Abstract
Decay-accelerating factor (DAF) is involved in the cell membrane attachment of many human enteroviruses. Presently, further specific active roles of DAF in mediating productive cell infection and in the pathogenesis of natural enterovirus infection are poorly understood. In an attempt to more fully understand the role of DAF in lytic cell infection we examined the specific interactions of the prototype strain of coxsackievirus A21 (CVA21) with surface-expressed DAF. Investigations into discrete DAF-CVA21 interactions focused on viral binding; viral particle elution with respect to the parameters of time, temperature, and pH; and subsequent cell infection. Radiolabeled-virus binding assays revealed that peak elution of CVA21 from DAF occurred within 15 min of initial attachment and that the DAF-eluted virus increased in a linear fashion with respect to temperature and pH. CVA21 eluted from endogenous surface-expressed DAF was highly infectious, in contrast to CVA21 eluted from intercellular adhesion molecule 1 (ICAM-1), which retained little to no infectivity. Using an adenovirus transduction system, we demonstrate that CVA21 can remain infectious for up to 24 h after DAF binding and is capable of initiating a multicycle lytic infection upon delayed ICAM-1 surface expression. Taken together, the data suggest that a major role of DAF in cell infection by the prototype strain of CVA21 is to provide membrane concentration of infectious virions, effectively increasing viral interactions with endogenous or induced ICAM-1.
Coxsackievirus A21 (CVA21) is a human enterovirus and a causative agent of upper respiratory tract infections (7). The prototype strain of CVA21 (Kuykendall) utilizes two separate host cell receptors, decay-accelerating factor (DAF) for cell binding and intercellular adhesion molecule 1 (ICAM-1) for both attachment and cell internalization (24). The use of two receptors by CVA21 is thought to provide multiple cell attachment sites and alternative mechanisms of viral cell entry, thereby increasing the chances of a productive infection. Not all cellular receptors that mediate enteroviral attachment to the cell surface facilitate cell entry. Coxsackievirus B3 (CVB3) can bind to DAF or the coxsackievirus-adenovirus receptor (CAR), with only interaction with CAR permitting successful infection (27). CVA9 recognizes the vitronectin receptor αvβ3 and β2-microglobulin but requires the heat shock protein GRP78 and interactions with major histocompatibility complex class I molecules for internalization (31).
A direct involvement for ICAM-1 in viral infection has been established for the major group of human rhinoviruses (8) and numerous group A coxsackieviruses (16, 24, 25). The ICAM-1 binding footprint on the CVA21 virion capsid maps to a deep surface depression or “canyon” surrounding each fivefold vertex (1, 5, 32). In contrast to ICAM-1, DAF is postulated to bind in a more exposed region on the capsid surface (11). Many human enteroviruses use DAF as a cellular attachment receptor; these include echovirus 3 (EV3), -6, -7, -11, -12, -13, -19, -20, -21, -24, -29, and -33; enterovirus 70; CVB1, -3, and -5; and CVA21 (2, 12, 23). DAF appears to play a passive role during enteroviral infection, as expression of DAF alone on rodent cells is capable of facilitating cellular binding but not infection by CVB1, CVB3, CVB5, EV7, and CVA21 (2, 23, 24).
Specific roles for DAF during virus-induced lytic infection, other than passive viral attachment, have at present not been established. The inability of DAF to facilitate enteroviral infection is possibly due its failure to induce capsid conformational changes (22). More recently, to various degrees, low-cell-culture-passage clinical isolates of CVA21 were shown to be capable of lytically infecting DAF-expressing rhabdomyosarcoma (RD) cells in the absence of ICAM-1, most probably mediated by capsid cross-linking of DAF in a manner similar to that of the anti-DAF monoclonal antibodies (17).
The study herein focuses on investigations addressing the active role(s) of DAF during infection by the CVA21 prototype strain. Characterization of DAF binding and the relative rates of CVA21 elution from DAF with respect to time, temperature, and pH were investigated. Furthermore, a virion concentration role for DAF in enteroviral infection was investigated by examining the capacity of DAF-bound CVA21 to maintain infectivity prior to and following elution and evaluating its subsequent potential in facilitating productive cell infection via interactions with delayed ICAM-1 expression.
Binding of CVA21 and its elution from DAF.
Picornaviral cell attachment and subsequent cell entry are characterized by elution of high levels of viral particles from their specific cell surface receptors following initial attachment (6). Similar to what is found for many picornaviruses, when the prototype strain of CVA21 is eluted from its natural internalizing receptor, ICAM-1, it possesses a significantly reduced infectivity, hence minimizing its capacity to initiate subsequent infections (25). A focused characterization of the relative kinetics and infectivity of prototype CVA21 particles eluted directly from surface-expressed DAF has not previously been undertaken. We concentrated our investigations on the effects of time, temperature, and pH on elution of CVA21 from DAF, with radiolabeled-virus binding assays performed using DAF-expressing CHO cells as the CVA21 binding substrate. The prototype strain of CVA21 used in these investigations had been passaged approximately 10 times in HeLa and/or human lung fibroblasts and three or four times in ICAM-1-expressing RD cells. Elution of CVA21 from DAF reached maximal levels at 15 min, with no further significant elution increase after this time (Fig. 1A). The amount of CVA21 eluted from DAF was increased by gradually elevating the temperature from 4 to 42°C while maintaining a constant elution time of 30 min (Fig. 1B). In this environment, maximal levels of CVA21 were eluted at 42°C. Not surprisingly, the infectivity of virus eluted at 42°C was significantly less than that of virus eluted at 37°C (data not shown). Temperatures above 37°C may have an adverse effect on the integrity of the virion capsid, resulting in reduced receptor binding and hence diminished infective capacity. The significant increases in CVA21 elution levels occurring at temperatures in excess of ambient levels may be representative of temperature-dependent A-particle formation, as is the case for poliovirus (10).
FIG. 1.
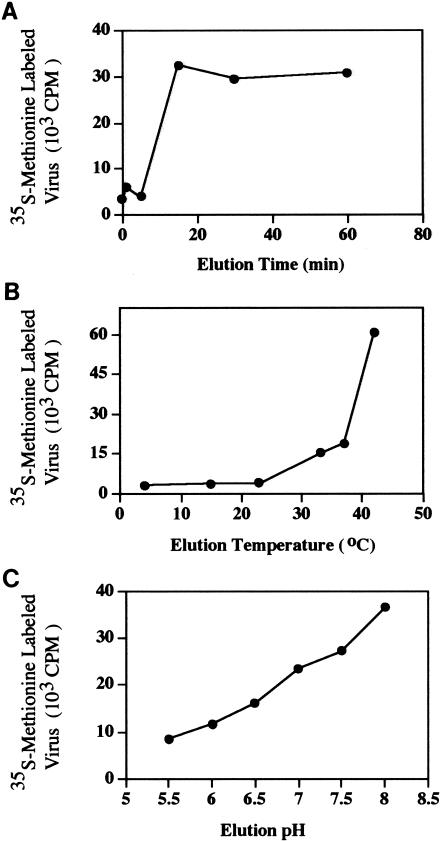
CVA21 elution from DAF-expressing CHO cells in response to time, temperature, and pH of the incubation media. (A) Radiolabeled CVA21 (5 × 105 cpm) was incubated with DAF-expressing CHO cells (approximately 2 × 107) for 2 h at 4°C; following three washes with phosphate-buffered saline (PBS) to remove unbound virions, cell aliquots were resuspended in 200 μl of PBS and incubated at 37°C for 0, 1, 5, 15, 30, and 60 min to induce viral-particle elution. Levels of CVA21 eluted were determined by liquid scintillation counting (23). Approximately 10 to 15% of input radiolabeled CVA21 bound to DAF-expressing CHO cells (data not shown). (B) Radiolabeled CVA21 was bound to cell surface-expressed DAF at 4°C for 2 h and then eluted by incubation at the appropriate temperature for a further 30 min. (C) Radiolabeled CVA21 was bound to cell surface-expressed DAF at 4°C for 2 h and then eluted by incubation in media of the appropriate pH at 37°C for a further 30 min.
DAF is expressed on the surfaces of cells in the gastrointestinal tract (acidic pH environment) and cells in the respiratory tract (neutral pH environment) (14, 19). Cells from both of these areas are potential targets for CVA21 membrane attachment, and we assessed whether changes in environmental pH impacted CVA21 DAF elution. Increasing the pH of the elution environment from 5.5 to 8.0 while maintaining an elution time of 30 min and a temperature of 37°C resulted in a continual increase in the level of CVA21 eluted from DAF (Fig. 1C).
CVA21 eluted from DAF retains infectivity.
A large proportion of eluted picornaviral particles are generally recognized to be noninfectious because cell-bound virions undergo specific receptor-induced capsid conformational changes (9). To compare the relative effects that binding to and elution from either surface-expressed DAF or ICAM-1 exert on CVA21 infectivity and to determine whether DAF interactions induced temperature-dependent A-particle formation, radiolabeled-virus binding and cell lytic assays were performed using CHO DAF- and CHO-ICAM-1-expressing cells as the CVA21 binding substrates. Flow-cytometric analysis revealed high-level expression of DAF and ICAM-1 on the appropriate transfected-CHO-cell surface (data not shown). Similar levels of radiolabeled CVA21 bound to and eluted from both DAF and ICAM-1 on the surfaces of the transfected cells (Fig. 2A). In contrast to virus eluted from ICAM-1, only CVA21 eluted from DAF displayed a significant retention of infectivity (>105 50% tissue culture infective doses [TCID50]/100 cpm) (Fig. 2B).
FIG. 2.
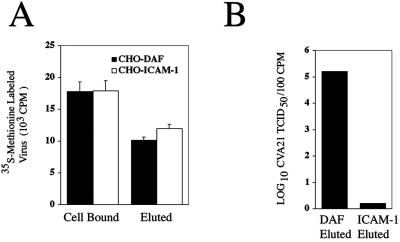
Infectivity of the prototype strain of CVA21 following elution from DAF and ICAM-1. (A) CHO cells (approximately 107) expressing either ICAM-1 or DAF were incubated with 200 μl of [35S]methionine-labeled CVA21 (5 × 105 cpm) in serum-free Dulbecco's modified Eagle medium for 2 h at 4°C. Following removal of unbound virions, the cells were incubated at 37°C for 1 h. Levels of [35S]methionine-labeled virus bound and eluted were measured by liquid scintillation counting on a 1450 Microbeta TRILUX (Wallac, Turku, Finland) (23). Results are expressed as means of triplicate samples plus standard deviations. (B) The infectivity of virions eluted from both cell lines was determined by lytic-end point dilution on 96-well monolayers of ICAM-1-expressing RD cells. Cell survival was quantitated from quadruplicate wells by staining with a crystal violet-methanol solution, and the relative absorbance of stained cell monolayers was read on a multiscan enzyme-linked immunosorbent assay plate reader (Flow Laboratories, McLean, Va.) at 540 nm. Fifty percent end point titers were calculated by the method of Reed and Muench (21), where a well was scored as positive if the absorbance was less than that of the no-virus control minus three standard deviations. Results are expressed as log10 TCID50 of CVA21 per 102 cpm.
CVA21 can bind to DAF, retain infectivity, and initiate productive infection following delayed expression of ICAM-1.
Once it was established that CVA21 eluted from surface-expressed DAF retains a high level of infectivity (Fig. 2B), investigations focused on whether DAF-eluted virus could play an active role in the pathogenesis of natural CVA21 infections. The specific question to be addressed was to determine whether virus bound by surface-expressed DAF could initiate a productive infection utilizing a delayed induction of cell surface ICAM-1. In an attempt to simulate such an environment, DAF-expressing RD cells (ICAM-1 negative), normally refractive to CVA21 lytic infection, were transduced to express ICAM-1 or CD36 (using recombinant adenovirus vectors) at 0, 6, and 24 h following initial CVA21 binding to RD cell surface DAF. Flow-cytometric analysis revealed significant levels of surface ICAM-1 expression at 4 h posttransduction, with expression increasing to maximal levels approximately 16 h after adenovirus inoculation (Fig. 3A). Additional flow-cytometric analysis (Fig. 3B) and Western blot assays (data not shown) confirmed high-level expression of both ICAM-1 and CD36 at 24 h after transduction of the RD cells by the appropriate receptor-bearing recombinant adenovirus, while levels of endogenous DAF expression in all cells were comparable. Viral infectivity assays were performed to compare the levels of progeny CVA21 propagated in the presence of transduced ICAM-1 and CD36 receptor expression at 0, 6, and 24 h following viral binding to endogenous RD cell DAF. The RD cells transduced to express ICAM-1 at 0, 6, or 24 h after initial inoculation with CVA21 produced significantly higher viral yields (approximately 200-fold) than cells induced to express a mock receptor (CD36) or nontransduced RD cells (Fig. 3C). Multicycle replication of CVA21 in RD cells transduced to express ICAM-1 at 0, 6, or 24 h after DAF binding resulted in complete lytic destruction of the cell monolayers, whereas no cell lysis was observed in cells expressing CD36 or in nontransduced cells (Fig. 4; data not shown). No cell lysis of non-CVA21-infected RD cells transduced with recombinant adenoviruses containing ICAM-1 and CD36 cDNAs was observed (Fig. 4).
FIG. 3.
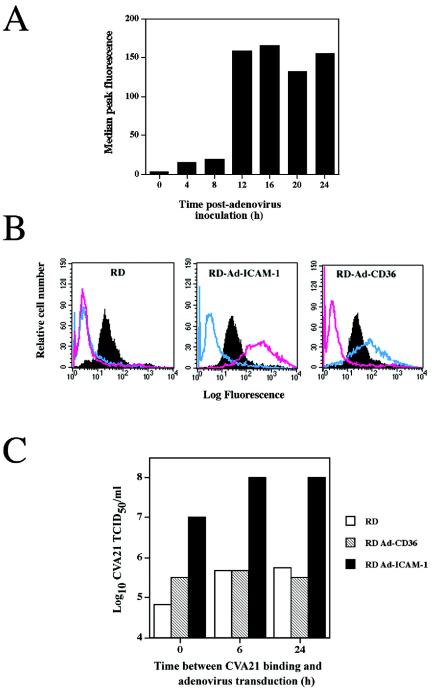
CVA21 induced lytic infection of RD cells following delayed induction of ICAM-1 expression. (A) Time course of ICAM-1 expression following adenovirus transduction. RD cells were induced to express human ICAM-1 by transduction with 2.5 × 106 TCID50 of a recombinant adenovirus containing human ICAM-1 cDNA. Cells were assessed by flow cytometry for ICAM-1 expression at various times after adenovirus inoculation with the anti-ICAM-1 domain monoclonal antibody (WEHI). (B) Flow-cytometric analysis of RD cells showing surface expression of DAF, ICAM-1, and CD36 24 h following mock transduction or transduction with recombinant adenoviruses containing human ICAM-1 or CD36 cDNA. The solid histograms represent DAF expression, while the pink histograms represent ICAM-1 expression and the blue histograms represent CD36 expression. The recombinant adenoviruses containing ICAM-1 cDNA or CD36 cDNA were constructed with an Adeno-quest kit (Quantum Biotechnologies Inc.) in accordance with the manufacturer's instructions. (C) CVA21 lytic infection of RD cells via the delayed expression of ICAM-1. Monolayers of DAF-expressing RD cells in six-well culture plates were infected with the prototype strain of CVA21 (multiplicity of infection = 1.0 TCID50) for 1 h at 37°C. Following removal of non-DAF-bound CVA21 virions by four washes with serum-free Dulbecco's modified Eagle medium (DMEM), cell monolayers were transduced with 2.5 × 106 TCID50 of a recombinant adenovirus containing ICAM-1 or CD36 cDNA immediately or at 6 and 24 h after CVA21 inoculation. At 6 and 24 h after CVA21 inoculation, cell monolayers were washed with serum-free DMEM prior to adenovirus transduction. Following adenovirus transduction, cell monolayers were incubated at 37°C for 24 h, at which time cell supernatants were harvested. RD cells that were inoculated with CVA21 but mock transduced served as background cell controls. Levels of progeny CVA21 in the cell supernatants were determined by lytic-end point dilution on 96-well monolayers of ICAM-1-expressing RD cells. Cell survival from quadruplicate wells was quantitated by staining with a crystal violet-methanol solution, and the relative absorbance of stained cell monolayers was read on a multiscan enzyme-linked immunosorbent assay plate reader (Flow Laboratories) at 540 nm. Fifty percent end point titers were calculated by the method of Reed and Muench (21), where a well was scored as positive if the absorbance was less than the no-virus control minus three standard deviations.
FIG. 4.
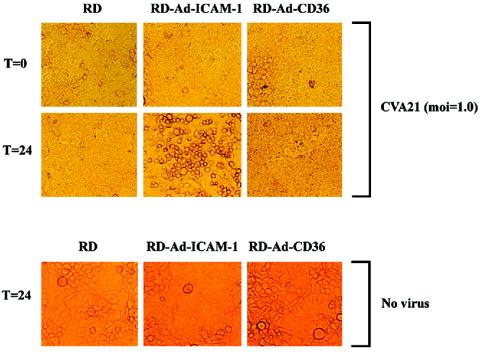
CVA21-induced lytic infection of RD cells transduced with ICAM-1- or CD36-expressing adenovirus. DAF-expressing RD cells were infected with the prototype strain of CVA21 (multiplicity of infection [moi] = 1.0 TCID50) for 1 h at 37°C. Following removal of non-DAF-bound CVA21 virions by four washes with serum-free Dulbecco's modified Eagle medium, cell monolayers were transduced with 2.5 × 106 TCID50 of a recombinant adenovirus (Ad) containing ICAM-1 or CD36 cDNA. Following incubation for 24 h at 37°C, cell monolayers were microscopically examined for cell lysis. Photomicrographs were taken at a magnification of ×200. Time (T) zero represents cell monolayers immediately after adenovirus transduction, while time 24 represents cell monolayers 24 h following adenovirus transduction.
Prototype and clinical isolates appear to vary with respect to enteroviral interactions with DAF (3, 15, 17, 29). In the absence of ICAM-1 expression and antibody-cross-linked DAF, clinical isolates of CVA21, to various degrees, achieve host cell lytic infection, possibly by cross-linking DAF via specific viral capsid interactions (17). Despite detailed descriptions of DAF interactions for numerous clinical enterovirus isolates, actively defined roles for DAF during lytic infection of prototype enteroviral strains have not been forthcoming. The general consensus from many studies investigating enterovirus-DAF interactions is that DAF functions as a viral concentration receptor, simply accumulating virions on the cell surface for interaction with additional functional internalizing receptors (26, 28). It has been suggested that the nature of DAF binding to enteroviral capsids is of low affinity because of a very fast dissociation rate constant (13). In contrast, interactions of ICAM-1 with viral capsids of similar architecture show comparable affinities but with significantly slower kinetics, consistent with binding to a relatively inaccessible site, the capsid canyon (4).
Studies addressing the impact of biophysical parameters, such as time, temperature, and pH, on the elution of CVA21 from DAF highlight that CVA21 particles are eluted relatively rapidly from DAF, and this elution is susceptible to increases in temperature and pH (Fig. 1). Elution of CVA21 from ICAM-1 is characterized by a dramatic reduction in viral infectivity compared to that of virions eluted from DAF (Fig. 2). CVA21 virions eluted from ICAM-1 undergo irreversible capsid conformational changes as a result of receptor binding, leaving them incapable of binding and initiating lytic infection (22, 25). The capacity of DAF-eluted particles to remain infectious is most probably a result of the inability of DAF to induce CVA21 capsid conformational changes (22). CVA21 particles eluted from DAF-expressing CHO cells possessed a sedimentation coefficient in sucrose gradients similar to that of infectious 160S particles, whereas CVA21 particles eluted from ICAM-1-expressing CHO cells exhibited a reduced sedimentation coefficient, closer to that of 135S altered particles (data not shown).
The reversible nature of the CVA21 interaction with DAF was highlighted by the capacity of CVA21 to bind to DAF (on ICAM-1-negative cells) and remain in an infectious state for up to 24 h. The retention of infectivity allowed DAF-bound virions to undergo cell entry and subsequent lytic infection when presented with delayed ICAM-1 expression (Fig. 3 and 4). In the absence of detectable changes in cell cytopathology, relatively high levels of infectious CVA21 on monolayers of RD cells and RD cells transduced with adenovirus expressing CD36 (Fig. 3C) persisted throughout the course of investigations, most likely because the residual viral inoculum bound to DAF retained infectivity (Fig. 2). Alternatively, it may be due to the presence of a subpopulation of virions within the CVA21 prototype preparation that possess an enhanced DAF usage phenotype, allowing cross-linking of DAF and initiating a subsequent slow infection, a finding previously described for clinical isolates of CVA21 (17).
The capacity of the prototype strain of CVA21 to use DAF as an attachment receptor and retain a highly infectious capacity is an extremely advantageous mechanism given the widespread distribution of DAF throughout the mammalian body, particularly on erythrocytes (18). In this environment, DAF-expressing erythrocytes provide the virus with a ready transport vehicle through the body; bound infectious virus can leave the erythrocyte surface and interact with ICAM-1-expressing cells for lytic infection. It is generally accepted that surface expression of endogenous ICAM-1 throughout the human body is relatively low, awaiting induction by the action of inflammatory cytokines such as tumor necrosis factor alpha and interleukin-1β (30). During natural human rhinovirus infections, infected cells release such cytokines (20), which mediate enhanced ICAM-1 expression on surrounding cells. As some low-cell-culture-passage clinical isolates of CVA21 are able to infect cells via DAF alone, the question arises as to whether multiple cell passages have led to the usage of ICAM-1 as an internalizing receptor. The finding that low-passage clinical CVA21 isolates exhibit more rapid and dramatic lytic infection of ICAM-1-bearing cells than those expressing DAF alone (17) suggests that ICAM-1 usage is most likely not a phenotype acquired through in vitro propagation.
The data presented herein confirm two major roles of DAF during CVA21 lytic infection. First the virus can bind to DAF and elute while still retaining receptor binding capacity and, hence, cell infectivity. Second, CVA21 can bind to DAF and wait for the availability of significant levels of ICAM-1 expression on the same cell or proximal cells to allow viral internalization and subsequent lytic infection. In aspects of both viral evolution and pathogenesis, the capacity to bind to DAF must be viewed as most advantageous for the prototype strain of CVA21 and other DAF-binding enteroviruses in maximizing cell infectivity, a phenotype that is retained and even enhanced in virulent clinical CVA2I isolates.
ADDENDUM IN PROOF
Characterization of the DAF interactions with a bioselected variant of the prototype strain of coxsackievirus A21 is described in this issue by Johansson et al. (E. S. Johansson, L. Xing, R. H. Cheng, and D. R. Shafren, J. Virol. 78:12603-12612, 2004).
REFERENCES
- 1.Belnap, D. M., B. M. McDermott, D. J. Filman, N. Cheng, B. L. Trus, H. J. Zuccola, V. R. Racaniello, J. M. Hogle, and A. C. Steven. 2000. Three-dimensional structure of poliovirus receptor bound to poliovirus. Proc. Natl. Acad. Sci. USA 97:73-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergelson, J. M., M. Chan, K. R. Solomon, N. F. St John, H. Lin, and R. W. Finberg. 1994. Decay-accelerating factor (CD55), a glycosylphosphatidylinositol-anchored complement regulatory protein, is a receptor for several echoviruses. Proc. Natl. Acad. Sci. USA 91:6245-6248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergelson, J. M., J. F. Modlin, W. Weiland-Alter, J. A. Cunningham, R. L. Crowell, and R. W. Finberg. 1997. Clinical coxsackievirus B isolates differ from laboratory strains in their interaction with two cell surface receptors. J. Infect. Dis. 175:697-700. [DOI] [PubMed] [Google Scholar]
- 4.Casasnovas, J. M., and T. A. Springer. 1995. Kinetics and thermodynamics of virus binding to receptor. J. Biol. Chem. 270:13216-13224. [DOI] [PubMed] [Google Scholar]
- 5.Colonno, R. J., J. Condra, H. S. Mizutani, P. C. Callahan, M. E. Davies, and M. A. Murcko. 1988. Evidence for the direct involvement of the rhinovirus canyon in receptor binding. Proc. Natl. Acad. Sci. USA 85:5449-5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crowell, R. L., B. J. Landau, and L. Philipson. 1971. The early interaction of coxsackie virus B3 with HeLa cells. Proc. Soc. Exp. Biol. Med. 137:1082-1088. [DOI] [PubMed] [Google Scholar]
- 7.Dalldorf, G., and J. L. Melnick. 1969. Coxsackieviruses, p. 474-512. In F. L. J. Horsfall and I. Tamm (ed.), Viral and rickettsial infections of man, 4th ed. J. B. Lippincott, Philadelphia, Pa.
- 8.Greve, J. M., G. Davis, A. M. Meyer, C. P. Forte, S. C. Yost, C. W. Marlor, M. E. Kamarck, and A. McClelland. 1989. The major human rhinovirus receptor is ICAM-1. Cell 56:839-847. [DOI] [PubMed] [Google Scholar]
- 9.Greve, J. M., C. P. Forte, C. Marlor, A. M. Meyer, H. Hoover-Litty, D. Wunderlich, and A. McClelland. 1991. Mechanisms of receptor-mediated rhinovirus neutralization defined by two soluble forms of ICAM-1. J. Virol. 65:6015-6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guttman, N., and D. Baltimore. 1977. A plasma membrane component able to bind and alter virions of poliovirus type 1: studies on cell-free alteration using a simplified assay. Virology 82:25-36. [DOI] [PubMed] [Google Scholar]
- 11.He, Y., F. Lin, P. Chipman, C. M. Bator, T. S. Baker, M. Shoham, R. J. Kuhn, E. M. Medof, and M. G. Rossmann. 2002. Structure of decay-accelerating factor bound to echovirus 7: a virus receptor complex. Proc. Natl. Acad. Sci. USA 99:10325-10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karnauchow, T. M., S. Dawe, D. M. Lublin, and K. Dimock. 1998. Short consensus repeat domain 1 of decay-accelerating factor is required for enterovirus 70 binding. J. Virol. 72:9380-9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lea, S., R. Powell, T. McKee, D. J. Evans, D. Brown, D. Stuart, and P. van der Merwe. 1998. Determination of the affinity and kinetic constants for the interaction between the human echovirus 11 and its cellular receptor, CD55. J. Biol. Chem. 273:30443-30447. [DOI] [PubMed] [Google Scholar]
- 14.Lublin, D. M., and J. P. Atkinson. 1989. Decay-accelerating factor: biochemistry, molecular biology, and function. Annu. Rev. Immunol. 7:35-58. [DOI] [PubMed] [Google Scholar]
- 15.Martino, T. A., M. Petric, M. Brown, K. Aitken, C. J. Gauntt, C. D. Richardson, L. H. Chow, and P. P. Liu. 1998. Cardiovirulent coxsackieviruses and the decay-accelerating factor (CD55) receptor. Virology 244:302-314. [DOI] [PubMed] [Google Scholar]
- 16.Newcombe, N., E. S. Johansson, G. G. Au, A. M. Lindberg, R. D. Barry, and D. R. Shafren. 2003. Cellular receptor interactions of C-cluster human group A coxsackieviruses J. Gen. Virol. 84:3041-3050. [DOI] [PubMed] [Google Scholar]
- 17.Newcombe, N. G., E. S. Johansson, A. M. Lindberg, R. D. Barry, and D. R. Shafren. 2004. Enteroviral capsid interactions with decay-accelerating factor mediate lytic cell infection J. Virol. 78:1431-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicholson-Weller, A., J. P. March, C. E. Rosen, D. B. Spicer, and K. F. Austen. 1985. Surface membrane expression by human blood leukocytes and platelets of decay-accelerating factor, a regulatory protein of the complement system. Blood 65:1237-1244. [PubMed] [Google Scholar]
- 19.Nicholson-Weller, A., and C. E. Wang. 1994. Structure and function of decay accelerating factor CD55. J. Lab. Clin. Med. 123:485-491. [PubMed] [Google Scholar]
- 20.Papi, A., and S. L. Johnston. 1999. Rhinovirus infection induces expression of its own receptor intercellular adhesion molecule-1 (ICAM-1) via increased NF-KB-mediated transcription. J. Biol. Chem. 274:9707-9720. [DOI] [PubMed] [Google Scholar]
- 21.Reed, L. J., and H. A. Muench. 1938. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 22.Shafren, D. R. 1998. Viral cell entry induced by cross-linked decay-accelerating factor. J. Virol. 72:9407-9412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shafren, D. R., R. C. Bates, M. V. Agrez, R. L. Herd, G. F. Burns, and R. D. Barry. 1995. Coxsackieviruses B1, B3 and B5 use decay accelerating factor as a receptor for cell attachment. J. Virol. 69:3873-3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shafren, D. R., D. Dorahy, R. A. Ingham, G. F. Burns, and R. D. Barry. 1997. Coxsackievirus A21 binds to decay-accelerating factor but requires intercellular adhesion molecule 1 for cell entry. J. Virol. 71:4736-4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shafren, D. R., D. J. Dorahy, S. J. Greive, G. F. Burns, and R. D. Barry. 1997. Mouse cells expressing human intercellular adhesion molecule-1 are susceptible to infection by coxsackievirus A21. J. Virol. 71:785-789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shafren, D. R., D. J. Dorahy, R. F. Thorne, T. Kinoshita, R. D. Barry, and G. F. Burns. 1998. Antibody binding to individual short consensus repeats of decay-accelerating factor enhances enterovirus cell attachment and infectivity. J. Immunol. 160:2318-2323. [PubMed] [Google Scholar]
- 27.Shafren, D. R., D. T. Williams, and R. D. Barry. 1997. A decay-accelerating factor-binding strain of coxsackievirus B3 requires the coxsackievirus-adenovirus receptor protein to mediate lytic infection of rhabdomyosarcoma cells. J. Virol. 71:9844-9848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stuart, A. D., H. E. Eustace, T. A. McKee, and T. D. K. Brown. 2002. A novel cell entry pathway for a DAF-using human enterovirus is dependent on lipid rafts. J. Virol. 76:9302-9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stuart, A. D., T. A. McKee, P. A. Williams, C. Harley, S. Shen, D. I. Stuart, T. D. K. Brown, and S. M. Lea. 2002. Determination of the structure of a decay-accelerating factor-binding clinical isolate of echovirus 11 allows mapping of mutants with altered receptor requirements for infection. J. Virol. 76:7694-7704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tosi, M. F., J. M. Stark, C. W. Smith, A. Hamedani, D. C. Gruenert, and M. D. Infeld. 1992. Induction of ICAM-1 expression on human airway epithelial cells by inflammatory cytokines: effects on neutrophil-epithelial cell adhesion. Am. J. Respir. Cell Mol. Biol. 7:214-221. [DOI] [PubMed] [Google Scholar]
- 31.Triantafilou, K., D. Fradelizi, K. Wilson, and M. Triantafilou. 2002. GRP78, a coreceptor for coxsackievirus A9, interacts with major histocompatibility complex class I molecules which mediate virus internalization. J. Virol. 76:633-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiao, C., C. M. Bator, V. D. Bowman, E. Reider, Y. He, B. Hebert, J. Bella, T. S. Baker, E. Wimmer, R. J. Kuhn, and M. G. Rossman. 2001. Interaction of coxsackievirus A21 with its cellular receptor, ICAM-1. J. Virol. 75:2444-2451. [DOI] [PMC free article] [PubMed] [Google Scholar]


