Abstract
Background:
Zoonotic cutaneous leishmaniasis (ZCL) is a neglected disease with public health importance that is common in many rural areas of Iran. In recent years, behavioral resistance and/or bait shyness against the common rodenticide among reservoir hosts of ZCL have been reported. The aim of this study was to evaluate the effectiveness of Klerat® and zinc phosphide against natural reservoir of ZCL.
Methods:
This survey was carried out in four villages located 45 to 95 km far from Esfahan City Esfahan province, central Iran from April to November 2011. The rodent burrows were counted destroyed and reopened holes baited around all villages. Effect of rodent control operation on the main vector density and incidence of ZCL were evaluated.
Results:
The reduction rate of rodent burrows after intervention calculated to be at 62.8% in Klerat® and 58.15% in zinc phosphide treated areas. Statistical analysis showed no difference between the densities of the vector in indoors and outdoors in intervention and control areas. The incidence of the disease between treated and control areas after intervention was statistically different (P< 0.05).
Conclusion:
Klerat® could be a suitable alternative for zinc phosphide in a specific condition such as behavior resistance or occurrence of bait shyness.
Keywords: Iran, Klerat® Rodent control, Zinc phosphide, Zoonotic cutaneous leishmaniasis
Introduction
Zoonotic cutaneous leishmaniasis (ZCL) is a neglected disease and a major public health problem that is common in many rural areas of 17 out of 31 provinces in Iran (1, 2). Combating rodent reservoir of ZCL is effective in reducing the incidence of the disease and rodent populations (36). Certain numbers of gerbils including; Rhombymos opimus and Meriones libycus, play as the main and secondary reservoir hosts of ZCL in Iran (2, 7). Phlebotomus papatasi is the predominate and the main vector of ZCL and Leishmania major is the causative agent of the disease (8). L. major has been isolated from P. papatasi, P. caucasicus, R. opimus, M. libycus and human in this endemic area (2, 8–10).
P. papatasi plays role of the main vector of cutaneous leishmaniasis due to L. major in Turkmenistan, Uzbekistan, Saudi Arabia, Iran, southern Morocco and central Tunisia (11, 12). Natural disasters such as earthquake, urbanization, urban and rural development near colonies of the rodents, population displacement between endemic and non-endemic areas, caused an exponential increase in disease cases (13, 14).
To control the disease different strategy including residual spraying with DDT, spraying powder of DDT in the rodent burrows (15), poisoning the reservoir hosts and using deltamethrin-impregnated bed nets and curtains (16) have been employed. Moreover, a successful leishmanization has been carried out in Iran (17). International efforts to achieve an effective vaccine to prevent leishmaniasis have not been successful yet (18, 19).
In Karshinskaya Steppes, Uzbekistan, Rh. opimus colonies were destroyed using heavy machinery in a radius of 2 to 3 km from the city in a 3-yr period. The results showed that, regional rodent control operation is not effective due to re-invasion (20). An extensive study to eliminate Rh. opimus in a vast area surrounded by mountain ranges and rivers showed a significant reduction in the density of sand flies. Moreover, no cases of ZCL reported at least 4 years after operation (21).
In recent years, some research projects have been conducted to evaluate rodent control measures in the country by Iranian scientists. In 1997, during a field study, rodent burrows within a radius of 500 meters from houses destroyed and reopened burrows were baited with zinc phosphide 2.5%. The operation was performed once a month in May, June, July and September. The results indicated 12-fold reduction in incidence of ZCL in intervention area compared to the control area at the end of first year and 5 fold at the end of second year of the study (4). To evaluate the conducted operation (1999 to 2002) the number of burrows was counted in May and October. The rodent re-opened holes were baited, if the number reached to 30% of initial active burrows. As a result, changes in the numbers of rodent burrows and incidence rate of ZCL in the treated and control village were statistically significant (3).
From January 2011 to January 2012, a study with the aim of introducing alternative rodenticide to control the reservoirs host of ZCL was performed. Along with the study, the effect of the intervention on the vector density and the incidence were evaluated. In this comprehensive study effectiveness of three different rodinticide were evaluated. Rodents’ burrows were baited with zinc phosphide, Coumavec® or phostoxin. Active case of ZCL was found by household questioning once every season. Furthermore, phlebotominae were collected every 15 days by sticky traps. The results showed phostoxin had a weaker ability in reducing rodent populations comparing to Coumavec® and zinc phosphide. Overall, Coumavec® and phostoxin could be suitable alternatives for zinc phosphide while bait shyness or behavioral resistance are observed (5, 6, 22).
Behavioral resistance and/or bait shyness against the conventional rodenticide among reservoir hosts of ZCL have been reported in some endemic foci including Esfahan (unpublished data, Esfahan Health Centre, Iran). Thus, it looks essential to introduce some new effective alternative rodenticides to control gerbil population and subsequently the disease in endemic area of ZCL in the country.
The aim of the present study was to introduce a new alternative rodenticide to control the reservoirs of the disease, its effect on seasonal density of the vector and thus, disease incidence. In this field trial the effect of Klerat® (Brodifacoum) on the reservoir host and vector population of the disease were compared with zinc phosphide 2.5%.
Materials and Methods
Study area
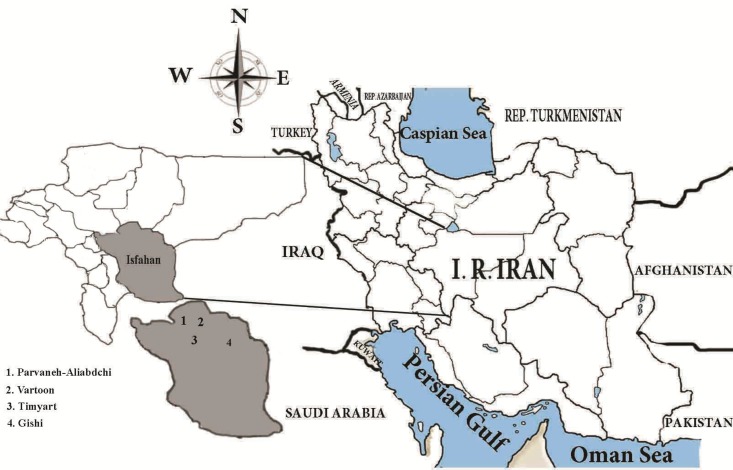
This survey was carried out in four villages located 45 to 95 km far from Esfahan City, Esfahan Province, Iran from April to November 2011. Timyart (32°32˙44.24″N/-52°01˙07.08″E) and Gishi (32°29˙24.07″N, / 52°21˙47.06″E) were chosen as intervention areas for Klerat® and zinc phosphide respectively. Vartoon (32°50˙11.48″N/ 52°06˙45.93″E) and Parvaneh-Aliabadchi (32°47˙44.36″N/ 51°58˙27.19″E) were chosen as control areas (Fig. 1).
Fig. 1:
Map of the Esfahan County, Esfahan Province, Iran, showing the geographical location and study areas
The study areas have dry climate. In 2010, the maximum mean temperature was 39.1°C in July and minimum mean temperatures were −1.6°C in December. The total rainfall was 72.2 mm. The minimum mean monthly relative humidity was 7% (July) and maximum was 82% (January).
The Klerat® (Brodifacoum) wax blocks formulation was used in the current study. Zinc phosphide 2.5% was used based on the previous studies (3).
Rodent control operation
In the late April 2011, before the emerging of adult sand flies, the rodent burrows were counted and destroyed in a radius of 500 m from houses around all villages.
After 48 h reopened holes were counted again. In treated areas, the reopened burrows were baited by Klerat® wax blocks or zinc phosphide baits and then closed. For Klerat® 2 to 4 wax blocks and for zinc phosphide 12–15 g of the poisoned baits were inserted in a depth of 10 cm in each burrow. For the following week, the study areas were revisited and in treated areas, the reopened burrows were counted, baited and closed. This operation was carried out monthly in May, June, July and August. The data including date of baiting and the number of reopened holes were recorded. No control operation was done in control village (Vartoon), but for comparison, only the numbers of reopened holes were counted in each stage.
Entomological study
Effect of the rodent control operation on the main vector (P. papatasi) density was evaluated through an entomological survey. Sand flies were collected by sticky paper traps (castor oil impregnated papers) twice a month in their active season from the beginning (April) to the end (October). The sticky traps were installed before sunset and collected before sunrise in the following day. The captured sand flies were removed from sticky traps, washed in absolute acetone and preserved in 70% ethanol for preparing microscopic slides. The slides were prepared using Pauri’s medium (23) and identified by valid morphological keys (24, 25).
Human infection
The effect of rodent control operation on the incidence of the disease was evaluated. Active case findings were done in treated (Timyart and Gishi) and control (Vartoon and Parvaneh-Aliabachi) areas before and after rodent control operation in January 2011 and once every season in 2012. One hundred and fifty households in treated villages and all inhabitants in control villages (less than 150 households) were survived. Information of the people including presence or absence of scar (s) or active lesion (s), number of the lesion (s) or scar (s), and history of traveling to the other ZCL foci, was collected for each household. Imported cases from other foci of ZCL were excluded from the study. Only new cases of ZCL with active lesion (s) were included. Either incidence of the disease in treated or control villages were calculated at the end of 2011 and 2012. For disease incidence calculation, the persons with previous scars were excluded from at risk population.
Statistical analysis
The data were analyzed using STATA and SPSS 22 IBM software and graphs were drawn using Excel software. Chi-squared test and the kruskal-Wallis non-parametric tests were used to compare rodent holes changes and density of sand flies in intervention and control areas, respectively.
Results
The treated area of Timyart (Klerat® intervention area) was around 210 hectares and the total burrows before intervention was 5175 (25 per h). Forty-eight h after the rodent burrows destroying, 1231 (5.8 active holes per h) of the burrows were reopened. The number of reopened holes decreased to 935 one week after control operations. The number of reopened burrows was counted at 613, 858 and 458 in June, July and August respectively. The treated area of Gishi (zinc phosphide intervention) was around 193 h. The numbers of holes before the intervention counted at 4729 (24.5 per h). After 48 h of distraction the colonies, the reopened holes numbers decreased to 1682 (8.7 active holes per h). The number of holes reduced to 600 one week after distraction. The number of the burrows was counted at 493, 424 and 704 in June, July and August respectively. Vartoon (the control area) was around 173 hectare. Before intervention, the number of burrows was 2297 (13.3 per h) and 48 h after distraction 196 (1.1 active holes per h) of these holes were reopened. The number of reopened holes in control area (Vartoon) increased to 281 after a week. The number of burrows increased to 365, 557 and 1306 in June, July and August, respectively. The number of holes in each stage in control village showed an increasing trend comparing to the intervention areas. The reduction rate of rodent burrows after intervention calculated at 62.8% in Timyart (Klerat® treated area) and 58.15% in Gishi (zinc phosphide treated area). Before and after the survey the number of rodent burrows in control area (Vartoon) showed 6.66 folds increasing (Table 2).
Table 2:
Comparison of the number of rodent holes in the intervention and control villages, Esfahan County, Esfahan, Iran, 2011
| Place | Treated area (hectare) | May before treatment | May, 24 hour after burrow destruction | May, One week after first baiting | June | July | August |
|---|---|---|---|---|---|---|---|
| Timyart | 210 | 5175 | 1231 | 935 | 613 | 858 | 458 |
| Gishi | 193 | 4729 | 1682 | 600 | 493 | 424 | 704 |
| Vartoon | 173 | 2297 | 196 | 281 | 365 | 557 | 1306 |
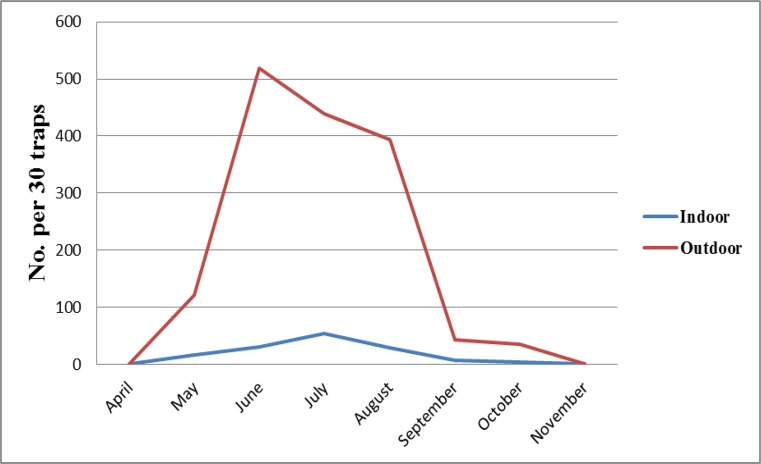
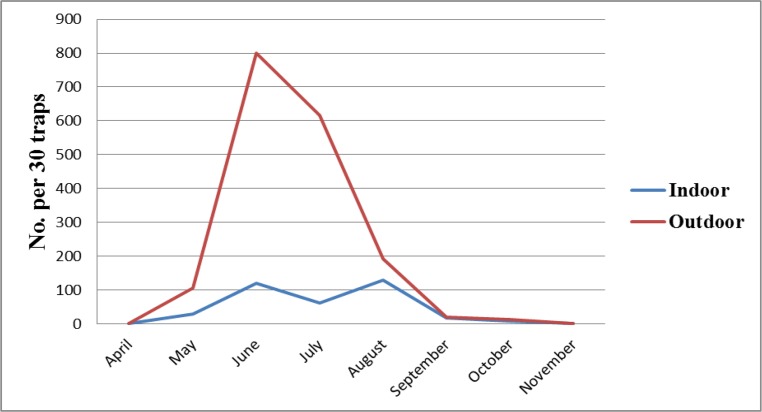
During May to October 2011, totally 3894 adult sand flies (3340 from outdoors and 554 from indoors) were collected. Phlebotomus. papatasi, Sergentomyia sintoni and P. sergenti in indoors and P. papatsi, S. sintoni, P. sergenti, Phlebotomus ansari and P. mongolensis in outdoors resting place were identified. In the late April and in the late October sand flies emerged and disappeared respectively. P. papatasi was the most common and dominant species in indoor and outdoor resting places. Monthly density of P. papatasi in both intervention areas with Klerat® and zinc phosphide was compared (Fig. 2 and Fig. 3). Statistical analysis showed no difference between the densities of the vector in indoors and outdoors in intervention and control areas (P> 0.05).
Fig. 2:
Monthly fluctuation of Phlebotomus papatasi in treated village with Klerat® (Timyart), Esfahan County, Esfahan Province, Iran, 2011
Fig. 3:
Monthly fluctuation of Phlebotomus papatasi in treated village with zinc phosphide (Gishi), Esfahan County, Esfahan Province, Iran, 2011
Table 1 shows the yearly incidence of the disease in treated and control villages. In Timyart and Gishi (intervention areas) the incidence was calculated at 14.52 and 39.14 per thousand and in Vartoon and Parvaneh-Aliabadchi (control areas) 18.40 and 76.19 per thousand, in 2011 respectively. After intervention, the incidence of the disease in Timyart and Gishi (treated villages) dropped to 5 and 11.40 per thousand (34.43% and 29.12% of its original level before the intervention), respectively. No significant difference was seen between reduction rate of the incidence between Kleart and zinc phosphide (P> 0.05). Comparing the incidence of the disease between treated and control areas after intervention were statistically different (P< 0.05).
Table 1:
Comparison of the incidence (per thousand) of zoonotic cutaneous leishmaniosis in the intervention and control villages, Esfahan County, Esfahan, Iran, 2010–2011
| 2011 | 2012 | |||
|---|---|---|---|---|
| Name of the village | No. with AL | Incidence | No. with AL | Incidence |
| Timyart (intervention area with Klerat®) | 6 | 14.52 | 2 | 5 |
| Gishi (intervention area with Zinc phosphide) | 11 | 39.14 | 3 | 11.40 |
| Vartoon (control area) | 3 | 18.40 | 2 | 10.92 |
| Parvaneh-Ali abdchi (control area) | 8 | 76.19 | 5 | 49.5 |
Discussion
Until now, various approaches have been introduced to ZCL control such as rodent control, bed nets impregnated with pyrethroids, repellents, residual spraying, health education and leishmanization in the country (15, 17), although, there is no success in developing an effective vaccine to prevent leishmaniasis as well.
The results of this study show rodent control operation has significant impact on the rodent population and incidence of the disease. Both rodenticides were effective on reservoir host population. After intervention, the reduction rate of rodent holes calculated at 62.8% and 58.14% in the Klerat® and in zinc phosphide treated areas, respectively. It seems that Klerat® was more effective than zinc phosphide in rodent population decrease. On the other hand, in control area (Vartoon) number of rodent colonies reached to 6.66 fold. Changing the number of rodent burrows showed a decreasing trend in the intervention areas while in the control area showed an increasing trend. The incidence reduction rates of ZCL were 65.56% and 70.87% in Timyart (treated with Klerat®) and Gishi (treated with zinc phosphide) respectively, thus both pesticides were effective to reduce incidence of the disease. The incidence of the disease from 2011 to 2012 decreased in all studied areas including control area as well. In 1997, a rodent control operation was conducted to control ZCL. During this intervention zinc phosphide 2.5% was used. Incidence of ZCL decreased 12-fold at the end of the first year and 5-fold at the end of the second year of the operation in intervention area compared to the control area (4). Another study was conducted to show the effect of rodent control operation on the disease incidence at the same intervention area from 1999 to 2002. Results showed the numbers of rodent holes trend in the treated and control areas were statistically significant. Moreover, trends in the incidence of ZCL between the intervention and control village were significant as well (3).
In both treated areas, the density of P. papatasi in indoors was lower than outdoor resting places. Comparing the density of P. papatasi trend in control and intervention villages revealed that, rodents control operation has no significant effect on the P. papatasi density.
Concurrent with this survey, in two other areas Coumavec® (a mixture of Coumatetralyl 0.5% and Etofenprox 0.5%) and phostoxin were evaluated and compared with zinc phosphide. The reduction rate of rodent burrows in treated areas with Coumavec®, phostoxin and zinc phosphide calculated at 48.46, 32.7% and 58.15%, respectively. The ZCL incidence significantly reduced in the all treated areas. In zinc phosphide intervention areas, the density of P. papatasi was higher in outdoors in contrary to what was seen in treated with Coumavec® and phostoxin which the density of sand flies was higher in indoors (5, 6).
As mentioned above, Klerat® caused 62.8% reduction in rodent burrows after intervention, although, this rodenticide caused 65.56% reduction in incidence of the disease.
As Klerat® is an anticoagulant chronic rodenticide and allows rodent to be live 1–2 weeks after ingestion, we do not recommend it as an alternative for zinc phosphide to control the reservoir hosts of ZCL in conventional situation. Because the sand fly vectors have enough time for transmitting the disease among rodents or from rodents to human, especially during sand flies peak activity.
Conclusion
Klerat® could be a suitable alternative to zinc phosphide in the case of bait shyness or behavioral resistance. After recovering bait shyness or behavioral resistance, zinc phosphide should be used again.
Acknowledgements
Authors would like to express their appreciation to the staff of Esfahan Health Station, National Institute of Health Research, Tehran University of Medical Sciences (TUMS) for their collaboration along the study. This research was financially supported by School of Public Health, TUMS, Institute for Environmental Research (IER), TUMS and Department of Zoonosis, CDC, Ministry of Health and Medical Education, Islamic Republic of Iran. The authors declare that they have no conflicts of interest.
References
- 1. Akhavan A. Immune Response of Great Gerbil against Phlebotomus papatasi Saliva. Lap Lambert Academic Publishing, Saarbrücken, Germany: 2011. [Google Scholar]
- 2. Yaghoobi-Ershadi M. Phlebotomine Sand Flies (Diptera: Psychodidae) in Iran and their Role on Leishmania Transmission. J Arthropod Borne Dis. 2012; 6 (1): 1– 17. [PMC free article] [PubMed] [Google Scholar]
- 3. Yaghoobi-Ershadi M, Zahraei-Ramazani AR, Akhavan AA, Jalali-Zand AR, Abdoli H, Nadim A. Rodent control operations against zoonotic cutaneous leishmaniasis in rural Iran. Ann Saudi Med. 2005; 25 (4): 309– 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yaghoobi-Ershadi M, Akhavan A, Zahraei-Ramazani A, Javadian E, Motavalli-Emami M. Field trial for the control of zoonotic cutaneous leishmaniosis in Badrood, Iran. Ann Saudi Med. 2000; 20 (5/6): 386– 389. [DOI] [PubMed] [Google Scholar]
- 5. Akhavan AA, Veysi A, Arandian M, Vatandoost H, Yaghoobi-Ershadi M, Hosseini M, Abdoli H, Heidari K, Sadjadi A, Fadaei R, Ramazanpour J, Aminian K, Shirzadi MR, Jafari R. Field evaluation of phostoxin and zinc phosphide for the control of zoonotic cutaneous leishmaniasis in a hyperendemic area, central Iran. J Vector Borne Dis. 2014; 51 (4): 307– 12. [PubMed] [Google Scholar]
- 6. Veysi A, Vatandoost H, Yaghoobi-Ershadi M, Arandian M, Jafari R, Hosseini M, abdoli H, Rassi Y, Heidari K, Sadjadi A, Fadaei R, Ramazanpour J, Aminian K, Shirzadi MR, Akhavan AA. Comparative Study on the Effectiveness of Coumavec® and Zinc Phosphide in Controlling Zoonotic Cutaneous Leishmaniasis in a Hyperendemic Focus in Central Iran. J Arthropod Borne Dis. 2012; 6 (1): 18. [PMC free article] [PubMed] [Google Scholar]
- 7. Akhavan AA, Yaghoobi-Ershadi M, Khamesipour A, Mirhendi H, Alimohammadian M, Rassi Y, Arandian MH, Jafari R, Abdoli H, Shareghi N, Ghanei M, Jalalizand N. Dynamics of Leishmania infection rates in Rhombomys opimus (Rodentia: Gerbillinae) population of an endemic focus of zoonotic cutaneous leishmaniasis in Iran. Bull Soc Pathol Exot. 2010; 103 (2): 84– 9. [DOI] [PubMed] [Google Scholar]
- 8. Nadim A, Mesghali A, Amini H. Epidemiology of cutaneous leishmaniasis in the Isfahan province of Iran: III. The vector. Trans R Soc Trop Med Hyg. 1968; 62 (4): 543– 9. [DOI] [PubMed] [Google Scholar]
- 9. Yaghoobi-Ershadi M, Javadian E. Studies on sandflies in a hyperendemic area of zoonotic cutaneous leishmaniasis in Iran. Indian J Med. Res. 1997; 105: 61– 6. [PubMed] [Google Scholar]
- 10. Akhavan AA, Mirhendi H, Khamesipour A, Alimohammadian MH, Rassi Y, Bates P, Kamhawi S, Valenzuela JG, Arandian MH, Abdoli H, Jalali-zand N, Jafari R, Shareghi N, Ghanei M, Yaghoobi-Ershadi MR. Leishmania species: Detection and identification by nested PCR assay from skin samples of rodent reservoirs. Exp Parasitol. 2010; 126 (4): 552– 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Killick-Kendrick R. Phlebotomine vectors of the leishmaniases: a review. Med Vet Entomol. 1990; 4 (1): 1– 24. [DOI] [PubMed] [Google Scholar]
- 12. Dejeux P. Information on the epidemiology and control of the leishmaniasis, by country or territory. Geneva: WHO; 1991. [Google Scholar]
- 13. Shirzadi M. Guideline for control of cutaneous leishmaniasis. Department of Zoonosis, CDC, Ministry of Health and Medical Education, Tehran, Iran; 2010. [Google Scholar]
- 14. Shirzadi MR, Mollalo A, Yaghoobi-Ershadi MR. Dynamic Relations between Incidence of Zoonotic Cutaneous Leishmaniasis and Climatic Factors in Golestan Province, Iran. J Arthropod Borne Dis. 2015; 9 (2): 148– 60. [PMC free article] [PubMed] [Google Scholar]
- 15. Nadim A, Amini H. The effect of antimalaria spraying on the transmission of zoonotic cutaneous leishmaniasis. Trop Geogr Med 1970; 22 (4): 479– 81. [PubMed] [Google Scholar]
- 16. Noazin S, Shirzadi MR, Kermanizadeh A, Yaghoobi-Ershadi M-R, Sharifi I. Effect of large-scale installation of deltamethrinimpregnated screens and curtains in Bam, a major focus of anthroponotic cutaneous leishmaniasis in Iran. Trans R Soc Trop Med Hyg. 2013; 107 (7): 444– 50. [DOI] [PubMed] [Google Scholar]
- 17. Nadim A, Javadian E, Tahvildar-Bidruni G, Ghorbani M. Effectiveness of leishmanization in the control of cutaneous leishmaniasis. Bull Soc Pathol Exot Filiales. 1983; 76 (4): 377– 383. [PubMed] [Google Scholar]
- 18. Gradoni L. Canine Leishmania vaccines: still a long way to go. Vet Parasitol. 2015; 208 (1): 94– 100. [DOI] [PubMed] [Google Scholar]
- 19. Alvar J, Croft SL, Kaye P, Khamesipour A, Sundar S, Reed SG. Case study for a vaccine against leishmaniasis. Vaccine. 2013; 31: B244– B9. [DOI] [PubMed] [Google Scholar]
- 20. Eliseev L, Principles and methods of control of zoonotic cutaneous leishmaniasis. USSR Ministry of Health and WHO Seminar on Control of Leishmaniases, Moscow; 1980. [Google Scholar]
- 21. Dergacheva I, Zherikhina I. Changes in the population density and species composition of Phlebotomidae as a result of eradication of great gerbils in a focus of zoonotic cutaneous leishmaniasis in the Karshi Steppe. Med Parazitol (Mosk). 1980; 49: 50– 5. [PubMed] [Google Scholar]
- 22. Veysi A, Vatandoost H, Arandian MH, Jafari R, Yaghoobi-Ershadi MR, Rassi Y, Akhavan AA. Laboratory Evaluation of a Rodenticide-insecticide, Coumavec®, against Rhombomys opimus, the Main Reservoir Host of Zoonotic Cutaneouse Leishmaniasis in Iran. J Arthropod Borne Dis. 2013; 7 (2): 188– 93. [PMC free article] [PubMed] [Google Scholar]
- 23. Smart J JK, Whittick RJ. Insects of medical importance. 4th ed British Museum, Natural History: Adien press; 1965. [Google Scholar]
- 24. Theodor O, Mesghali A. On the phlebotominae of Iran. J Med Entomol. 1964; 1 (3): 285– 300. [DOI] [PubMed] [Google Scholar]
- 25. Seyedi-Rashti M, Nadim A. The genus Phlebotomus (Diptera: Psychodidae: Phlebotominae) of the countries of the eastern mediterranean region. Iran J Public Health. 1992; 21 (1–4): 11– 50. [Google Scholar]