Abstract
Background:
The current study was designed to evaluate immune responses induced by DNA vaccines encoding 8-kDa subunit of antigen B (HydI) of Echinococcus granulosus and murine interleukin 12 (IL-12) as genetic adjuvants in BALB/c mice.
Methods:
Expression plasmid pcDNA3.1 containing HydI (pcHyd1) as vaccine along with the murine interleukin 12 (pcMIL12) as adjuvant were used. Thirty-five mice in the five experimental groups received PBS, empty pcDNA3.1, pcHydІ, pcMIL-12, and pcHydІ+ pcMIL-12 in days zero, 14th and 28th. Two weeks after the last immunization, evaluation of the immune response was performed by evaluating the proliferation of splenic lymphocytes, IFN-γ and IL-4, determination of IgG isotyping titer.
Results:
Mice that received the pcHydI+pcMIL12 exhibited higher levels of lymphocyte proliferation compared to mice that received the pcHydI alone (P<0.001), and produced significantly more IFN-γ in comparison to other groups (P< 0.001). In addition, they produced significantly less IL-4 than mice receiving the PBS and the empty plasmid (P<0.023). The IgG2a levels were clearly higher in pcHydI+pcMIL12 group in comparison with the groups of pcHydI alone, empty plasmid, and PBS. In contrast, IgG1 was elevated in the group of pcHydI.
Conclusion:
Co-delivery of IL-12 with DNA encoding 8-kDa subunit of antigen B was effective significantly in inducing the immune response in mice.
Keywords: Hydatid disease, Th1/Th2, Cytokines, Immunization, Genetic adjuvant, Antigen B
Introduction
Cystic echinococcosis (CE), also known as hydatid disease, is a zoonotic and helminthic disease with worldwide distribution caused by the larval stage of Echinococcus granulosus (1). It is found in seven continents, with the highest prevalence in parts of Eurasia (especially Mediterranean and Middle Eastern countries and China), north and east Africa, Australia, and South America, where human incidence rates are as high as 50 per 100,000 person-years (2–4). This disease has a significant impact on both human and animal health and causes important public health-economic problem with annual estimated cost around three billion US dollars in endemic areas (5, 6).
Echinococcosis is preventable (7). Even although hydatid control programs have been successful in some countries but the worldwide distribution and the important socioeconomic problem of hydatid cyst has not seriously changed (8). Unfortunately, in many regions hydatid control programs is not applicable and available for some reason such as the budget deficit, foreign and domestic wars, mismanagement, the issues of security and political-social-environmental changes (9, 10).
Vaccination is the most effective and efficient manner for diseases prevention caused by infectious agents (11). Mathematical models show approaches to control and prevention of hydatidosis, including an effective vaccine for intermediate host and more effective anti-parasitic treatments in definitive host (11, 12). Genetic/DNA vaccines are the new generation of vaccines that have high potential to stimulate both cellular and humoral immune response as well as to polarize T-cell helper toward type 1 or type 2 by direct injection plasmid expressing the DNA sequence of antigen(s) into appropriate tissues of animal (13).
Antigen B (AgB), major antigen in hydatid cyst fluid, is a thermo-stable lipoprotein with a molecular mass of 160 kDa that separate in SDS-PAGE into three bands of 8–12, 16, and 24-kDa; in fact AgB is a combination of monomers 8-kDa. AgB is encoded by at least five main gene clusters, EgAgB1-5 (14, 15).
The current study was designed to evaluate of induced immune responses by DNA vaccines encoding 8-kDa subunit of antigen B (HydI) of E. granulosus and mouse interleukin-12 (IL-12) as genetic adjuvants in BALB/c mice.
Materials and Methods
Mice
Thirty-five female BALB/c mice of 6–8 week age were purchased from Razi Institute (Karaj, Iran). Mice were maintained under specific-pathogen-free, feeding and rearing standard conditions. All experiments were performed according to the Animal Care and Use Protocol of Tehran University of Medical Sciences (Tehran, Iran) and the Ethical Committee of the university approved the study.
Plasmid construction
The eukaryotic expression plasmid pcDNA/Hyd1(pcHydI) has been previously constructed and expressed in eukaryotic cells successfully in our laboratory (16).
Mouse IL-12 was synthesized and sub-cloned into the dual promoter IL-12p70 expression vector pcDNA3.1 (pcMIL-12) (Cinnagen, Tehran, Iran). The mouse IL-12 p35 subunit (accession number NM_001303244.1) is expressed under control of the human CMV promoter and bovine growth hormone (BGH) polyadenylation signal, while the mouse IL-12 p40 subunit (accession number NM_001159424.2) is expressed under control of the simian CMV promoter and simian virus 40 (SV40) polyadenylation signal.
The NIH 3T3 cells, mouse embryonic fibroblast cell line, transfected with plasmid pcMIL-12 were tested for the presence of IL-12 protein by antibody capture ELISA. The plasmid DNA was prepared using the Qiagen Plasmid DNA Purification Kit (Qiagen, USA) according to the manufacturer’s protocol.
DNA immunization
Five groups of mice (seven per group) were bilaterally inoculated intramuscularly in the thigh skeletal muscle with 100 μg of plasmid DNA suspended in 100 μl sterile PBS, whereas control mice received PBS alone. Group I was injected with PBS as control, group II with empty pcDNA3.1 vector also as control, group III with pcHydI, group IV with pcMIL12, group V with pcHydI plus pcMIL12 (50 μg each). Every mouse was injected with same protocol on days zero, 14th and 28th. At two weeks post the last vaccination, blood samples were collected from the heart for serological tests and the spleens of the mice were removed under sterile conditions for lymphocyte proliferation assay and cytokine measurements.
Lymphocyte proliferation assays
The lymphocyte proliferation rate was measured using an MTT (3[4,5-dimethylthiazol-2-ml]-2,5-diphenyltetrazolium bromide; thiazolyl-blue, Sigma, Germany) dye assay, two weeks after the last immunization. Briefly, splenocyte suspensions were prepared from each group of mice by squishing the spleens through a wire mesh under sterile conditions. After the red blood cells was removed using ammonium chloride. Splenocytes were resuspended in RPMI 1640 medium supplemented with 10% FCS. One hundred ml of diluted cell suspensions were then plated in 96-well flat-bottom culture plates at a density of 1×1010 cells per ml in each well and cultured with concanavalin A (Con A; 5 μg/ml; Sigma; positive control), or medium alone (negative control) or 10 μl of the Ag B at 37 °C with 5% CO2 (17).
After 72 h, cell proliferation was determined using an MTT assay (17). Briefly, each well was added with 20 μl of MTT and the plates were further incubated at 37 °C for 4 h. then, the supernatant was harvested from each well and formazan crystals were solubilized by adding 100 ml of dimethyl sulfoxide (DMSO). After 20 min, the absorbance of each well was measured at a wavelength of 540 nm. Stimulation indices were determined and expressed as differences between the absorbance of treated and untreated wells.
Cytokine assays
To evaluate cytokines levels, splenocytes from immunized mice were cultured and restimulated in vitro with 10 μl of the Ag B suspension as described for the lymphoproliferation assay. Cell-free supernatants were removed and assayed for interleukin-4 (IL-4), and for gamma-interferon (IFN-γ) activity at 72 h. IFN-γ and IL-4, were measured using ELISA kits according to the manufacturer’s instructions (eBscience, USA). Cytokine concentrations were determined by reference to standard curves constructed with known amounts of mouse recombinant IFN-γ and IL-4. All assays were performed in triplicate.
Antibody assays
The humoral IgG1 and IgG2a subclass in serum samples, which were taken in two weeks after the final immunization, were measured by ELISA using 96-well micro-titer plates (18). In brief, following coating micro-titer plates at 4 °C overnight with 100 μl Ag B (with the concentration of 10 μg/ml) in 0.05 M bicarbonate buffer, pH 9.6, the plates were washed with PBS containing 0.05% Tween 20 (PBST-20), pH 7.4, 2 times and non-specific binding sites were blocked with PBS containing 5% FCS for 2 h at 37 °C. After washing the plates with PBST, sera were diluted in 1% PBST-20 (1:100) and added to each well. Plates were incubated for 2 h at room temperature, washed three times and incubated with HRP-conjugated rabbit anti-mouse IgG2a, IgG1. After sufficient washes, the reaction was developed by adding 200 ml of TMB/H2O2 substrate. The reaction was terminated by the addition of 50 ml of 2.0N H2SO4 and the optical density (OD) was read at 450 nm in a microplate reader (Thermo Scientific Multiskan FC, USA). All assays were performed in triplicate.
Statistical analysis
The lymphocyte proliferation assay and cytokine and antibody levels were analyzed by one-way analysis of variance (ANOVA) technique followed by Tukey’s post-test. Significance was set at P<0.05. Using SPSS 16 (SPSS Inc., Chicago, IL, USA).
Results
Lymphocyte proliferation
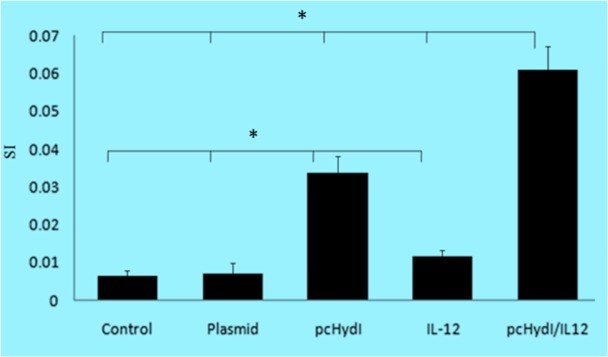
Lymphocyte proliferation is generally considered a measure of cell-mediated immunity; therefore, Ag B-specific lymphocytic proliferation was evaluated using an MTT assay in two weeks after the last immunization. As shown in Fig. 1, the two groups immunized with pcHydI plus pcMIL12, and pcHydI alone, increased lymphocyte response, compared with the negative control (which received only PBS) (P<0.001). Mice that received the pcHydI plus pcMIL12 exhibited higher levels of lymphocyte proliferation compared to mice that received the pcHydI alone (P< 0.001).
Fig. 1:
Splenocyte proliferation assay, spleens from individual mice (seven per group) was removed and lymphocyte proliferation was evaluated by the MTT method. Values represent the mean ± SE. SI=Stimulation Index. Statistically significant differences (P<0.05) are indicated by (*) compared with control, plasmid, pcHydl, IL-12 or pcHydl/IL-12 group
Cytokine pattern
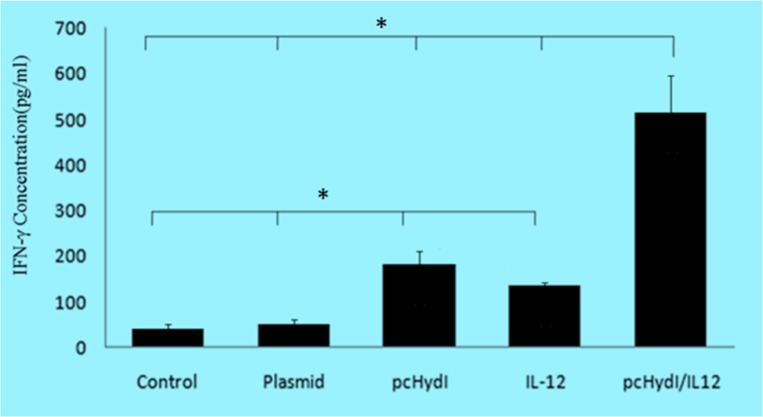
We investigated the effect of pcHydI with and without co-injection of pcMIL12 on changes in Th1 and Th2 phenotypes. As shown in Fig. 2, immunized mice with the pcHydI plus pcMIL12 produced significantly more IFN-γ (Th1-type) in comparison to mice that received either PBS, the pcHydI alone, the pcMIL12 alone or empty plasmid (for all P<0.001).
Fig. 2:
Comparison of production levels of IFN-γ from splenocytes in groups control (PBS and empty plasmid), pcHydI+pcMIL12 and pcHydI and pcMIL12. Values are shown as the mean± SE. Statistically significant differences (P < 0.05) are indicated by (*) compared with control, plasmid, pcHydl, IL-12 or pcHydl/IL-12 group
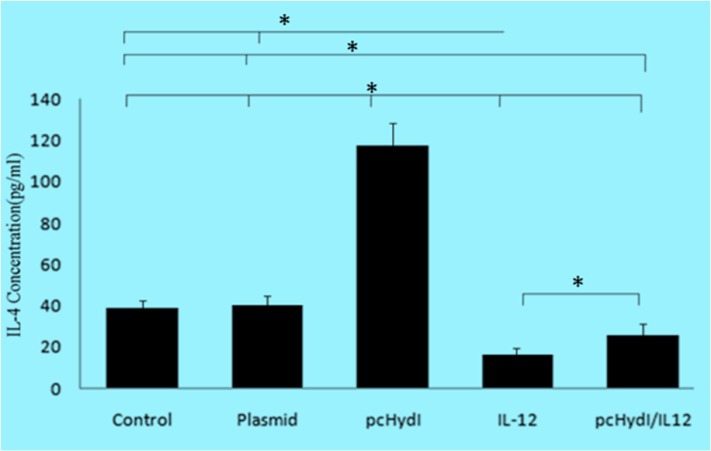
Fig. 3:
Comparison of production levels of IL-4 from splenocytes in groups control (PBS and empty plasmid), pcHydI+pcMIL12 and pcHydI and pcMIL12. Values are shown as the mean± SE. Statistically significant differences (P < 0.05) are indicated by (*) compared with control, plasmid, pcHydl, IL-12 or pcHydl/IL-12 group
In contrast, lymphocytes from vaccinated mice with the pcHydI alone produced larger amounts of IL-4 (Th2-type) than mice that received PBS, the pcHydI plus pcMIL12, the pcMIL12 alone or empty plasmid (for all P<0.001).
Immunized mice with the pcHydI+ pcMIL12 produced significantly loss IL-4 than mice receiving the PBS and the empty plasmid (for both P<0.023). The results show that the IL-4 cytokine levels were decreased in the presence of IL-12.
Antibody titer
Considering that IFN-γ and IL-4 are important to conduct immunoglobulin class switching for IgG2a and IgG1, respectively, the production of antigen-specific immuneglobulin isotypes were evaluated in the mice at two weeks after final immunization.
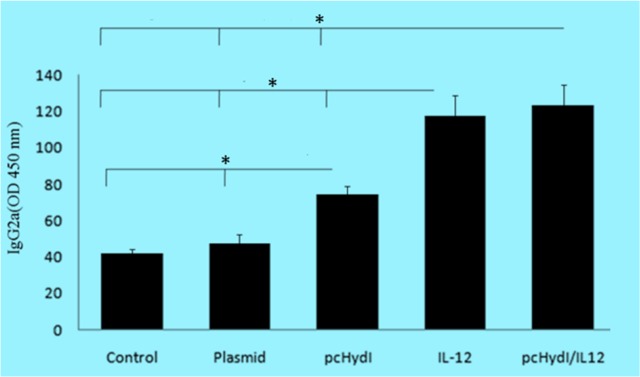
The IgG2a levels, as shown in Fig. 4, were clearly higher in the sera of mice treated with the pcHydI plus pcMIL12 in comparison with mice that received the pcHydI alone, empty plasmid and PBS (P<0.002, P<0.001, P<0.001, respectively). The vaccinated mice with the pcHydI plus pcMIL12 had more IgG2a compared to the mice that received the pcMIL12; however, the differences were not statistically significant.
Fig. 4:
The levels of IgG2a antibody in the sera obtained two weeks after the last immunization were determined. Values represent the mean ± SE. Sera were used at 1/100 dilutions. O.D., optical density at 450 nm. Statistically significant differences (P < 0.05) are indicated by (*) compared with control, plasmid, pcHydl, IL-12 or pcHydl/IL-12 group
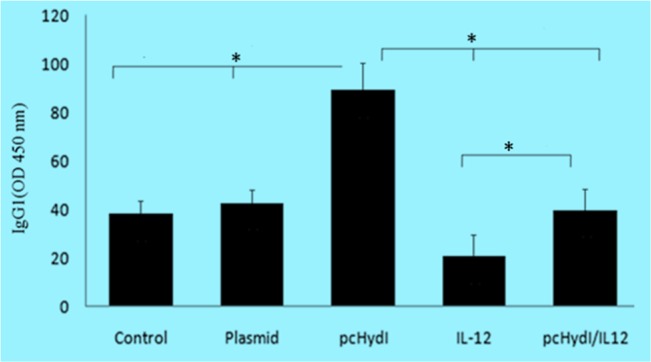
In contrast, antigen-specific IgG1 was elevated in the group of mice that received the pcHydI alone and in comparison with groups receiving pcHydI plus pcMIL12, pcMIL12 alone, empty plasmid and PBS(control) had significant difference (for all groups P<0.001) (Fig. 5).
Fig. 5:
The levels of IgG1 antibody in the sera obtained two weeks after the last immunization were determined. Values represent the mean ± SE. Sera were used at 1/100 dilutions. O.D., optical density at 450 nm. Statistically significant differences (P < 0.05) are indicated by (*) compared with control, plasmid, pcHydl, IL-12 or pcHydl/IL-12 group
Discussion
Vaccination of intermediate host is one of the main ways to control of hydatid cyst disease (19). In order to access an appropriate vaccine against hydatid cyst, many studies were performed on various-parasite antigens alone or in combination with different adjuvant such as genetic adjuvant (20–22).
DNA vaccines have several interesting features for researchers, such as long-lasting immunity, priming both cellular and humoral specific immune responses, and production of the native form of a given antigen. In addition, the production costs are low and they are easy and quick to product (23). After the first report of successful DNA immunization (24), great efforts have been done in developing DNA vaccines for parasitic infections.
In hydatid cyst disease, immunity induced is complex and containing both antibody and cellular components. However, protective outcome is corresponding to Th1 responses, while Th2 responses not only non-protective but also are associated with progress of the disease (25). Therefore, the potency of the antigen to shift the immune response to specifically the T helper (Th) subtype is important in vaccine design against hydatid cyst agent. Here, for the first time the potential of Ag B (pcHyd1) alone and in compound with murine interleukin (pcMIL-12) to induce protective immune response was investigated in mouse models.
Overall, DNA vaccines produce a Th1 rather than Th2 type immune responses (13), but our results showed that the Ag B (pcHyd1) alone is adequately immunogenic and induce a mixture of humoral and cellular immunity but induce Th2 response more than Th1 response. This might be due to the nature of antigen B that has both B cell and T-cell epitopes. In mice that had been vaccinated with pcHyd1, levels of IL-4 and IgG1 signficantly increased in contrast to IFN-γ and IgG2a respectively. Antibodies seem have little or no importance protection for host against infection by oncosphere of E. granulosus (8). In the current study, we approved that antigen B is immunogenic but has potential to direct the immune system towards Th2 type.
Due to lack of available research on the DNA vaccine of antigen B, our findings were compared to other studies that had been carried out on parasitic antigens with same conditions including; the amount of vaccine, laboratory animals, site of injection, repeated injection number and interval time. Comparisons showed that depending on the type epitope(s) into antigen, different results (cellular immunity, humoral or a combination of both) might be obtained (26–29).
Although DNA vaccines have many advantages, their ability in inducing immune responses in the absence of suitable adjuvants is poor (23). Enhancing and shifting immune response of vaccinated animal into Th1 or Th2 regulated profiles by the co-administration of genetic adjuvants is one of the advantages of DNA vaccines (23). In this regard, numerous studies have been performed on the effectiveness of co-administration of Th1 cytokine-encoding plasmids, such as IL-2, IL-15, IL-18, IL-23, and IFN-γ (30–32). IL-12 is produced by activated antigen-presenting cells such as dendritic cells and macrophages. It facilitates the development of Th1 responses and is a powerful inducer of IFN-γ production by T and NK cells (33). Hence, in this study IL-12 was used as genetic adjuvant to enhance immune response to Ag B.
Our observations indicated mice vaccinated with composed pcHydI plus pcMIL12 produce higher IFN-γ. The amount of IFN-γ in pcHydI-vaccinated mice certified that simultaneous injection of IL-12 with the pcHydI increased and expanded the Th1 cytokines responses but not Th2 cytokines like IL-4.
IFN-γ promotes isotype switching towards IgG2 in mouse. Our results show that vaccinated mice with pcMIL12 with pcHydI significantly increased IgG2a production than immunized mice with pcHydI alone. This results show a shift toward a Th1 immune response.
This finding is in accordance with other studies where co-administration of IL-12 and antigen-encoding plasmid augmented the ability of vaccine to induce the production of IFN-γ and IgG2a against Toxoplasma gondii (34, 35).
These results clearly indicate that pcMIL12 is a powerful adjuvant that along with vaccine develops a strong Th1 polarization in the immune response in mice. Other studies used murine IL12 as genetic adjuvant reported similar findings (36–39).
Conclusion
Co-delivery of IL-12 with DNA encoding the 8-kDa subunit of antigen B hydatid cyst was effective in inducing Th1 type immune response. The higher titers of anti-antigen B IgG2a class antibodies plus the Th1 polarization response may be effective for protecting mice. Therefore, is complementary studies are suggested towards the use of Ag B-DNA vaccines combined with IL12 plasmid to protection against oncosphere of Echinococcus granulosus.
Acknowledgements
The study was supported financially by a grant from Tehran University of Medical Sciences No. 21780. The authors declare that there is no conflict of interests.
References
- 1. Pakala T, Molina M, Wu GY. Hepatic Echinococcal Cysts: A Review. J Clin Transl Hepatol. 2016; 4 (1): 39– 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Qingling M, Guanglei W, Jun Q, Xinquan Z, Tianli L, Xuemei S, et al. Prevalence of hydatid cysts in livestock animals in Xinjiang, China. Korean J Parasitol. 2014; 52 (3): 331– 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dakkak A. Echinococcosis/hydatidosis: a severe threat in Mediterranean countries. Vet Parasitol. 2010; 174 (1): 2– 11. [DOI] [PubMed] [Google Scholar]
- 4. Mandal S, Mandal MD. Human cystic echinococcosis: epidemiologic, zoonotic, clinical, diagnostic and therapeutic aspects. Asian Pac J Trop Med. 2012; 5 (4): 253– 60. [DOI] [PubMed] [Google Scholar]
- 5. Torgerson P. Economic effects of echinococcosis. Acta Tropica. 2003; 85 (2): 113– 18. [DOI] [PubMed] [Google Scholar]
- 6. Torgerson P, Budke C. Echinococcosis–an international public health challenge. Res Vet Sci. 2003; 74 (3): 191– 202. [DOI] [PubMed] [Google Scholar]
- 7. Heath D, Yang W, Li T, Xiao Y, Chen X, Huang Y, et al. Control of hydatidosis. Parasitol Int. 2006; 55: S247– S252. [DOI] [PubMed] [Google Scholar]
- 8. Lightowlers MW, Gauci CG. Vaccines against cysticercosis and hydatidosis. Vet Parasitol 2001; 101 (3): 337– 52. [DOI] [PubMed] [Google Scholar]
- 9. Lightowlers MW. Vaccines against cysticercosis and hydatidosis: foundations in taeniid cestode immunology. Parasitol Int. 2006; 55: S39– S43. [DOI] [PubMed] [Google Scholar]
- 10. Craig PS, McManus DP, Lightowlers MW, Chabalgoity JA, Garcia HH, Gavidia CM, et al. Prevention and control of cystic echinococcosis. Lancet Infect Dis. 2007; 7 (6): 385– 94. [DOI] [PubMed] [Google Scholar]
- 11. Torgerson PR. Mathematical models for the control of cystic echinococcosis. Parasitol Int. 2006; 55: S253– S258. [DOI] [PubMed] [Google Scholar]
- 12. Torgerson P. The use of mathematical models to simulate control options for echinococcosis. Acta Tropica. 2003; 85 (2): 211– 21. [DOI] [PubMed] [Google Scholar]
- 13. Khan KH. DNA vaccines: roles against diseases. Germs. 2013; 3 (1): 26– 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chow C, Gauci CG, Cowman AF, Lightowlers MW. A gene family expressing a host-protective antigen of Echinococcus granulosus. Mol Biochem Parasitol. 2001; 118 (1): 83– 88. [DOI] [PubMed] [Google Scholar]
- 15. Mamuti W, Sako Y, Bart J-M, Nakao M, Ma X, Wen H, et al. Molecular characterization of a novel gene encoding an 8-kDa-subunit of antigen B from Echinococcus granulosus genotypes 1 and 6. Parasitol Int. 2007; 56 (4): 313– 316. [DOI] [PubMed] [Google Scholar]
- 16. Azizi H, Kazemi B, Bandehpour M, Mohebali M, Khamesipour A, Aryaeipour M, et al. Molecular Cloning and Expression an 8-kDa Subunit of Antigen B from G1 strain of Echinococcus granulosus. Iran J Public Health. 2015; 44 (7): 962– 8. [PMC free article] [PubMed] [Google Scholar]
- 17. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65 (1–2): 55– 63. [DOI] [PubMed] [Google Scholar]
- 18. Bansal A, Paliwal PK, Sagi SS, Sairam M. Effect of adjuvants on immune response and protective immunity elicited by recombinant Hsp60 (GroEL) of Salmonella typhi against S. typhi infection. Mol Cell Biochem. 2010; 337 (1–2): 213– 21. [DOI] [PubMed] [Google Scholar]
- 19. Woollard DJ, Gauci CG, Heath DD, Lightowlers MW. Protection against hydatid disease induced with the EG95 vaccine is associated with conformational epitopes. Vaccine. 2000; 19 (4): 498– 507. [DOI] [PubMed] [Google Scholar]
- 20. LI ZJ, WANG YN, Qi W, Wei Z. Echinococcus granulosus 14-3-3 protein: a potential vaccine candidate against challenge with Echinococcus granulosus in mice. Biomed Environ Sci. 2012; 25 (3): 352– 58. [DOI] [PubMed] [Google Scholar]
- 21. Chow C, Gauci CG, Vural G, Jenkins DJ, Heath DD, Rosenzvit MC, et al. Echinococcus granulosus: variability of the host-protective EG95 vaccine antigen in G6 and G7 genotypic variants. Exp Parasitol. 2008; 119 (4): 499– 505. [DOI] [PubMed] [Google Scholar]
- 22. Pirestani M, Dalimi A, Sarvi S, Khoramabadi N. Evaluation of Immunogenicity of Novel Isoform of EG95 (EG95-5G1) From Echinococcus granulosus in BALB/C Mice. Iran J Parasitol. 2014; 9 (4): 491– 502. [PMC free article] [PubMed] [Google Scholar]
- 23. Wedrychowicz H. Antiparasitic DNA vaccines in 21st century. Acta Parasitol. 2015; 60 (2): 179– 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ulmer JB, Donnelly JJ, Parker SE, Rhodes GH, Felgner PL, Dwarki V, et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993; 259 (5102): 1745– 49. [DOI] [PubMed] [Google Scholar]
- 25. Zhang W, Wen H, Li J, Lin R, McManus DP. Immunology and immunodiagnosis of cystic echinococcosis: an update. Clin Dev Immunol. 2012; 2012: 101895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Smooker PM, Kennedy NJ, Steeper KR, Christopoulos H, Spithill TW. Fasciola: kinetics and quality of humoral responses to fatty acid binding protein and cathepsin l following delivery as DNA vaccines in mice. Exp Parasitol. 2001; 97 (3): 154– 60. 11312577 [Google Scholar]
- 27. Lança ASC, de Sousa KP, Atouguia J, Prazeres DMF, Monteiro GA, Silva MS. Trypanosoma brucei: immunisation with plasmid DNA encoding invariant surface glycoprotein gene is able to induce partial protection in experimental African trypanosomiasis. Exp Parasitol. 2011; 127 (1): 18– 24. [DOI] [PubMed] [Google Scholar]
- 28. Yan H-K, Yuan Z-G, Petersen E, Zhang X-X, Zhou D-H, Liu Q, et al. Toxoplasma gondii: protective immunity against experimental toxoplasmosis induced by a DNA vaccine encoding the perforin-like protein 1. Exp Parasitol. 2011; 128 (1): 38– 43. [DOI] [PubMed] [Google Scholar]
- 29. Xue M, He S, Cui Y, Yao Y, Wang H. Evaluation of the immune response elicited by multi-antigenic DNA vaccine expressing SAG1, ROP2 and GRA2 against Toxoplasma gondii. Parasitol Int. 2008; 57 (4): 424– 29. [DOI] [PubMed] [Google Scholar]
- 30. Wozniak TM, Ryan AA, Triccas JA, Britton WJ. Plasmid interleukin-23 (IL-23), but not plasmid IL-27, enhances the protective efficacy of a DNA vaccine against Mycobacterium tuberculosis infection. Infect Immun. 2006; 74 (1): 557– 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Calarota SA, Weiner DB. Enhancement of human immunodeficiency virus type 1-DNA vaccine potency through incorporation of T helper 1 molecular adjuvants. Immunol Rev. 2004; 199 (1): 84– 99. [DOI] [PubMed] [Google Scholar]
- 32. Liu Q, Shang L, Jin H, Wei F, Zhu X-Q, Gao H. The protective effect of a Toxoplasma gondii SAG1 plasmid DNA vaccine in mice is enhanced with IL-18. Res Vet Sci. 2010; 89 (1): 93– 97. [DOI] [PubMed] [Google Scholar]
- 33. Wolf SF, Sieburth D, Sypek J. Interleukin 12: a key modulator of immune function. Stem Cells. 1994; 12 (2): 154– 68. [DOI] [PubMed] [Google Scholar]
- 34. Letscher-Bru V, Villard O, Risse B, Zauke M, Klein J-P, Kien TT. Protective effect of vaccination with a combination of recombinant surface antigen 1 and interleukin-12 against toxoplasmosis in mice. Infect Immun. 1998; 66 (9): 4503– 06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xue M, He S, Zhang J, Cui Y, Yao Y, Wang H. Comparison of cholera toxin A2/B and murine interleukin-12 as adjuvants of Toxoplasma multi-antigenic SAG1-ROP2 DNA vaccine. Exp Parasitol. 2008; 119 (3): 352– 57. [DOI] [PubMed] [Google Scholar]
- 36. Cui Yl, He Sy, Xue Mf, Zhang J, Wang Hx, Yao Y. Protective effect of a multiantigenic DNA vaccine against Toxoplasma gondii with co-delivery of IL-12 in mice. Parasite Immunol. 2008; 30 (5): 309– 13. [DOI] [PubMed] [Google Scholar]
- 37. Chen G, Chen H, Guo H, Zheng H. Protective effect of DNA-mediated immunization with a combination of SAG1 and IL-2 gene adjuvant against infection of Toxoplasma gondii in mice. Chin Med J (Engl). 2002; 115 (10): 1448– 52. [PubMed] [Google Scholar]
- 38. Schadeck EB, Sidhu M, Egan MA, Chong S-Y, Piacente P, Masood A, et al. A dose sparing effect by plasmid encoded IL-12 adjuvant on a SIVgagplasmid DNA vaccine in rhesus macaques. Vaccine. 2006; 24 (21): 4677– 87. [DOI] [PubMed] [Google Scholar]
- 39. Chow Y-H, Chiang B-L, Lee Y-L, Chi W-K, Lin W-C, Chen Y-T, et al. Development of Th1 and Th2 populations and the nature of immune responses to hepatitis B virus DNA vaccines can be modulated by codelivery of various cytokine genes. J Immunol. 1998; 160 (3): 1320– 29. [PubMed] [Google Scholar]