Abstract
Very little is known about the interaction of bryophytes with bacteria. Therefore, we analyzed bacteria associated with three bryophyte species, Tortula ruralis, Aulacomnium palustre, and Sphagnum rubellum, which represent typical moss species of three nutrient-poor plant communities at the southern Baltic Sea coast in Germany. By use of two cultivation-independent techniques, denaturing gradient gel electrophoresis and single-strand conformation polymorphism analysis of the 16S ribosomal DNA, a high degree of moss specificity was found for associated bacterial communities. This specificity could be further evidenced by a cultivation-dependent approach for the following parameters: (i) plate counts of bacteria on R2A medium, (ii) proportion of antagonistic isolates, (iii) antagonistic activity as well as spectrum against pathogens, and (iv) diversity and richness of antagonistic isolates. The proportion of isolates with antagonistic activity against the pathogenic model fungus Verticillium dahliae was highest for S. rubellum (31%), followed by A. palustre (17%) and T. ruralis (5%). A high percentage (99%) of moss-associated antagonistic bacteria produced antifungal compounds. The high recovery of antagonistic isolates strongly suggests that bryophytes represent an ecological niche which harbors a diverse and hitherto largely uncharacterized microbial population with yet unknown and untapped potential biotechnological applications, e.g., for biological control of plant pathogens.
The bryophytes, including liverworts, hornworts, and mosses, are a diverse group of land plants that usually colonize habitats with moist or extremely variable conditions. One of their most important features is their life cycle, which involves alternation between a diploid sporophyte and a dominant, free-living haploid gametophyte generation (43). Bryophytes are unique host plants for microorganisms in numerous ways. For example, the small size of mosses results in limited availability of the substratum. Additionally, their indefinite growth in the form of dense overwintering colonies, mats, and cushions without periodic leaf fall enables colonization for long periods. Also, because bryophytes absorb water over much of their surface from atmospheric moisture, most of them display an extraordinarily high tolerance to extreme desiccation and resume normal metabolism very rapidly after rehydration (poikilohydric organization). Hence, successful microbial colonization requires adaptation to these special conditions (11).
Bryophytes represent the simplest extant land plant group and are phylogenetically very old (14). Miller (27) regards them as the oldest extant terrestrial plants, which represent the level of evolution associated with transmigration to the land. Bacterium-host interactions can be symbiotic, commensal, or pathogenic, and while the processes leading to the evolution of symbionts or pathogens are similar, the establishment of a symbiosis requires more time and evolutionary processing (17, 38). Plant-associated bacteria that are able to antagonize other (pathogenic) microorganisms belong to the symbiotic fraction of microorganisms (37, 41).
Traditionally, because of their antimicrobial activity, mosses were used as a natural medicine in Indian culture (15) and as natural diapers (3). Today, mosses represent interesting tools for biotechnological use in medicine, agriculture, and pharmacology (10, 15). However, although mosses are becoming increasingly important in many fields and Physcomitrella patens is used as a model organism for genetic studies (30), little is known about moss-associated microorganisms, beneficial as well as pathogenic. The colonization of mosses by ascomycetes is a very frequent though generally neglected phenomenon (for a review, see reference 11). However, no moss-specific pathogenic fungi are known. Therefore, Verticillium dahliae KLEB., the causative agent of verticillium wilt, which has an extremely broad spectrum of host plants (39), was selected as the model pathogen for our antagonism studies. The diverse antagonistic bacteria associated with potential Verticillium host plants are known to be strongly plant species specific (7, 36). However, much remains to be discovered about the specificity and diversity of moss-associated bacteria (9), their role in influencing the development of bryophytes (19), and their antagonistic and biotechnological potential.
Here, our aim is to analyze and characterize the associated bacterial communities of three different moss species with regard to their diversity and biotechnological potential. The mosses Tortula ruralis, Aulacomnium palustre, and Sphagnum rubellum grow in different natural habitats on the southern Baltic Sea coast (northeast Germany). To examine the impact of the moss species and their ecological background on moss-associated bacteria, two cultivation-independent approaches were used—to our knowledge, for the first time for this purpose. Denaturing gradient gel electrophoresis (DGGE) and single-strand conformation polymorphism (SSCP) analysis of the 16S ribosomal DNA (rDNA) were used to analyze nonculturable bacteria, which generally constitute a high percentage of plant-associated microorganisms (24, 36). Additionally, to investigate the abundance and diversity of Verticillium antagonists, gametophyte samples of the mosses were analyzed by a cultivation-dependent approach. A comprehensive phenotypic and genotypic characterization of the antagonists allowed us to provide new data on the diversity of moss-dependent Verticillium antagonists.
MATERIALS AND METHODS
Sampling and isolation of the bacterial fraction.
The mosses were sampled in three different natural habitats on the southern Baltic Sea coast (northeast Germany). T. ruralis HEDW. (family Pottiaceae) formed lax patches on sand dunes. The plant community in which the moss occurred belongs to Corynephorus dune grassland, characterized by grey hairgrass (Corynephorus canescens). A. palustre HEDW. (family Aulacomniaceae) grows on the edge of a noncalcareous mire behind the dunes. In the Sphagnum-rich birchwood, the dominant plants are birch trees (Betula pubescens), glossy buckthorn (Frangula alnus), and purple moorgrass (Molinia caerulea). The third moss species, S. rubellum HEDW. (family Sphagnaceae), was found in a more open part of the mire, in the center, together with common cottongrass (Eriophorum angustifolium), small cranberries (Vaccinium oxycoccus), and crossleaf heath (Erica tetralix) in a Sphagnum-Eriophorum mire plant community. These three habitats were characterized by a gradient of abiotic conditions, especially with regard to soil reaction (pH values: moderately acidic, acidic, and strongly acidic, respectively), moisture (dry, medium wet, and wet, respectively), and nutrient content (poor, medium poor, and poor, respectively), described by Precker (28). The gradients were confirmed by species indicator values according to the work of Ellenberg et al. (12). For example, indicator values for soil reaction, expressed on a scale of 1 to 9, are different for T. ruralis (6 [moderate]), A. palustre (3 [acidic]), and S. rubellum (1 [strongly acidic]).
Adult gametophytes of the mosses T. ruralis, A. palustre, and S. rubellum were collected in November 2001 and March 2002 from the natural reserve “Ribnitzer Großes Moor” near Rostock (54°18′N, 12°16′E), Germany. Additionally, T. ruralis was collected in March 2002 from the dune in Rostock-Warnemünde (54°05′N, 12°07′E) and in May 2002 from the island of Vilm (54°20′N, 13°30′E); both areas are located in northern Germany directly on the Baltic Sea coast. Gametophytes with adhering soil were placed in sterile petri dishes and transported to the laboratory, and then 5 g was transferred to a sterile stomacher bag. To extract the moss-associated bacteria from the gametophytes, 45 ml of sterile 0.85% NaCl was added, and samples were homogenized in a stomacher laboratory blender for 60 s at high speed (BagMixer; Interscience, St. Nom, France). This suspension was used for cultivation as well as for cultivation-independent investigation procedures. For the latter, after centrifugation at low speed (2 min, 500 × g), the supernatant was collected and the resulting pellet was suspended in distilled water, followed by stomacher blending and low-speed centrifugation. This step was repeated once. The supernatants of the three centrifugation steps were combined before centrifugation at high speed (10,000 × g) for 30 min to collect the microbial pellet. The resulting microbial pellet was stored at −70°C.
Total-community DNA isolation.
DNA of bacterial-cell consortia was extracted by using the FastDNA Spin Kit for Soil (Bio 101, Carlsbad, Calif.) according to the manufacturer's protocol.
Scanning electron microscopy.
Gametophytes were fixed in glutaraldehyde-phosphate buffer (2%; 0.1 M; pH 7.2) and postfixed in 2% glutaraldehyde buffer. After removal of the fixative by a wash in phosphate buffer, samples were dehydrated, critical point dried, and coated with gold before undergoing scanning electron microscopy (Carl Zeiss, Oberkochen, Germany).
Molecular analysis by DGGE.
Fingerprinting of the bacterial moss communities by DGGE was carried out as described by Heuer and Smalla (18). Briefly, 16S rDNA fragments (positions 968 to 1401 [Escherichia coli rDNA sequence]) were amplified by PCR using bacterial DNA isolated from moss tissue as a template, with the primer pair F984GC-R1378 (36). The amplicons were separated in a 40 to 56% denaturing gradient of 7 M urea and 40% (vol/vol) formamide at 60°C. Acid silver staining was used to detect DNA in DGGE gels (31).
Molecular analysis by SSCP.
Fingerprinting of the moss communities by SSCP was carried out as described by Schwieger and Tebbe (33). Briefly, 16S rDNA fragments (positions 968 to 1401 [E. coli rDNA sequence]) were amplified by PCR using bacterial DNA isolated from moss tissue as the template, with the UniBac 927r primer (18) along with specific primers for Burkholderia (32), Pseudomonas (42), and Serratia (23) spp. The amplicons were separated by using the TGGE Maxi system (Biometra, Göttingen, Germany) at 400 V and 26°C. Silver staining was used to detect DNA in SSCP gels (4).
Identification of SSCP bands.
Dominant bands were excised from SSCP gels as described by Schwieger and Tebbe (33). Gel-extracted DNA was reamplified, and products were then ligated into a pGEM-T vector and transformed into E. coli DH5α (Promega, Mannheim, Germany). Transformed cells with inserts were selected by blue-white screening. Cloned DNA fragments were amplified from the vector by PCR (under the conditions recommended by the manufacturer) using primers matching the flanking regions of the vector (forward, 5′-CAC GAC GTT GTA AAA CGA C-3′; reverse, 5′-GGA TAA CAA TTT CAC ACA GG-3′). The sizes of the PCR products were determined by agarose gel electrophoresis (0.8% [wt/vol] agarose). Inserts of the expected size were then sequenced by cycle sequencing using the DTCS CEQ Quick Start kit (Beckman Coulter, Fullerton, Calif.). By using primers usp (5′-GTA AAA CGA CGG CCA GT-3′) and rsp (5′-CAG GAA ACA GCT ATG ACC-3′), the fragments were sequenced with the Beckman Coulter system. The sequences were edited and aligned with CEQ 2000 XL analysis systems. For phylogenetic analysis and identification of related sequences, the BLAST algorithm according to Altschul et al. (1) was used. Additionally, sequences were loaded into the ARB program and database (http://www.arb-home.de).
Isolation of moss-associated bacteria.
Microbial suspensions obtained by the procedure explained above were serially diluted with sterile 0.85% NaCl and plated onto R2A medium (Difco, Detroit, Mich.). Plates were incubated for 5 days at 20°C, after which CFU were counted to calculate the mean number of colonies (log10 CFU) based on fresh weight. Data were analyzed for significance by using the Mann-Whitney U test (P ≤ 0.05) by Statistical Product and Service Solutions for Windows, release 9.0.1 (SPSS Inc., Chicago, Ill.). Isolates obtained by plating were purified and stored at −70°C in sterile broth containing 50% glycerol.
Screening of antagonistic bacteria.
Bacterial isolates were screened for their activity toward V. dahliae KLEB. by a dual-culture in vitro assay on Waksman agar (WA) containing 5 g of proteose-peptone (Merck, Darmstadt, Germany), 10 g of glucose (Merck), 3 g of meat extract (Chemex, Munich, Germany), 5 g of NaCl (Merck), 20 g of agar (Difco), and distilled water (to 1 liter) (pH 6.8). Zones of inhibition were measured after 3 and 7 days of incubation at 20°C according to the method of Berg (5). All strains were tested in three independent replicates with V. dahliae V25 (isolated from Brassica napus L. by K. Zeise and kept in the culture collection of the University of Rostock, Department of Microbiology). The fungus was routinely grown on Sabouraud medium (Gibco, Paisley, Scotland) and stored at −70°C in sterile broth containing 50% glycerol. In vitro inhibition of Xanthomonas campestris DZM 3586 was determined by a dual-culture assay in Luria-Bertani (LB) agar (Difco) in microtiter plates. From an overnight culture of Xanthomonas, 10 μl was mixed with LB agar, and bacterial isolates were spotted onto the solidified agar surface. Zones of inhibition were measured after incubation at 20°C for 24 and 48 h.
Identification of bacterial antagonists.
Antagonists were identified based on whole-cell fatty acids derivatized to methyl esters (fatty acid methyl esters [FAME]) and analyzed by gas chromatography using the MIDI system (Microbial ID, Inc., Newark, N.J.). In addition, the majority of strains were identified by 16S rDNA sequencing and aligned with the reference 16S rRNA gene sequence by using the BLAST algorithm according to Altschul et al. (1). Species richness, expressed as the number of species (S) as a function (ratio) of the total number of individuals (N), was determined by the index (d) proposed by Menhinick (26). Diversity indices were calculated according to the method of Shannon and Weaver (35). Only isolates which were identified with a similarity index higher than 0.5 by FAME analysis or 97% by sequencing of 16S rDNA genes were used for the calculations of indices.
Screening for strains with macromolecular hydrolytic activity.
Chitinase activity (β-1,4-glucosamine polymer degradation) was tested in chitin minimal medium by the method of Chernin et al. (8). Clearing zones were detected 5 days after incubation at 20°C. β-Glucanase activity was tested by using chromogenic (azurine-dyed, cross-linked [AZCL]) substrates (Megazyme, Bray, Ireland). Formation of blue halos was recorded until 5 days after incubation. Protease activity (casein degradation) was determined from clearing zones in skim milk agar (50 ml of sterilized skim milk mixed at 55°C with 50 ml of 1/5 tryptic soy agar and 4% agar) after 5 days of incubation at 20°C.
Production of secondary metabolites with potential antagonistic activity.
Antibiosis against V. dahliae by the bacterial strains was assayed on WA plates (15 ml) containing 5 ml of sterile culture filtrate (64-h culture, nutrient broth II [Sifin, Berlin, Germany]). The pH was adjusted to between 7 and 8. A 3-mm-diameter plug from a V. dahliae agar plate was placed in the center of a WA plate. As a control, WA plates (20 ml) were similarly inoculated with mycelial plugs. Colony diameters were measured daily for 10 days, and the percent reduction in linear growth of the fungi was calculated. Siderophore production was assayed according to the method of Schwyn and Neilands (34).
BOX-PCR genomic fingerprints.
Bacterial DNA was prepared by following the protocol of Anderson and McKay (2) modified for genomic DNA. BOX-PCR (fingerprinting based on repetitive BOX elements, of unknown function, in the bacterial genome) was carried out as described by Rademaker and De Bruijn (29). By using the BOXA1R primer (5′-CTA CGG CAA GGC GAC GCT GAC G-3′), PCR amplification was performed with a Peltier Thermal Cycler PTC-200 (Biozym Diagnostic, Hessisch Oldendorf, Germany) with an initial denaturation step at 95°C for 6 min; 35 cycles of denaturation at 94°C for 1 min, annealing at 53°C for 1 min, and extension at 65°C for 8 min; and a final extension at 65°C for 16 min. A 10-μl aliquot of the amplified PCR product was separated by gel electrophoresis on 1.5% agarose gels in 0.5× Tris-borate-EDTA buffer for 6 h, stained with ethidium bromide, and then photographed under UV transillumination. The reproducibility of the results was verified in three independent experiments.
Computer-assisted cluster analysis.
Computer-assisted evaluation of bacterial community profiles obtained by DGGE and SSCP, and of fingerprints generated by BOX-PCR, was performed by using the GelCompar program (version 4.1; Applied Maths, Kortrijk, Belgium). Cluster analysis was performed with the UPGMA (unweighted pair group method with arithmetic averages) algorithm.
Nucleotide sequence accession numbers.
The nucleotide sequences determined in this study have been deposited in the EMBL Data Library under accession numbers AJ575061 to AJ575093, AJ574888 to AJ574896, and AJ5774931 to AJ574938.
RESULTS
Microscopic observation of surfaces of gametophytes.
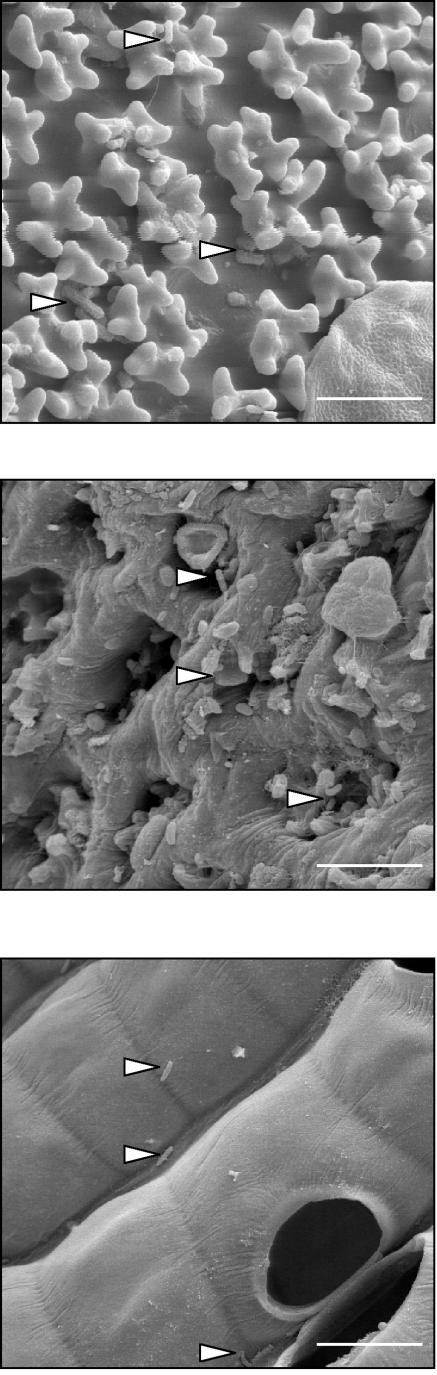
Impressions of the specific microenvironment were obtained by analyzing gametophytes by electron microscopy (Fig. 1). The top panel of Fig. 1 represents the surface of a Tortula leaf with characteristic papillae that form a very special microenvironment for bacteria. These unique structures improve the movement of water and its uptake into the cells and are important for mosses living under changing water conditions such as those in the dune. In the central panel of Fig. 1, a typical leaf surface of Aulacomnium with conical papillae, colonized by bacteria, is shown. In contrast, Sphagnum formed a regular pattern of alternating green and hyaline cells which act as a very special microenvironment for bacteria. The hyaline cells with large pores, normally filled with water, are dead at maturity and now serve to provide water (Fig. 1, bottom panel).
FIG.1.
Scanning electron micrographs of the surfaces of gametophytes of T. ruralis (top), A. palustre (center), and S. rubellum (bottom). Bars, 8 μm; arrowheads indicate bacterial cells.
Molecular fingerprinting of moss-associated bacterial communities.
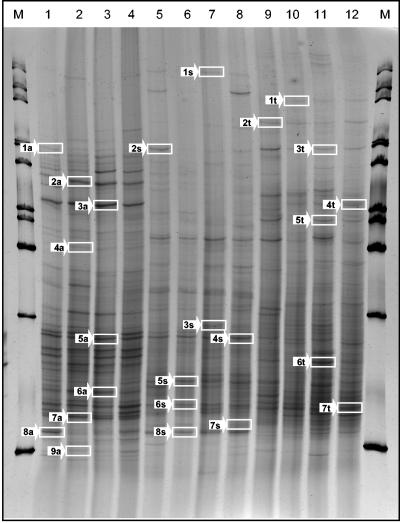
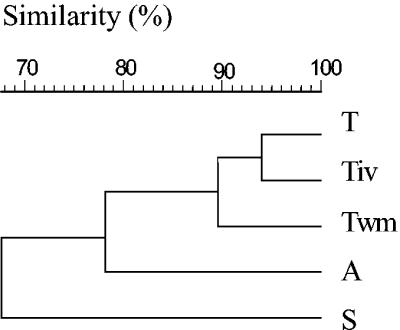
For cultivation-independent analysis, total-community DNA was extracted from the microbial pellets recovered from different mosses. 16S rDNA fragments amplified by PCR were analyzed by DGGE and by SSCP. Generally, the patterns of different mosses produced with eubacterial as well as specific primers showed a high degree of diversity. For example, Fig. 2 presents four replicates of DNAs from each of the three bacterial moss-associated communities amplified with universal primers and separated by SSCP. Very similar patterns were obtained for the eubacterial communities by DGGE and SSCP. According to a cluster analysis using the UPGMA algorithm, DGGE patterns exhibited a lower similarity (54%) for all mosses than patterns separated by SSCP (68%). By use of universal primers, the SSCP patterns obtained for Tortula from different locations displayed a similarity of 90% and were more similar to each other than to those of the other moss species (Fig. 3). The similarity among all mosses was higher (85%) with Pseudomonas-specific primers than with eubacterial primers and confirmed the influence of moss species on the bacterial community. Tortula-associated Pseudomonas community patterns from different locations exhibited 92% similarity to each other. In contrast, Serratia-specific patterns and especially Burkholderia-specific patterns showed lower degrees of similarity (48 and 28%, respectively) among the three species and among the three locations (80 and 68%, respectively).
FIG. 2.
SSCP profiles showing the bacterial communities of the bryophytes A. palustre (lanes 1 to 4), S. rubellum (lanes 5 to 8), and T. ruralis (lanes 9 to 12). The fingerprints were generated by separation of 16S rDNA fragments amplified with universal primers. As a marker (lanes M), a 1-kb ladder was used. Bands indicated by arrows were purified and sequenced (for results, see Table 1).
FIG. 3.
Dendrogram based on amplified 16S rDNA fragments of the moss-associated communities from T. ruralis from Rostock-Warnemünde (Twm), T. ruralis from the island of Vilm (Tiv), and T. ruralis (T), S. rubellum (S), and A. palustre (A) from Ribnitzer Großes Moor, obtained by using universal primers and separated by SSCP. The patterns obtained were grouped by UPGMA.
As indicated in Fig. 2, typical bands were excised and sequenced to obtain further information about dominant bacterial populations of the three different moss species. The resulting species list, along with partial sequence analysis data and tentative phylogenetic affiliations, is given in Table 1. DNA sequencing of these bands showed similarities in the range of 90 to 100% to sequences from the database, although five sequences were related to uncultured or unidentified bacteria. For Aulacomnium, a high proportion of uncultured or unidentified eubacteria were found. Photorhabdus luminescens (SSCP band 1a; 99% similarity), Collimonas fungivorans (band 2a; 99% similarity), and diverse Pseudomonas spp. (bands 7a and 9a) were identified. The sequences of two dominant bands of Sphagnum could be assigned to Pseudomonas grimontii with 100% similarity (band 2s) and to Methylobacterium mesophilicum (band 4s) with only 93% similarity. Species of Acetobacter, Frateuria, and Acidocella, bacterial genera known for their occurrence in acidic environments, were found for Sphagnum. Tortula-specific bands were identified as Pseudomonas aeruginosa (band 1t; 99% similarity), Rhodococcus erythropolis (band 3t; 99% similarity), or Acidovorax wohlfahrtii (band 4t; 98% similarity).
TABLE 1.
Results of partial sequence analyses and tentative phylogenetic affiliations of bands
| Origin and SSCP band | Most closely related sequence(s) | % Identity | GenBank accession no. |
|---|---|---|---|
| A. palustre | |||
| 1a | Photorhabdus luminescens | 99 | AY444555 |
| 2a | Collimonas fungivorans | 99 | AJ496444 |
| 3a | Uncultured eubacterium WD 247 | 94 | AJ292581 |
| 4a | Acinetobacter sp. | 94 | AJ633638 |
| 5a | Rhizosphere soil bacterium | 97 | AJ252602 |
| Flavobacterium heparinum | 96 | M11657 | |
| 6a | Unidentified eubacterium | 91 | AF009975 |
| 7a | Pseudomonas sp. | 99 | AY337597 |
| Pseudomonas grimontii | 99 | AF268029 | |
| 8a | Uncultured eubacterium TRB82 | 97 | AF047646 |
| 9a | Pseudomonas migulae | 99 | AF501370 |
| S. rubellum | |||
| 1s | Pseudomonas sp. | 99 | AY456705 |
| 2s | Pseudomonas grimontii | 100 | AF268029 |
| 3s | Uncultured eubacterium WD 229 | 91 | AJ292593 |
| 4s | Methylobacterium mesophilicum | 93 | AJ400919 |
| 5s | Acidocella sp. | 95 | AF376021 |
| 6s | Acetobacter malorum | 96 | AJ419844 |
| 7s | Gamma proteobacterium | 98 | AY135638 |
| Frateuria aurantia | 97 | AB091201 | |
| 8s | Uncultured eubacterium WD260 | 97 | AJ292673 |
| T. ruralis | |||
| 1t | Pseudomonas aeruginosa | 99 | AY360347 |
| 2t | Bacteroidetes bacterium | 96 | AY337604 |
| Sphingomonas-like sp. | 96 | X89912 | |
| 3t | Rhodococcus erythropolis | 99 | AY281114 |
| 4t | Acidovorax wohlfahrtii | 98 | AJ400840 |
| 5t | Cytophaga sp. | 96 | AB015550 |
| 6t | Bartonella henselae | 90 | AY513504 |
| 7t | Flexibacter sancti | 95 | AB078068 |
Isolation of bacteria from moss gametophytes.
CFU determined for moss samples were rather similar for the different species (counts, expressed as log10 CFU per gram [fresh weight] of plant, were 6.7 to 7.1 for T. ruralis, 5.9 to 6.4 for A. palustre, and 5.4 to 6.1 for S. rubellum). However, the highest (and statistically significant) averages were found for T. ruralis, with 6.8 log10 CFU g [fresh weight] of plant−1. No statistically significant differences at a P value of ≤0.05 could be found between sampling times and sites.
Screening for isolates antagonistic to V. dahliae.
A total of 710 bacterial isolates were screened for their ability to suppress V. dahliae in an in vitro dual-culture assay. Initially, 105 (15%) isolates which were active against V. dahliae were found; of these, 23 (22%) were strongly active, with inhibition zones larger than 10 mm. Although similar numbers of isolates from each of the treatments were tested, the proportions of isolates with antagonistic activity were different. The proportion of isolates with antifungal activity was highest for Sphagnum (31%), followed by Aulacomnium (17%) and Tortula (5%). Isolates from Sphagnum showed the strongest activity: 44% of the antifungal isolates caused inhibition zones of more than 10 mm. In contrast, no strong antagonist was observed for Tortula.
Diversity of Verticillium antagonists.
The majority of the in vitro antagonists (83) were identified by 16S rDNA sequencing and partly by fatty acid analysis (Table 2). Based on their sequences, 17 different bacterial species were identified; they belonged to nine different genera, among which Burkholderia, Pseudomonas, and Serratia were dominant. The highest numbers of different species with antagonistic activity were isolated from Sphagnum (11), whereas only seven and three were found on the gametophytes of Aulacomnium and Tortula, respectively. The richness and diversity of antagonistic species were moss species dependent. The highest diversity and richness were found for Sphagnum (diversity, 6.2; richness, 1.8). Conversely, the lowest indices were calculated for Tortula (diversity, 1.2; richness, 0.9). Aulacomnium-associated bacteria were characterized by a diversity index of 3.2 and a richness index of 1.5.
TABLE 2.
Taxonomic classification and characterization of bacterial isolates with antagonistic properties
| Strain | Identification by 16S rDNA sequencing or FAMEa | GenBank accession no. | SIb | Taxonomic grouping | Activityc against:
|
Enzyme, siderophore, or antibiotic productiond
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| V. dahliae | X. campestris | Glucan- ases | Prote- ases | Chitin- ases | Sidero- phores | Antibi- otics | |||||
| 1T1 | Pseudomonas putida* | 0.429 | γ-Proteobacteria | ++ | +++ | − | + | − | + | 0 | |
| 1T2 | Pseudomonas putida* | 0.919 | γ-Proteobacteria | ++ | +++ | − | + | − | + | 55 | |
| 1T3 | Pseudomonas putida* | 0.919 | γ-Proteobacteria | + | − | − | + | − | + | 59 | |
| 1T4 | Pseudomonas reactans | AF320987 | 97 | γ-Proteobacteria | ++ | − | − | + | − | + | 55 |
| 1T5 | Pseudomonas putida* | 0.877 | γ-Proteobacteria | ++ | − | − | + | + | + | 40 | |
| 1T6 | Pseudomonas reactans | AF320987 | 98 | γ-Proteobacteria | ++ | − | − | + | − | + | 34 |
| 1T7 | Pseudomonas putida* | 0.937 | γ-Proteobacteria | ++ | − | − | + | − | + | 22 | |
| 1T8 | Pseudomonas putida* | 0.888 | γ-Proteobacteria | ++ | − | − | + | − | + | 10 | |
| 1T9 | Pseudomonas reactans | AF320987 | 98 | γ-Proteobacteria | + | − | − | + | − | − | ND |
| T5 | Pseudomonas sp. | AY089990 | 98 | γ-Proteobacteria | + | − | + | + | − | + | 20 |
| T6 | Pseudomonas sp. | AY089990 | 98 | γ-Proteobacteria | + | − | − | + | − | + | 23 |
| Tiv17 | Pseudomonas sp. | AY089990 | 97 | γ-Proteobacteria | ++ | +++ | − | + | + | + | 36 |
| Twm31 | Xanthomonas sp. | AB016762 | 97 | γ-Proteobacteria | ++ | − | + | + | + | − | 26 |
| Twm74 | Pseudomonas fluorescens | AF368760 | 97 | γ-Proteobacteria | + | − | + | + | + | − | 20 |
| 1A1 | Serratia proteamaculans | AY040208 | 98 | Enterobacteria | +++ | − | − | + | + | + | 31 |
| 1A2 | Serratia liquefaciens* | 0.862 | Enterobacteria | +++ | − | − | + | + | + | 61 | |
| 1A3 | Serratia liquefaciens* | 0.902 | Enterobacteria | ++ | − | − | + | + | + | 7 | |
| 1A4 | Serratia proteamaculans | AY040208 | 98 | Enterobacteria | ++ | − | − | + | + | + | 76 |
| 1A5 | Serratia liquefaciens* | 0.802 | Enterobacteria | ++ | − | − | + | + | + | 40 | |
| 1A6 | Serratia proteamaculans | AF286867 | 98 | Enterobacteria | +++ | − | − | + | + | + | 10 |
| 1A7 | Serratia liquefaciens* | 0.680 | Enterobacteria | +++ | − | − | + | + | + | 19 | |
| 1A8 | Serratia liquefaciens* | 0.902 | Enterobacteria | +++ | − | − | + | + | + | 10 | |
| 1A9 | Serratia liquefaciens* | 0.887 | Enterobacteria | +++ | − | − | + | + | + | 16 | |
| 1A10 | Serratia liquefaciens* | 0.762 | Enterobacteria | +++ | − | − | + | + | + | 31 | |
| 1A11 | Burkholderia fungorum | AAAC01000192 | 97 | β-Proteobacteria | + | − | − | − | − | + | 3 |
| 1A16 | Burkholderia phenazinium | AY154375 | 98 | β-Proteobacteria | + | − | − | + | − | + | 3 |
| A1 | Burkholderia phenazinium | U96936 | 98 | β-Proteobacteria | ++ | − | + | + | − | − | 20 |
| A2 | Burkholderia sp. | AJ300693 | 98 | β-Proteobacteria | ++ | − | − | − | − | − | 52 |
| A3 | Burkholderia phenazinium | U96936 | 97 | β-Proteobacteria | ++ | + | + | + | − | − | 23 |
| A5 | Burkholderia sp. | AJ300693 | 98 | β-Proteobacteria | ++ | − | − | − | − | − | ND |
| A6 | Burkholderia phenazinium | AY154375 | 98 | β-Proteobacteria | ++ | − | + | + | − | − | 13 |
| A8 | Bacillus pumilus | AY167882.1 | 99 | β-Proteobacteria | ++ | − | − | − | − | − | ND |
| A11 | Pseudomonas sp. | AY089990 | 98 | γ-Proteobacteria | + | − | − | − | − | − | ND |
| A12 | Burkholderia phenazinium | AB021394 | 97 | β-Proteobacteria | ++ | − | + | + | − | − | 30 |
| A14 | Burkholderia terricola | AY040362 | 97 | β-Proteobacteria | +++ | − | − | − | − | − | 42 |
| A15 | Xanthomonas sp. | AB016762 | 97 | γ-Proteobacteria | ++ | − | − | − | − | − | ND |
| A16 | Pseudomonas sp. | AY089990 | 97 | γ-Proteobacteria | + | − | − | − | − | − | 39 |
| A17 | Burkholderia phenazinium | AY154375 | 98 | β-Proteobacteria | + | − | − | − | − | − | ND |
| A20 | Burkholderia sp. | AJ300693 | 98 | β-Proteobacteria | + | − | − | − | − | − | ND |
| A21 | Burkholderia phenazinium | U96936 | 97 | β-Proteobacteria | + | − | − | − | − | − | ND |
| A22 | Burkholderia sp. | AJ300693 | 97 | β-Proteobacteria | + | − | − | − | − | + | 36 |
| A23 | Collimonas fungivorans | AJ496444 | 98 | β-Proteobacteria | + | − | − | − | − | − | 39 |
| A24 | Burkholderia sp. | AJ300693 | 98 | β-Proteobacteria | + | − | − | − | − | + | 23 |
| A25 | Burkholderia terricola | AY040362 | 98 | β-Proteobacteria | + | − | − | − | − | − | ND |
| 1S1 | Burkholderia phenazinium | U96936 | 98 | β-Proteobacteria | +++ | − | − | − | − | + | 10 |
| 1S2 | Micrococcus lylae* | 0.770 | +++ | − | − | − | − | + | 31 | ||
| 1S3 | Burkholderia gladioli* | 0.436 | β-Proteobacteria | +++ | − | − | − | − | + | 13 | |
| 1S4 | Burkholderia phenazinium | U96936 | 98 | β-Proteobacteria | +++ | − | − | − | − | + | 10 |
| 1S5 | Burkholderia terricola | AY040362 | 99 | β-Proteobacteria | + | − | − | + | − | + | 31 |
| 1S8 | Burkholderia gladioli* | 0.437 | β-Proteobacteria | +++ | − | − | − | − | − | 64 | |
| 1S9 | Burkholderia phenazinium | U96936 | 98 | β-Proteobacteria | ++ | − | − | − | − | + | 66 |
| 1S10 | Burkholderia gladioli* | 0.680 | β-Proteobacteria | +++ | − | − | − | − | + | 64 | |
| 1S11 | Burkholderia phenazinium | U96936 | 98 | β-Proteobacteria | +++ | − | − | − | − | + | 10 |
| 1S12 | Burkholderia phenazinium | U96936 | 98 | β-Proteobacteria | ++ | − | − | − | − | + | ND |
| 1S13 | Paenibacillus pabuli* | 0.827 | Firmicutes | +++ | − | − | − | − | + | 16 | |
| 1S15 | Burkholderia phenazinium | U96936 | 98 | β-Proteobacteria | +++ | − | − | − | − | + | 13 |
| 1S16 | Burkholderia phenazinium | U96936 | 98 | β-Proteobacteria | +++ | − | − | − | − | + | 10 |
| 1S18 | Burkholderia cepacia | 0.399 | β-Proteobacteria | +++ | − | − | − | − | + | 10 | |
| S1 | Burkholderia phenazinium | U96936 | 98 | β-Proteobacteria | +++ | − | − | − | − | + | 55 |
| S2 | Burkholderia phenazinium | U96936 | 98 | β-Proteobacteria | +++ | − | − | − | − | ++ | 19 |
| S3 | Rahnella aquatilis | AY253919.1 | 98 | Enterobacteria | +++ | − | − | − | − | ++ | 7 |
| S4 | Pseudomonas sp. | AF094725 | 97 | γ-Proteobacteria | ++ | − | − | − | − | + | 55 |
| S5 | Burkholderia terricola | AY040362 | 97 | β-Proteobacteria | +++ | − | − | − | − | ++ | 10 |
| S6 | Rahnella aquatilis | U90758 | 98 | Enterobacteria | ++ | − | − | − | − | − | ND |
| S7 | Burkholderia phenazinium | U96936 | 98 | β-Proteobacteria | ++ | − | − | − | − | + | 10 |
| S9 | Burkholderia phenazinium | U96939 | 98 | β-Proteobacteria | ++ | − | − | + | − | + | 23 |
| S11 | Burkholderia sp. | AY134849 | 97 | β-Proteobacteria | +++ | − | − | − | − | − | 7 |
| S12 | Burkholderia phenazinium | U96936 | 98 | β-Proteobacteria | ++ | + | − | − | − | + | 20 |
| S13 | Burkholderia phenazinium | U96936 | 98 | β-Proteobacteria | ++ | − | − | − | − | + | 26 |
| S14 | Burkholderia phenazinium | U96936 | 98 | β-Proteobacteria | +++ | − | − | − | − | + | 10 |
| S15 | Burkholderia phenazinium | U96936 | 98 | β-Proteobacteria | ++ | − | − | − | − | ++ | 23 |
| S16 | Burkholderia phenazinium | U96936 | 98 | β-Proteobacteria | ++ | − | − | − | − | ++ | 20 |
| S17 | Burkholderia phenazinium | U96936 | 98 | β-Proteobacteria | + | − | − | − | − | − | 4 |
| S18 | Burkholderia phenazinium | U96936 | 98 | β-Proteobacteria | + | − | − | − | − | ++ | 23 |
| S19 | Serratia fonticola | AF286869 | 97 | Enterobacteria | ++ | − | − | − | − | + | 26 |
| S20 | Burkholderia phenazinium | U96936 | 99 | β-Proteobacteria | ++ | − | − | − | − | + | 26 |
| S22 | Burkholderia phenazinium | U96936 | 99 | β-Proteobacteria | ++ | + | − | − | − | ++ | 17 |
| S23 | Burkholderia phenazinium | U96936 | 98 | β-Proteobacteria | ++ | + | − | − | − | ++ | 20 |
| S25 | Burkholderia phenazinium | U96936 | 97 | β-Proteobacteria | ++ | + | − | − | − | ++ | ND |
| S26 | Burkholderia phenazinium | U96936 | 97 | β-Proteobacteria | +++ | − | − | − | − | ++ | 26 |
| S28 | Burkholderia fungorum | AAAC01000192 | 98 | β-Proteobacteria | ++ | − | − | − | − | + | 23 |
| S31 | Burkholderia phenazinium | U96936 | 97 | β-Proteobacteria | ++ | + | − | − | − | ++ | 25 |
| S32 | Burkholderia phenazinium | U96936 | 98 | β-Proteobacteria | ++ | − | − | − | − | ++ | 23 |
Asterisks indicate isolates identified by FAME analysis.
SI, similarity index. For isolates identified by FAME analysis, values range from 0 to 1; for isolates identified by 16S rDNA sequencing, values range from 0 to 100%.
Antagonism toward V. dahliae and X. campestris was determined by dual-culture assay. +, 0 to 5-mm-wide zone of inhibition; ++, 5 to 10-mm-wide zone; +++, 10 to 15-mm-wide zone.
Production of glucanases, proteases, chitinases, and siderophores was determined by plate assays. +, hydrolysis; −, no hydrolysis. Antibiotic production was determined by in vitro bioassays with sterilely filtered culture supernatants; results are expressed as percentages of fungal growth inhibition. ND, not determined.
No common single species was obtained from the gametophytes of all three mosses. Three species were found on Sphagnum and Aulacomnium: Burkholderia fungorum, Burkholderia phenazinium, and Burkholderia terricola. For Sphagnum and Tortula, only Pseudomonas fluorescens was detected. No common species were found for Aulacomnium and Tortula. The most abundant bacterial species were Burkholderia phenazinium (31 isolates) and Serratia liquefaciens (8 isolates). The dominant species for the single mosses were B. phenazinium (25 isolates) for Sphagnum, S. liquefaciens (8 isolates) for Aulacomnium, and Pseudomonas putida (7 isolates) for Tortula. A high percentage of species (65%) was detected only on one single moss, and a large proportion of species (7) were isolated only once, e.g., Budvicia aquatica, Rahnella aquatilis, and Serratia fonticola.
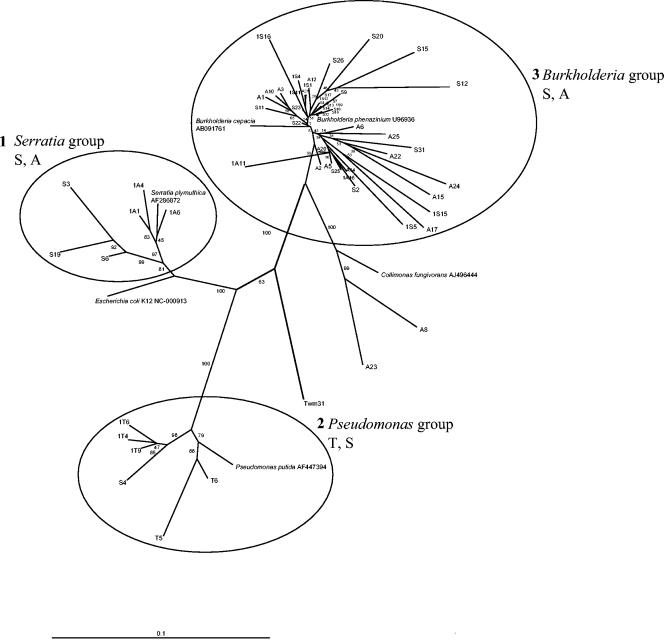
Moss-associated antagonists belong mainly to β-Proteobacteria (50 isolates) as well as γ-Proteobacteria (18 isolates) and enteric bacteria (13 isolates). Gram-positive antagonistic isolates accounted for only a small proportion of the Verticillium antagonists (two isolates). The phylogenetic relationships among moss-associated isolates with antagonistic properties are depicted in Fig. 4. According to the 16S rDNA sequences of the isolates, three cluster groups were found: the Serratia cluster, the Pseudomonas cluster, and the Burkholderia cluster. The Serratia cluster contained isolates from Sphagnum and Aulacomnium, the Pseudomonas cluster contained isolates from Sphagnum and Tortula, and the Burkholderia cluster contained isolates from Sphagnum and Aulacomnium.
FIG. 4.
Phylogenetic dendrogram calculated with all 16S rDNA sequences that were recovered from bacteria associated with the mosses T. ruralis (T), A. palustre (A), and S. rubellum (S), grouped by the neighbor-joining method. Additionally, type strain sequences of Burkholderia cepacia, Collimonas fungivorans, E. coli, P. putida, and Serratia plymuthica were added. Most of the isolates belong to three different cluster groups. Bar indicates 10% sequence divergence.
Characterization of Verticillium antagonists belonging to the Burkholderia and Pseudomonas groups by BOX-PCR.
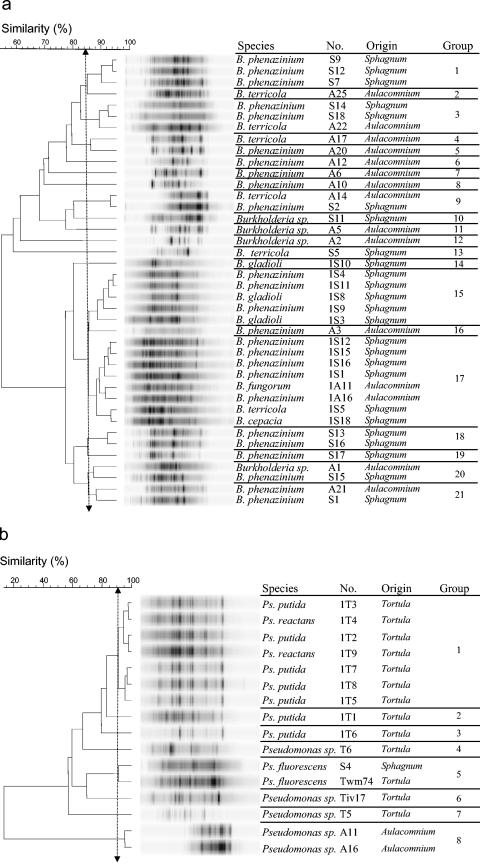
Verticillium antagonists assigned by 16S rDNA sequencing to the genus Pseudomonas were isolated from all three of the moss species, whereas, in contrast, Burkholderia isolates were isolated only from Sphagnum and Aulacomnium. A total of 40 Burkholderia and 16 Pseudomonas isolates were genotypically characterized by their BOX fingerprints to detect moss-specific genotypes (Fig. 5). The intraspecies diversity of BOX patterns analyzed in three independent replicates for each isolate was shown at 89% similarity. Analysis of BOX patterns for Burkholderia of more than 85% similarity resulted in 21 different cluster or genotype groups, although 10 of them contained only one isolate. Only five groups contained isolates from Sphagnum and Aulacomnium. Analysis of BOX patterns for Pseudomonas of more than 85% similarity resulted in eight different cluster or genotype groups, although five of them contained only one isolate. The majority of groups contained isolates from only one moss species. Overall analysis of genotypes demonstrated a high degree of moss specificity.
FIG. 5.
Dendrogram showing the relationships of 38 Burkholderia isolates (a) and 16 Pseudomonas isolates (b) from Tortula, Aulacomnium, and Sphagnum based on BOX-PCR fingerprints grouped by UPGMA. Double-headed vertical arrows indicate the similarity for the groupings.
Antifungal activity and production of antifungal metabolites of Verticillium antagonists.
All 83 antagonists were screened in vitro for their activities against the fungal pathogen V. dahliae and the bacterial pathogen X. campestris (Table 2). Generally, the fungus grew as well as the bacterial isolates on Waksman agar. Inhibition was clearly discerned by limited growth, or the complete absence of fungal mycelium or bacterial growth in the inhibition zone surrounding a bacterial colony. Only nine isolates possessed antifungal as well as antibacterial activity. Three strong Xanthomonas antagonists were selected from Tortula and were found to be pseudomonads. Additionally, in vitro production of antagonistic metabolites was analyzed. Only seven isolates with glucanolytic activity were found; none of the Sphagnum antagonists was able to produce glucanase. Production of proteases was moss specific: all of the Tortula-associated antagonists were proteolytic, but only two proteolytic antagonists were isolated from Sphagnum. Twenty-two percent of the isolates possessed chitinolytic properties, and all strongly chitinolytic isolates were associated with Aulacomnium. The production of siderophores (77% of the isolates) and antifungal antibiotics (99% of the isolates) was extremely widely distributed. Verticillium antagonists assigned to the same species often displayed different patterns of antagonistic activity, as was the case, for example, with S. liquefaciens.
Concluding assessment of the in vitro screening.
All of the parameters tested were used to evaluate the strains by means of a point system. For antagonistic activity, three points were awarded for high activity against either of the two pathogens (totaling a possible six points). One point each was awarded for production of an enzyme (chitinase, glucanase, or proteinase) or siderophore (for a possible four points), and antibiotic production received zero to three points depending on the level. Based on this system, the number of points for each isolate was established in order to evaluate the strains. Among the Sphagnum isolates, B. phenazinium S22 was the most efficient (nine points). Pseudomonas sp. strain Tiv17 was the most efficient isolate from the Tortula gametophyte (11 points). Three Serratia isolates, 1A1, 1A2, and 1A10, identified as Serratia proteamaculans/liquefaciens, were the most effective antagonists from Aulacomnium (nine points).
DISCUSSION
In the present study, a high degree of specificity of moss-associated bacteria was found. Here we combined cultivation-dependent techniques with two cultivation-independent methods to analyze the moss-associated bacterial populations. The latter were based on the extraction of bacterial DNA and amplification of the 16S rDNA fragments, which were then separated by DGGE and/or SSCP. Both methods gave the same picture, showing clearly the moss specificity of the associated bacterial communities. To our knowledge, this is the first report which documents an analysis of moss-associated bacteria with these particular molecular tools. For various crop species, the specificity of associated bacterial communities was demonstrated by Smalla et al. (36) and Berg et al. (7); that for wild plants was demonstrated by Kowalchuk et al. (20). Cultivation on R2A medium was also used to isolate bacteria and to investigate their properties. By this approach, moss specificity could be confirmed by using the following parameters: (i) plate counts of bacteria on R2A medium, (ii) percentage of antagonistic isolates, (iii) antagonistic activity as well as spectrum against pathogens, and (iv) diversity and richness of antagonistic isolates. Overall, the combination of methods was employed successfully to define and to illustrate the specificity and diversity of moss-associated bacteria.
For this study, samples were isolated from bryophytes inhabiting natural nutrient-poor habitats on the southern Baltic Sea coast. Interestingly, correlations were found between the natural abiotic gradients in the ecosystem pH, moisture, and nutrient content and the colonization of bryophytes. The abundance of culturable bacteria increased with pH. In contrast, the percentage of antagonistic bacteria, along with the intensity of antagonism, richness, and diversity, increased with decreasing pH and moisture of these microenvironments. Different structures of leaf surfaces could be another reason for the specificity and diversity of moss-associated bacteria. As shown on micrographs, leaf surfaces formed highly specific microenvironments.
The second key aim of our investigation was to ascertain whether moss-associated bacteria displayed any biotechnological potential. Plant-associated bacteria and those with antagonistic properties are important not only for plant health and growth but also for biotechnological applications. For example, they can be used directly, for biological control of plant pathogens (41), or indirectly, for the production of active substances, e.g., antibiotics or enzymes (16). In comparison to plant-associated bacteria, approximately twofold higher active-substance production was detected for moss-associated bacteria, especially those from Sphagnum. The proportion of antagonistic bacteria was 3 to 9% in the rhizosphere of Verticillium host plants (7), 16% in the rhizosphere of oilseed rape (5), and as much as 18% for various weeds (22). The high proportion is much more surprising when the rhizosphere effect is taken into consideration. This is the well-known phenomenon that, in comparison to that in other plant-associated microenvironments or in bulk soil in the rhizosphere, the proportion of microorganisms (including those with antagonistic properties) is enhanced because of the rich exudation of roots (21, 36). For bryophytes, which possess only a root-like rhizoid, no exudation of nutrients is known. Most of the mosses are ectohydric, which means that the gametophytes can absorb water and dissolved minerals over their surfaces. In this way, the leaf surface of mosses resembles the rhizosphere. This may be one reason for bacterial colonization. While Pseudomonas and Serratia are well-known antagonistic genera (6, 25), Burkholderia is an interesting and rarely mentioned genus. In 2001, Estrada-De Los Santos et al. (13) demonstrated that nitrogen fixation is a common property in the genus Burkholderia. In addition, Burkholderia strains have been shown to be plant growth-promoting rhizobacteria (40). Here, we selected five isolates with very high biotechnological potential, namely, Pseudomonas sp. strain Tiv17, B. phenazinium S22, and the Serratia isolates 1A1, 1A2, and 1A10. Generally, a high percentage of antibiotic-producing bacteria was found.
Recently, mosses have been proposed as ideal models for genetic studies and biotechnological applications (10). Therefore, it is essential to establish whether the mosses were colonized with a variety and abundance of microorganisms as well as whether these microorganisms performed important functions in the mosses' life cycle, health, and growth. Additionally, moss-associated bacteria have great potential for biotechnological applications. Our results indicate that bryophyte-associated bacteria are important potential sources of antifungal antibiotics and that some of them are interesting candidates for biological control agents against plant pathogens.
Acknowledgments
We are grateful to Christian Berg (Rostock, Germany), an expert on bryophytes, who answered all our queries about these fascinating plants. We also thank Hella Goschke and Katja Scherwinski (Rostock, Germany) for valuable technical assistance and Anja Golly (Braunschweig, Germany) for performing FAME analysis for some strains. Additionally, we thank Gerd Fulda and Ludwig Jonas (Rostock, Germany) for help in electron microscopy.
The work was partly supported by the Deutsche Forschungsgemeinschaft.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, D. G., and L. L. McKay. 1983. Simple and rapid method for isolating large plasmid DNA from lactic streptococci. Appl. Environ. Microbiol. 46:549-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ando, H., and A. Mastuo. 1984. Applied bryology. Adv. Bryol. 2:133-224. [Google Scholar]
- 4.Bassam, B. J., G. Ceatano-Anolles, and P. M. Gresshoff. 2001. Fast and sensitive silver staining of DNA in polyacylamide gels. Anal. Biochem. 196:80-83. [DOI] [PubMed] [Google Scholar]
- 5.Berg, G. 1996. Rhizobacteria of oil seed rape antagonistic to Verticillium dahliae. J. Plant Dis. Prot. 103:20-30. [Google Scholar]
- 6.Berg, G. 2000. Diversity of antifungal and plant-associated Serratia plymuthica strains. J. Appl. Microbiol. 88:952-960. [DOI] [PubMed] [Google Scholar]
- 7.Berg, G., N. Roskot, A. Steidle, L. Eberl, A. Zock, and K. Smalla. 2002. Plant-dependent genotypic and phenotypic diversity of antagonistic rhizobacteria isolated from different Verticillium host plants. Appl. Environ. Microbiol. 68:3328-3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chernin, L., Z. Ismailov, S. Haran, and I. Chet. 1995. Chitinolytic Enterobacter agglomerans antagonistic to fungal plant pathogens. Appl. Environ. Microbiol. 61:1720-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costa, J., P. Paulsrud, J. Rikkinen, and P. Lindblad. 2001. Genetic diversity of Nostoc symbionts endophytically associated with two bryophyte species. Appl. Environ. Microbiol. 67:4393-4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Decker, E. L., G. Gorr, and R. Reski. 2003. Moss: an innovative tool for protein production. BioForum Eur. 2:2-3. [Google Scholar]
- 11.Döbbeler, P. 1997. Biodiversity of bryophilous ascomycetes. Biodivers. Conserv. 6:721-738. [Google Scholar]
- 12.Ellenberg, H., H. E. Weber, R. Düll, V. Wirth, W. Werner, and D. Paulißen. 1991. Indicator values of plants in Central Europe. Script. Geobotan. 18:1-248. [Google Scholar]
- 13.Estrada-De Los Santos, P. R., R. Bustillos-Cristales, and J. Caballero-Mellado. 2001. Burkholderia, a genus rich in plant-associated nitrogen fixers with wide environmental and geographic distribution. Appl. Environ. Microbiol. 67:2790-2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frahm, J.-P. 1994. Moose—lebende Fossilien. Biol. Unserer Zeit 24:120-124. [Google Scholar]
- 15.Frahm, J. P. 2001. Biologie der Moose, 1st ed. Spektrum Verlag, Berlin, Germany.
- 16.Fravel, D. R. 1988. Role of antibiosis in the biocontrol of plant diseases. Annu. Rev. Phytopathol. 26:75-91. [Google Scholar]
- 17.Hentschel, U., J. Hopke, M. Horn, A. B. Friedrich, M. Wagner, J. Hacker, and B. S. Moore. 2002. Molecular evidence for a uniform microbial community in sponges from different oceans. Appl. Environ. Microbiol. 68:4431-4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heuer, H., and K. Smalla. 1997. Application of denaturing gradient gel electrophoresis (DGGE) and temperature gradient gel electrophoresis (TGGE) for studying soil microbial communities, p. 353-373. In J. D. Van Elsas, J. T. Trevors, and E. M. H. Wellington (ed.), Modern soil microbiology. Marcel Dekker, Inc., New York, N.Y.
- 19.Hornschuh, M., R. Grotha, and U. Kutschera. 2002. Epiphytic bacteria associated with the bryophyte Funaria hygrometrica: effects of Methylobacterium strains on protonema development. Plant Biol. 4:682-687. [Google Scholar]
- 20.Kowalchuk, G. A., D. S. Buma, W. de Boer, P. G. Klinkhamer, and J. A. van Veen. 2002. Effects of above-ground plant species composition and diversity on the diversity of soil-borne microorganisms. Antonie Leeuwenhoek 81:509-520. [DOI] [PubMed] [Google Scholar]
- 21.Krechel, A., A. Faupel, J. Hallmann, A. Ulrich, and G. Berg. 2002. Potato-associated bacteria and their antagonistic potential towards plant pathogenic fungi and the plant parasitic nematode Meloidogyne incognita (Kofoid & White) Chitwood. Can. J. Microbiol. 48:772-786. [DOI] [PubMed] [Google Scholar]
- 22.Kremer, R. J., M. F. T. Begonia, L. Stanley, and E. T. Lanham. 1990. Characterization of rhizobacteria associated with weed seedlings. Appl. Environ. Microbiol. 56:1649-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lieber, A., B. Kiesel, and W. Babel. 2003. Microbial diversity analysis of soil by SSCP fingerprinting technique using TGGE Maxi System, p. 61-65. In W. Merbach, B. W. Hütsch, and J. Augustin. (ed.), Ökophysiologie des Wurzelraumes. Teubner Verlag, Stuttgart, Germany.
- 24.Liesack, W., P. Janssen, F. A. Rainey, N. L. Ward-Rainey, and E. Stackebrandt. 1997. Microbial diversity in the soil: the need for a combined approach using molecular and cultivation techniques, p 375-440. In J. D. Van Elsas, J. T. Trevors, and E. M. H. Wellington (ed.), Modern soil microbiology. Marcel Dekker, Inc., New York, N.Y.
- 25.Lugtenberg, B. J. J., L. Dekkers, and G. V. Bloemberg. 2001. Molecular determinants of rhizosphere colonization by Pseudomonas. Annu. Rev. Phytopathol. 39:461-490. [DOI] [PubMed] [Google Scholar]
- 26.Menhinick, E. F. 1964. A comparison of some species-individuals diversity indices applied to samples of field insects. Ecology 45:859-861. [Google Scholar]
- 27.Miller, H. A. 1982. Bryophyte evolution and geography. Biol. J. Linn. Soc. 18:145-169. [Google Scholar]
- 28.Precker, A. 2000. The nature reserve “Ribnitzer Großes Moor”—restitution and tourism in a complex mire ecosystem of northern Germany. Telma 30:43-75. [Google Scholar]
- 29.Rademaker, J. L. W., and F. J. De Bruijn. 1997. Characterization and classification of microbes by REP-PCR genomic fingerprinting and computer-assisted pattern analysis, p. 151-171. In G. Caetano-Anollés and P. M. Gresshoff (ed.), DNA markers: protocols, applications and overviews. Wiley & Sons, Inc., New York, N.Y.
- 30.Reski, R. 2003. Physcomitrella patens as a novel tool for plant functional genomics, p. 205-209. In I. K. Vasil (ed.). Plant biotechnology 2002 and beyond. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 31.Riesner, D., G. Steger, R. Zimmat, R. A. Owens, and K. Henco. 1989. Temperature-gradient gel electrophoresis of nucleic acids: analysis of conformational transitions, sequence variations and protein-nucleic acid interactions. Electrophoresis 10:377-389. [DOI] [PubMed] [Google Scholar]
- 32.Salles, J. F., F. A. De Souza, and J. D. van Elsas. 2002. Molecular method to assess the diversity of Burkholderia species in environmental samples. Appl. Environ. Microbiol. 68:1595-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwieger, F., and C. C. Tebbe. 1998. A new approach to utilize PCR-single-strand-conformation polymorphism for 16S rRNA gene-based microbial community analysis. Appl. Environ. Microbiol. 64:4870-4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwyn, B., and J. B. Neilands. 1987. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 160:47-56. [DOI] [PubMed] [Google Scholar]
- 35.Shannon, C. E., and W. Weaver. 1949. The mathematical theory of communication. University of Illinois Press, Urbana, Ill.
- 36.Smalla, K., G. Wieland, A. Buchner, A. Zock, J. Parzy, N. Roskot, H. Heuer, and G. Berg. 2001. Bacterial bulk and rhizosphere communities studied by denaturing gradient gel electrophoresis of PCR-amplified fragments of 16S rRNA genes: plant-dependent enrichment and seasonal shifts revealed. Appl. Environ. Microbiol. 67:4742-4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sørensen, J. 1997. The rhizosphere as a habitat for soil microorganisms, p. 21-45. In J. D. Van Elsas, J. T. Trevors, and E. M. H. Wellington (ed.), Modern soil microbiology. Marcel Dekker, Inc., New York, N.Y.
- 38.Steinert, M., U. Hentschel, and J. Hacker. 2000. Symbiosis and pathogenesis: evolution of the microbe-host interaction. Naturwissenschaften 87:1-11. [DOI] [PubMed] [Google Scholar]
- 39.Tjamos, E. C., R. C. Rowe, J. B. Heale, and D. R. Fravel. 2000. Advances in Verticillium research and disease management. American Phytopathological Society, St. Paul, Minn.
- 40.TrÂn Van, V., O. Berge, S. Ke, J. Balandreau, and T. Heulin. 2000. Repeated beneficial effects of rice inoculation with a strain of Burkholderia vietnamiensis on early and late yield components in low fertility sulphate acid soils of Vietnam. Plant Soil 218:273-284. [Google Scholar]
- 41.Weller, D. M. 1988. Biological control of soilborne plant pathogens in the rhizosphere with bacteria. Annu. Rev. Phytopathol. 26:379-407. [Google Scholar]
- 42.Widmer, F., R. J. Seidler, P. M. Gillevet, L. S. Watrud, and G. D. Di Giovanni. 1998. A highly selective PCR protocol for detecting 16S rRNA genes of the genus Pseudomonas (sensu stricto) in environmental samples. Appl. Environ. Microbiol. 64:2545-2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wyatt, R. 1982. Population ecology of bryophytes. J. Hattori Bot. Lab. 52:179-198. [Google Scholar]