RCA product. The following primer pairs (with the expected amplicon length, based on the sequence of HPV type 1a [HPV-1a]) were used: AR-E1F2-AR-E1R3 (371 bp), AR-L1F1-AR-L1R3 (600 bp), and AR-L1F1-AR-L1R5 (974 bp) (Table 1). We also tested the degenerate primer pair FAP59-FAP64 (478 bp), developed by Forslund and coworkers (10). PCR was carried out in a volume of 50 μl, containing a 200 μM concentration of each deoxynucleoside triphosphate, 0.75 μM concentrations of the forward and reverse primers, 1 U of Taq DNA polymerase (Perkin Elmer/Roche Molecular Systems), and 2.5 mM MgCl2 [pH 8.5], with 1 μl of 10-fold-diluted RCA product of the manatee sample or of water (negative control) as the template. PCR conditions were 10 min of denaturation at 94°C; 45 cycles of 1 min at 94°C, 1 min at 50°C, and 1 min at 72°C; and a final elongation step of 5 min at 72°C. Ten microliters of the PCR products was analyzed by electrophoresis on a 3% agarose gel. With all primer pairs used, amplicons suggestive of PV-specific amplification were generated (data not shown). These PCR products were purified through extraction of the PV-specific bands from the gel (QIAquick gel extraction kit; QIAGEN) and sequenced with the same degenerate primers as those used for PCR. Similarity searches, performed with the NCBI BLAST server (version 2.2.8) on GenBank DNA database release 147.0 (1), revealed that partial E1 and L1 sequences of a novel PV were amplified, showing only a limited degree of similarity with other PV sequences. Starting from these partial E1 and L1 sequences, the complete nucleotide sequence of the Florida manatee (Trichechus manatus latirostris) PV type 1 (TmPV-1) was determined by primer-walking sequencing directly on the multiply primed RCA product, with 31 sequencing primers to cover the complete genome on both strands. Sequencing was performed on an ABI Prism 3100 genetic analyzer (Perkin-Elmer Applied Biosystems), chromatogram sequencing files were inspected with Chromas 2.2 (Technelysium), and contigs were prepared by using SeqMan II (DNASTAR).
TABLE 1.
Degenerate primers used for amplification of partial TmPV-1 E1 and L1 sequences
| Primer | Sequence (5′-3′)a | Fold degeneracy | Position (nt)b |
|---|---|---|---|
| AR-E1F2 | ATGGTNCAGTGGGCNTATGA | 16 | 1778-1797 |
| AR-E1R3 | TTNCCWSTATTNGGNGGNCC | 1024 | 2148-2129 |
| AR-L1F1 | TTDCAGATGGCNGTNTGGCT | 48 | 5425-5444 |
| AR-L1R3 | CATRTCHCCATCYTCWAT | 24 | 6024-6007 |
| AR-L1R5 | CCATTRTTHWKDCCYTG | 144 | 6398-6382 |
| FAP 59 | TAACWGTNGGNCAYCCWTATT | 128 | 5558-5578 |
| FAP 64 | CCWATATCWVHCATNTCNCCATC | 576 | 6035-6013 |
N = A, G, C, or T; W = A or T; S = C or G; D = A, T, or G; R = A or G; H = A, T, or C; Y = C or T; K = T or G; V = A, C, or G.
Position relative to the sequence of HPV-la (GenBank accession number NC_001356).
The complete genome of TmPV-1 (Fig. S1 in the supplemental material) is made up of 7,722 bp and has a GC content of 45%. TmPV-1 contains seven major open reading frames (ORFs) coding for five early (E) proteins and two late (L) capsid proteins (Fig. 1). The putative TmPV-1 E6 protein contains two conserved zinc-binding domains, CXXC-X29-CXXC, separated by 36 amino acids, whereas the E7 contains one such domain. A putative retinoblastoma tumor suppressor-binding domain (DLLCHENLDDPE) is present in the E7 protein. The ATP-binding site of the ATP-dependent helicase (GPPDTGKS) is conserved in the carboxy-terminal part of E1. The E4 ORF is completely contained within the E2 gene, and an E4 start codon was identified for TmPV-1, which is not present in most other PVs. Like most PV E4 ORFs, the TmPV-1 E4 has a high percentage of cytosine di-, tri-, and tetranucleotides, resulting in a high proline content (27 proline residues out of 162 amino acids). The major (L1) and minor (L2) capsid proteins both show a nuclear localization signal at the 3′ end. A polyadenylation consensus sequence (AATAAA) for processing of the early viral mRNA transcript is present within the beginning of the L2 ORF (nucleotides [nt] 3973 to 3978). In TmPV-1, the upstream regulatory region (URR) between the stop codon of L1 and the first ATG of E6 is 818 bp long (nt 6914 to 6919). Five typical palindromic E2-binding sites with the consensus sequence AAC-N6-GGT were found, at nt positions 7203 to 7214, 7276 to 7287, 7383 to 7394, 7436 to 7447, and 7585 to 7596, and one modified putative E2-binding site (AAC-N6-GGT) was found at nt 7314 to 7325. The URR also contains a second polyadenylation site (AATAAA; nt 6964 to 6969) in the 5′ end, located 21 nt 5′ of a CA dinucleotide, for processing of the L1 and L2 capsid mRNA transcripts, and a TATA box at the 3′ end (nt 7698 to 7703) (Fig. 1).
FIG. 1.
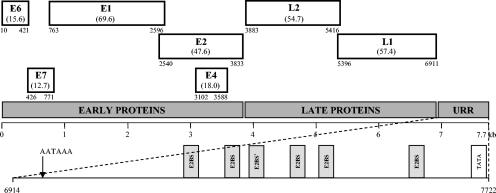
Linear representation of the ORFs of the TmPV-1 genome (with the molecular mass of the predicted proteins in kilodaltons). Numbers show the nucleotide positions of the start and stop codons. The position of the first nucleotide of the TmPV-1 genome corresponds to the start of the first major ORF in the early protein region. The enlarged section shows the distribution of E2-binding sites (E2BS) and a modified putative E2-binding site (E2BS*) and the position of the polyadenylation site (AATAAA) and TATA box in the TmPV-1 URR. E, early; L, late.
The sequence similarity between TmPV-1 and HPV-1a (a benign cutaneous PV), HPV-5 (an epidermodysplasia verruciformis-associated PV), HPV-16 (a mucosal high-risk PV), bovine PV type 1 (BPV-1) (a fibropapillomavirus), and parrot (Psittacus erithacus) PV (PePV) was investigated by pairwise alignments of the corresponding ORFs and their proteins, as calculated by using the GAP program of the Sequence Analysis Server at Michigan Technological University (http://genome.cs.mtu.edu/align/align.html) (Table 2). Only low percentages of similarity were found, and they were comparable for the cutaneous, mucosal, and fibropapillomatous PVs. The highest similarity (46 to 56%) was noted for the L1 ORF. This result places TmPV-1 in a new PV genus, since different genera of PV are defined by their sharing less than 60% nucleotide sequence identity in the L1 ORF (9).
TABLE 2.
Percentages of nucleotide and amino acid similarity of the different TmPV-1 ORFs to the ORFs of HPV-1a, HPV-5, HPV-16, BPV-1, and PePVa
| TmPV-1 ORF | % nt (amino acid) similarity to ORF of:
|
||||
|---|---|---|---|---|---|
| HPV-1a | HPV-5 | HPV-16 | BPV-1 | PePV | |
| E6 | 43 (30) | 38 (24) | 38 (25) | 35 (22) | No E6 |
| E7 | 32 (30) | 39 (29) | 37 (29) | 32 (21) | No E7 |
| E1 | 55 (44) | 52 (42) | 50 (40) | 48 (38) | 41 (30) |
| E2 | 40 (30) | 38 (28) | 30 (21) | 37 (30) | 31 (22) |
| E4 | 25 (11) | NA (NA) | NA (NA) | 25 (15) | NA (NA) |
| L2 | 39 (31) | 37 (34) | 37 (30) | 33 (28) | NA (NA) |
| L1 | 52 (50) | 56 (53) | 52 (46) | 52 (47) | 46 (42) |
The TmPV-1 L1 nucleotide sequence was aligned with 458-bp L1 fragments of Florida manatee PV that were previously determined by degenerate primer PCR from lesions in captive and free-range manatees (GenBank accession numbers AY455940, AY455941, AY496568 to AY496572, AY496574, and AY496575). After removal of the MY09 and MY11 primer sequences used for amplification of these fragments, these sequences were all 100% identical to the corresponding region of TmPV-1 (nt 6373 to 6790). One of the previously reported L1 fragments was isolated from a pedunculated skin lesion biopsy from the Florida manatee Lorelei (AY496572; isolate V375) (R. A. Woodruff et al., Abstr. 34th Annu. Conf. Int. Assoc. Aquat. Anim. Med.). This sequence is 100% identical to the corresponding region of TmPV-1, which was isolated from a sessile papillomatous skin lesion, suggesting that the same virus causes both types of papillomatous skin lesions in manatees.
To make an optimal nucleotide sequence alignment of 43 human and animal PVs (type species of the PV genera and species that were described in the new PV classification) (9), separate alignments were constructed for the different ORFs. Sequences were imported into DAMBE version 4.2.7 (23) and aligned at the amino acid level by using Clustal W (19), after which the nucleotide sequences were aligned according to the aligned amino acid sequences. Unambiguously aligned regions were compiled in one concatenated alignment of 2,556 nucleotides. Based on this alignment, a phylogenetic tree was constructed by using the neighbor joining method with MEGA version 2.1 (11), which clusters the PVs in the different genera suggested by de Villiers and colleagues (9) (Fig. 2). In this tree, TmPV-1 appears as a close-to-root PV, with only a distant relationship to other PV sequences, and therefore cannot be classified in an existing PV genus.
FIG. 2.
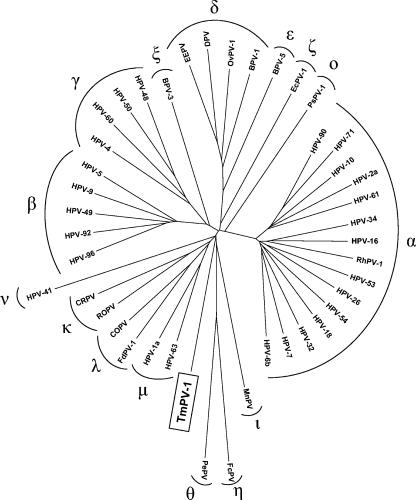
Neighbor-joining phylogenetic tree, based on a concatenated E1/E2/L2/L1 nucleotide sequence alignment of TmPV-1 and 42 other animal and human PVs. Nucleotide positions that were included in the alignment are nt 1504 to 1620, 1717 to 1932, 1969 to 2355, and 2386 to 2514 in E1, nt 2597 to 2941 in E2, nt 3904 to 4077, 4693 to 4713, and 4825 to 4929 in L2, and nt 5423 to 5533, 5579 to 5788, 5819 to 5902, 5960 to 6175, 6287 to 6439, 6491 to 6676, and 6731 to 6832 in L1, relative to the TmPV-1 sequence. The PVs (with their GenBank accession numbers) included bovine BPV-1 (X02346), BPV-3 (NC_004197), BPV-5 (NC_004195), canine oral PV (COPV) (NC_001619), cottontail rabbit PV (CRPV) (NC_001541), deer PV (DPV) (NC_001523), Equus caballus PV (EcPV-1) (NC_003748), European elk PV (EEPV) (NC_001524), Fringilla coelebs PV (FcPV) (NC_004068), Felis domesticus PV type 1 (FdPV-1) (AF480454), HPV-1a (NC_001356), HPV-2a (NC_001352), HPV-4 (NC_001457), HPV-5 (NC_001531), HPV-6b (X00203), HPV-7 (NC_001595), HPV-9 (NC_001596), HPV-10 (NC_001576), HPV-16 (NC_001526), HPV-18 (NC_001357), HPV-26 (NC_001583), HPV-32 (NC_001586), HPV-34 (NC_001587), HPV-41 (NC_001354), HPV-48 (NC_001690), HPV-49 (NC_001591), HPV-50 (NC_001691), HPV-53 (NC_001593), HPV-54 (NC_001676), HPV-60 (NC_001693), HPV-61 (NC_001694), HPV-63 (NC_001458), HPV-71 (NC_002644), HPV-90 (NC_004104), HPV-92 (NC_004500), HPV-96 (NC_005134), Mastomys natalensis PV (MnPV) (NC_001605), ovine PV type 1 (OvPV-1) (NC_001789), Psittacus erithacus PV (PePV) (NC_003973), Phocoena spinipinnis PV type 1 (PsPV-1) (NC_003348), rhesus monkey PV type 1 (RhPV-1) (NC_001678), rabbit oral PV (ROPV) (NC_002232), and Trichechus manatus latirostris PV type 1 (TmPV-1) (AY609301). The different PV genera are indicated with their Greek symbols.
It is assumed that PVs are ancient viruses that have coevolved and cospeciated with their host species, an idea which is supported by the fact that PVs of closely related host species—for instance, the parrot PePV and chaffinch (Fringilla coelebs) PV (18) and the canine oral PV and feline (Felis domesticus) PV type 1 (17)—are generally closely related themselves and are clustered together in the PV phylogenetic tree. The isolated position of TmPV-1 was therefore to be expected, since no PVs from species closely related to the manatee have been characterized thus far.
Given the species-specific nature of papillomaviruses, with interspecies transmission being very rare, it is unlikely that PVs have been transmitted to manatees in recent history. Manatees are probably latently infected with PV on their healthy skin, as is the case with many animal species (2). Latent PV infections are generally contained by the immune system and tend to become clinically apparent upon acquired, genetic, or iatrogenic deficiencies of cell-mediated immunity (6). The recent appearance of PV lesions in manatees might be due to impaired immunity, which is consistent with the finding that manatees with PV lesions had lowered lymphocyte proliferation stimulation indices compared to healthy controls (5). Causes for immunologic suppression in manatees include low water temperature (4) and a reduction in population size. Reduced diversity of genes that mediate immune defenses, particularly the major histocompatibility complex genes, due to genetic bottlenecks can lead to increased population sensitivity to infectious agents (12), and an increased prevalence and severity of PV-related pathology is often seen in populations that went through a historic genetic bottleneck (15, 20, 21).
The malignant potential of TmPV-1 could not be inferred from the sequence, but monitoring of the manatee lesions would be advisable, since malignant transformation is an important complication of PV infection in immunosuppressed individuals (6). If papillomatous skin lesions in manatees were to become more widespread and pose an important health problem for these endangered animals, the construction of a prophylactic (or even therapeutic) vaccine should be considered, and the TmPV-1 sequence reported in this study may be used for developing such a vaccine.
Nucleotide sequence accession number.
The complete genome of TmPV-1 was deposited in GenBank under accession number AY609301.
Supplementary Material
Acknowledgments
This work was supported by a fellowship from the University of Leuven to Annabel Rector and by the Flemish Fonds voor Wetenschappelijk Onderzoek (FWO grant G.0288.01).
Footnotes
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 215:403-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antonsson, A., and B. G. Hansson. 2002. Healthy skin of many animal species harbors papillomaviruses which are closely related to their human counterparts. J. Virol. 76:12537-12542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bossart, G. D. 1999. The Florida manatee: on the verge of extinction? J. Am. Vet. Med. Assoc. 214:1178-1183. [PubMed] [Google Scholar]
- 4.Bossart, G. D., R. Meisner, S. A. Rommel, S.-J. Ghim, and A. B. Jenson. 2003. Pathological features of the Florida manatee cold stress syndrome. Aquat. Mamm. 29:9-17. [Google Scholar]
- 5.Bossart, G. D., R. Y. Ewing, M. Lowe, M. Sweat, S. J. Decker, C. J. Walsh, S.-J. Ghim, and A. B. Jenson. 2002. Viral papillomatosis in Florida manatees (Trichechus manatus latirostris). Exp. Mol. Pathol. 72:37-48. [DOI] [PubMed] [Google Scholar]
- 6.Bouwes Bavinck, J. N., and R. J. M. Berkhout. 1997. HPV infections and immunosuppression. Clin. Dermatol. 15:181-198. [DOI] [PubMed] [Google Scholar]
- 7.Buergelt, C. D., and R. K. Bonde. 1983. Toxoplasmic meningoencephalitis in a West Indian manatee. J. Am. Vet. Med. Assoc. 183:1294-1296. [PubMed] [Google Scholar]
- 8.Buergelt, C. D., R. K. Bonde, C. A. Beck, and T. J. O'Shea. 1984. Pathologic findings in manatees in Florida. J. Am. Vet. Med. Assoc. 185:1331-1334. [PubMed] [Google Scholar]
- 9.de Villiers, E.-M., C. Fauquet, T. R. Broker, H.-U. Bernard, and H. zur Hausen. 2004. Classification of papillomaviruses. Virology 324:17-27. [DOI] [PubMed] [Google Scholar]
- 10.Forslund, O., A. Antonsson, P. Nordin, B. Stenquist, and B. Hansson. 1999. A broad range of human papillomavirus types detected with a general PCR method suitable for analysis of cutaneous tumours and normal skin. J. Gen. Virol. 80:2437-2443. [DOI] [PubMed] [Google Scholar]
- 11.Kumar, S., K. Tamura, I. B. Jakobsen, and M. Nei. 2001. MEGA2: molecular evolutionary genetics analysis software. Bioinformatics 17:1244-1245. [DOI] [PubMed] [Google Scholar]
- 12.O'Brien, S. J. 1994. Genetic and phylogenetic analyses of endangered species. Annu. Rev. Genet. 28:467-489. [DOI] [PubMed] [Google Scholar]
- 13.Rector, A., R. Tachezy, and M. Van Ranst. 2004. A sequence-independent strategy for detection and cloning of circular DNA virus genomes by using multiply primed rolling-circle amplification. J. Virol. 78:4993-4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sundberg, J. P. 1987. Papillomavirus infections in animals, p. 40-103. In K. Syrjänen, L. Gissmann, and L. Koss (ed.), Papillomaviruses and human disease. Springer-Verlag, Heidelberg, Germany.
- 15.Sundberg, J. P., M. Van Ranst, R. Montali, B. L. Homer, W. H. Miller, P. H. Rowland, D. W. Scott, J. J. England, R. W. Dunstan, I. Mikaelian, and A. B. Jenson. 2000. Feline papillomas and papillomaviruses. Vet. Pathol. 37:1-10. [DOI] [PubMed] [Google Scholar]
- 16.Tachezy, R., A. Rector, M. Havelkova, E. Wollants, P. Fiten, G. Opdenakker, A. B. Jenson, J. P. Sundberg, and M. Van Ranst. 2002. Avian papillomaviruses: the parrot Psittacus erithacus papillomavirus (PePV) genome has a unique organization of the early region and is phylogenetically related to the chaffinch papillomavirus. BMC Microbiol. 2:19. [Online.] http://www.biomedcentral.com/1471-2180/2/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tachezy, R., G. Duson, A. Rector, A. B. Jenson, J. P. Sundberg, and M. Van Ranst. 2002. Cloning and genomic characterization of Felis domesticus papillomavirus type 1. Virology 301:313-321. [DOI] [PubMed] [Google Scholar]
- 18.Terai, M., R. DeSalle, and R. D. Burk. 2002. Lack of canonical E6 and E7 open reading frames in bird papillomaviruses: Fringilla coelebs papillomavirus and Psittacus erithacus timneh papillomavirus. J. Virol. 76:10020-10023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Ranst, M., A. Fuse, H. Sobis, W. De Meurichy, S. M. Syrjänen, A. Billiau, and G. Opdenakker. 1991. A papillomavirus related to HPV type 13 in oral focal epithelial hyperplasia in the pygmy chimpanzee. J. Oral Pathol. Med. 20:325-331. [DOI] [PubMed] [Google Scholar]
- 21.Van Ranst, M., A. Fuse, P. Fiten, E. Beuken, H. Pfister, R. D. Burk, and G. Opdenakker. 1991. Human papillomavirus type 13 and pygmy chimpanzee papillomavirus type 1: comparison of the genome organizations. Virology 190:587-596. [DOI] [PubMed] [Google Scholar]
- 22.Van Ranst, M., J. B. Kaplan, and R. D. Burk. 1992. Phylogenetic classification of human papillomaviruses: correlation with clinical manifestations. J. Gen. Virol. 73:2653-2660. [DOI] [PubMed] [Google Scholar]
- 23.Xia, X., and Z. Xie. 2001. DAMBE: data analysis in molecular biology and evolution. J. Hered. 92:371-373. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


