Abstract
Background:
Cross antigenicity is the major problem in developing a reliable tool for immunodiagnosis and immunoprophylaxis of parasitic diseases. Mixed infection due to different types of gastrointestinal parasites is more common than single species infection under field condition.
Methods:
The present study was undertaken to detect antigenic cross-reactivity among Haemonchus contortus, Oesophagostomum columbianum and Trichuris ovis of goats by SDS-PAGE and western blot analysis using hyperimmune sera (HIS) rose in rabbit separately against the antigens of the three nematode species.
Results:
Thirteen, 16 and 14 polypeptides in crude somatic antigen (CSAg) of H. contortus (CSAg-Hc), O. columbianum (CSAg-Oc) and T. ovis (CSAg-To), respectively, were resolved in SDS PAGE analyses. It was revealed that 54 kDa peptide was shared by H.contortus and O. columbianum, whereas 47 kDa peptide was shared by O. columbianum and T. ovis. Western blot analyses revealed that three immunogenic polypeptides (MW 54, 49 and 42 kDa) in CSAg-Hc, five in CSAg-Oc (54, 47, 44, 38 and 35.5 kDa) and CSAg-To and five polypeptides (90, 51, 47, 39.5 and 31 kDa) in CSAg-To cross-reacted with the heterologous HIS. Four species-specific immunoreactive polypeptides (92, 85, 65 and 39 kDa) of H. contortus and two (72 & 26 kDa) in O. columbianum were also identified in the study.
Conclusion:
The shared polypeptides and species-specific polypeptides might be evaluated as protective antigen and subsequently exploitation for developing immunodiagnostic and for immunoprophylactic tools of for these common nematode species.
Keywords: Haemonchus contortus, Oesophagostomum columbianum, Trichuris ovis, Antigenic profiles, Cross-reactivity
Introduction
Small ruminant livestock are the frequent victim of mixed nematode infection throughout the world including India. Haemonchus contortus, Oesophagostomum columbianum and Trichuris ovis are the most common composition of the mixed infection in West Bengal, India (1, 2). Conventional methods can diagnose the infection only when it is patent and has already exerted part of its impact on the host. However ensuring sustainable productivity of livestock demands an early detection of the infection and adoption of timely therapeutic measures. Antigenic cross reactivity among the helminth species and lack of desirable specificity have been the major constraints for development of a reliable immunological tool as an alternative for diagnosis of helminth infections including the nematode.
There is evidence of cross-immunity between some gastrointestinal helminths of sheep (3, 4) and cross-antigenicity has been widely reported (5, 6). Since most ruminants are infected with multiple species of gastrointestinal nematodes with several parasitic stages, the antigens shared among the stages and species of the nematodes would make appropriate targets for multivalent vaccines. The presence of shared epitopes (7) and conservation of intestinal surface epitopes among gastrointestinal nematodes has also been demonstrated (8, 9). Employing the shared protein, the unique protein or the cocktail antigen comprising the unique proteins could offer a promising approach for developing a reliable diagnostic tool for single and multiple nematode species infection. It is therefore imperative to determine the antigen profile of the nematode species and their antigenic cross reactivity.
Earlier studies on the diagnosis of O. columbianum infection in goat by antibody detection ELISA revealed cross-reactivity of crude somatic antigen of O. columbianum with serum of goat infected with H. contortus and T. ovis (10).
The present study was carried out with a view to identify common antigenic polypeptide shared by the three nematode species and to identify species-specific antigen of the three nematodes.
Materials and Methods
Experimental Animals
Adult New Zealand white strain rabbits (n=8) were used for raising hyperimmune serum (HIS) against crude somatic antigens (CSAg) of H. contortus, O. columbianum and T. ovis were maintained under conventional conditions in the University Animal House. The entire experimental design was approved by the Institutional Animal Ethical Committee, West Bengal University of Animal and Fishery Sciences, India.
Collection of H. contortus, O. columbianum and T. ovis and preparation of their crude somatic antigens
For collection of H. contortus, the aboma-sums and for O. columbianum and T. ovis, the caecum and colon of slaughtered goats were collected from local abattoir. The worms from the excised organs were separately collected with the help of a forceps in 0.15M PBS (pH 7.2). The worms were then washed for 4–5 times in 0.15M PBS (pH 7.2). Finally, 300 worms of each species were homogenised separately in 10ml of chilled PBS containing phenyl methyl sufonyl fluoride (PMSF) @ 25 mM and ethylene diamine tetra acetic acid (EDTA) @ 24 mM in a glass tissue homogenizer. The homogenized materials were then sonicated in an Ultra Sonicator (NISSEI, Model-US50, Japan). The sonicated materials were then centrifuged at 2000g for 45 minutes at 4°C. Then the supernatant was collected (11) as crude somatic antigen (CSAg). Protein concentrations of CSAgs were estimated by the method of Lowry (12) and the protein content of CSAg of H. contortus (CSAg-Hc), O. columbianum (CSAg-Oc) and T. ovis (CSAg-To) were 3.46 mg/ml, 3.82 mg/ml and 3.94 mg/ml, respectively. The antigens were stored at −20°C for further use.
Raising of HIS against the prepared antigens
Hyperimmune sera against the parasitic antigens prepared as above (e.g. CSAg-Hc, CSAg-Oc and CSAg-To) were raised in New Zealand White strain rabbits (13). The antigen (0.5 mg antigenic protein) thoroughly emulsified with equal volume Freundscomplete adjuvant injected intramuscularly and it was followed by four booster doses (1.0, 1.5, 2.0, and 2.5 mg) in equal volume of Freunds incomplete adjuvant at weekly intervals. Blood from the hyperimmunized rabbits was collected 7 days following the last booster and then the separated serum was preserved at −20 °C until further use.
Determination of peptide profiles of the antigens
The crude somatic antigen of H. contortus, O. columbianum and T. ovis were fractionated to determine their peptide profiles by Sodium dodecyl polyacrylamide gel electrophoresis (SDS-PAGE) in 10% gel as per the method of Laemmli (14).
Broad range molecular weight marker (3.0 – 205 kDa, GeNei, Bangalore) was used. After completion of the run, the separating gel containing different antigenic proteins were stained by Coomassie brilliant blue stain. The molecular weight (MW) of the unknown polypeptides of the test protein mixtures was determined by Gel Documentation System, (BioRad, Japan).
Antigenic characterization of CSAg-Hc, CSAg-Oc and CSAg-To
Antigenic characterization of the prepared antigens was done by western blotting technique using HIS raised in rabbits against the antigens under the present study. After separating the antigenic polypeptides by SDS-PAGE the lane, having the marker was stained by Coomassie brilliant blue stain. The dilution of HIS for western blot was 1: 100 and the dilution of the conjugate (rabbit anti-goat horseradish peroxidase conjugate) was 1: 1000 in blocking buffer. The electrophoretic transfer of parasitic antigens resolved by SDS-PAGE to a nitrocellulose membrane and immunoreactions development was carried out as per the standard method (15).
Antigenic cross-reactivity among CSAg-Hc, CSAg-Oc and CSAg-To
Antigenic cross-reactivity among the antigens of the three nematode species was studied by performing western blotting technique. Antigen of one species of nematode was blotted against HIS raised against other two antigens separately.
Results
Polypeptide profiles of the antigens
Thirteen, 16 and 14 polypeptides in CSAg-Hc, CSAg-Oc and CSAg-To, respectively, were resolved in SDS PAGE analyses (Table 1). Five polypeptides (60, 54, 49, 27.5 and 12 kDa) in CSAg-Hc and six polypeptides each in CSAg-Oc (59, 54, 47, 44, 35.5 and 26 kDa) and CSAg-To (101, 90, 70, 51, 47 and 31 kDa) were evident as the major polypeptides in SDS-PAGE analyses (Fig. 1).
Table 1:
Polypeptide profiles of crude somatic antigens of the three nematode species
| Antigen | Molecular weight of polypeptides (kDa) |
|---|---|
| CSAg-Hc | 92, 85, 65, 60, 54,49, 42, 39, 30, 27.5, 12, 6.5, 3.0 |
| CSAg-Oc | 122, 116, 78, 72, 66, 59, 54, 47, 44, 38, 35.5, 28, 26, 24.5, 21, 4.0 |
| CSAg-To | 107, 101, 90, 70, 59, 51, 47, 39.5, 31, 24.5, 21, 12, 8.0, 5.0 |
Fig. 1:
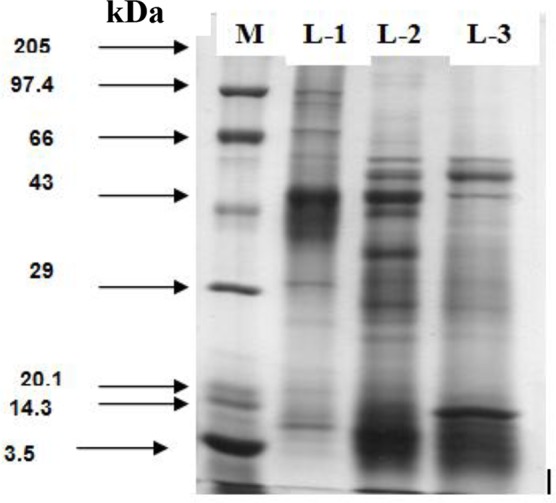
Comparative polypeptide profile CSAg-To (L-1), CSAg-Oc (L-2) and CSAg-Hc (L-3)
Interestingly, H. contortus and O. columbianum, the two most pathogenic nematodes shared a single polypeptide (54 kDa), whereas 47 and 59 kDa polypeptides were shared between O. columbianum and T. ovis.
Antigenic Characterization
Western blot analyses using the homologous antiserum detected ten, eight and six immunoreactive polypeptides in CSAg-Hc, CSAg-Oc and CSAg-To, respectively. Out of these, four polypeptides each in CSAg-Hc (54, 42, 39 and 27.5 kDa) and CSAg-Oc (54, 44, 38 and 35.5 kDa), and three in CSAg-To (47, 39.5 and 31 kDa) were detected as the major immunoreactive polypeptides (Table 2).
Table 2:
Cross-reacting immuno-reactive polypeptides in antigen of the three nematode species
| Antiserum against | CSAg-Hc | CSAg-Oc | CSAg-To |
|---|---|---|---|
| H. contortus | 92, 85, 65, 60, 54, 49, 42, 39, 27.5, 30 | 78, 54, 47, 44, 38, 35.5 | 90, 51, 47, 39.5, 31 |
| O. columbianum | 54, 49, 42, 39, 27.5 | 78, 72, 54, 47, 44, 38, 35.5, 26 | 90, 51, 47, 39.5, 31, 24.5 |
| T. ovis | 60, 54, 49, 42 | 54, 47, 44, 38, 35.5 | 90, 51, 47, 39.5, 31, 24.5 |
The antisera of H. contortus and T. ovis cross-reacted respectively with six and five immunogenic polypeptides of O. columbianum. The dominant polypeptides of O. columbianumgiving cross-reaction with the antiserum against H. contortus and T. ovis had the MW of 78, 54 and 44 kDa (Fig. 3), and 54, 38 and 35.5 kDa (Fig. 4), respectively. Five immunogenic polypeptides (e.g. 54, 47, 44, 38 and 35.5 kDa) of O. columbianum were cross-reacted upon by H. contortus and T. ovis antisera; however 54 kDa peptide was both common and the major one.
Fig. 3:
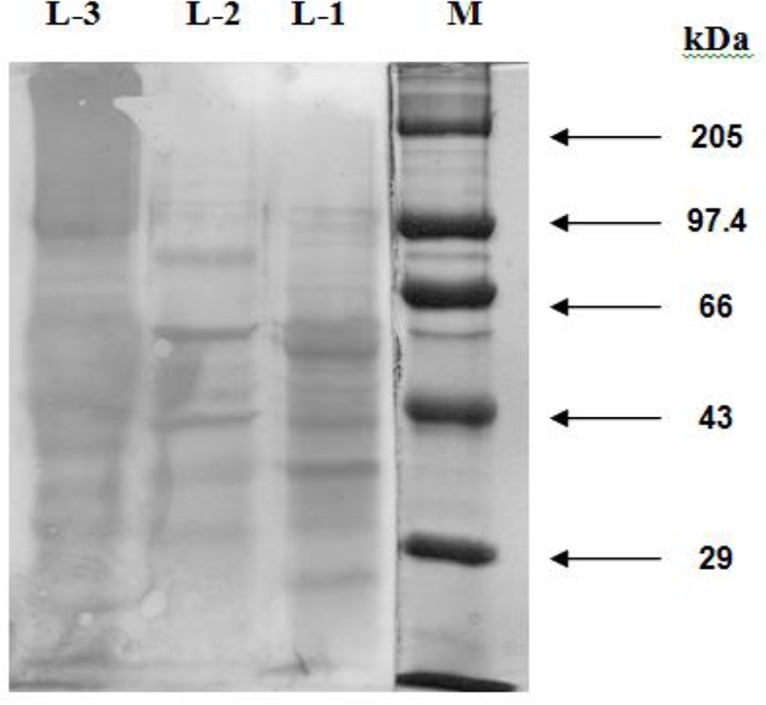
Western Blotting Pattern of CSAg-Hc (L-1), CSAg-Oc (L-2) and CSAg-To (L-3)
Fig. 4:
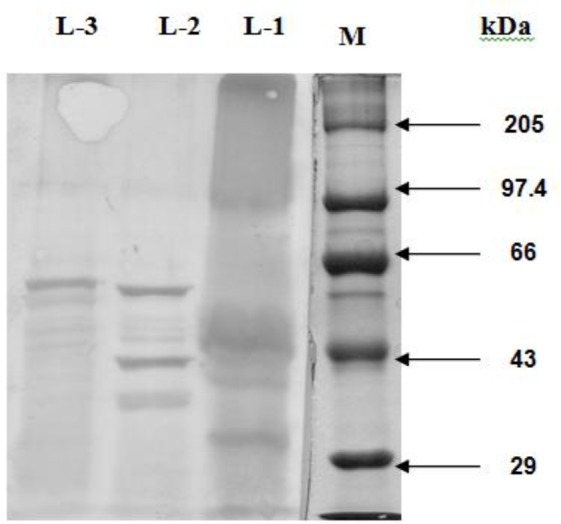
Western Blotting Pattern of CSAg-To (L-1), CSAg-Oc (L-2) and CSAg-Hc (L-3)
Cross-reactivity of the antigens
Five of the ten immunogenic polypeptides (i.e. 54, 49, 42, 30 and 27.5 kDa) in CSAg-Hc gave cross-reaction with the HIS against CSAg-Oc (Fig. 2) and four (60, 54, 49 and 42 kDa) with the HIS against CSAg-To (Fig. 4). Although three polypeptides (54, 49 and 42 kDa) were recognized by both the antisera only the 54 kDa polypeptide was the major one (Table- 2).
Fig. 2:
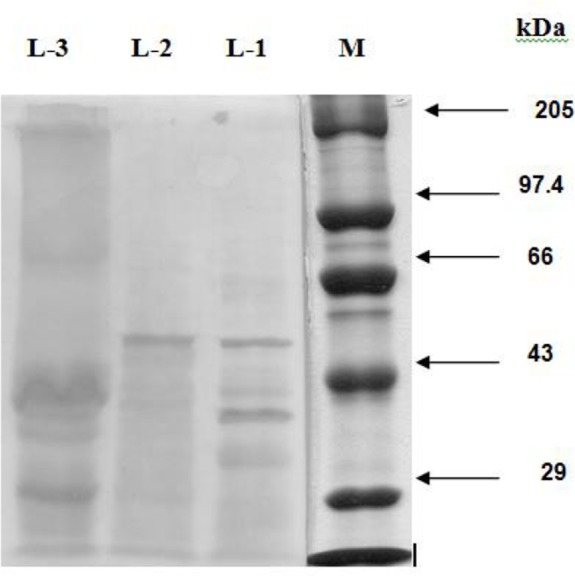
Western Blotting Pattern of CSAg-Oc (L-1), CSAg-Hc (L-2) and CSAg-To (L-3)
Five immuno-reactive polypeptides (90, 51, 47, 39.5 and 31 kDa), out of the six in CSAg-To, ex-hibited cross-reaction with the antisera against both O. columbianum and H. contortus (Fig. 2 and 3). Out of these five cross-reacting polypeptides only two polypeptides (47 and 31 kDa) were identified as the dominant by antisera of both H. contortus and O. columbianum.
From the results of western blot analyses and cross-reactivity analysis assay it was evident that four immunogenic polypeptides (MW, 92, 85, 65 and 39 kDa) were species-specific for H. contortus and two (MW, 72, and 26 kDa) for O. columbianum. However, none of the six immunogenic polypep-tides of T. ovis was species-specific.
Discussion
Antigenic complexity is a major challenge for immunological approaches to diagnosis and control of parasitic infections, especially the helminths (16), which are metazoan organisms. However, identification of the target antigens in different helminths and their exploitation as immunodiagnostic and immunoprophylactic agents has become the recent trend in immunological studies of helminth parasites (17).
Characterization and cross antigenicity studies of the three commonly occurring caprine nematode species revealed that four out of ten immunogenic peptides of H. contortus were specific for the species. However, two out of eight immunopolypeptides of O. columbianum were species- specific. Interestingly, T. ovis sp. did not reveal any species-specific antigenicity. This study has therefore opened up the scope for future development of specific immuno diagnostic method for the two most widely prevalent and economically important nematode (H. contortus and O. columbianum) infections by exploiting the species-specific antigens as identified in this study. Future studies in this direction, however, should first identify the target antigen(s), which must express in in vivo conditions, in each species separately.
Concurrent infection of two or more nematode species is usually more common than the infection with a single species of nematode and in the state of West Bengal mixed infection of H. contortus, O. columbianum and T. ovis is quite frequent (Annual Report, 2008-’09, A.I.N.P. on G.I.P., Kolkata Centre, WBUAFS, Kolkata). Therefore, a polyvalent antigen with the potential of detecting the monospecific or mixed infection of the three nematode species, at the early stage, will have immense practical value. Thus the cross reacting peptides as identified in this study would ideally constitute such a polyvalent antigen for diagnosis of either monospecific or mixed infection of these three nematode species.
The polypeptides between H. contortus and O. columbianum(54 kDa) and between O. columbianum and T. ovis (47 kDa) as observed in the present study might be evaluated for having any protective epitope and subsequently exploitation of those peptides for development of reliable immunodiagnostic as well as immunoprophylactic tools for the commonly occurring gastrointestinal nematodoses in small ruminants.
Immunological methods for early detection of H. contortus infection has justifiably received inadequate research attention as it can be diagnosed as early as 19 days after the infection, when it becomes patent, using conventional parasitological methods. Major pathogenic impact of the parasite is caused after this period. On the contrary, oesophagostomosis, the second most pathogenic nematode in small ruminants in India, although deserves due attention in this regard, has been overlooked. O. columbianum infection has a long prepatent period of 41 days (18) during which this infection exerts its major pathogenic effects. Thus, conventional methods detect the infection when the major damage is already done and hence there is a pressing need for a reliable method to detect the infection at the early stage (10, 19) for curtailing the associated economic losses. The target proteins of O. columbianum identified in this study will thus pave a promising way for early and reliable detection of gastrointestinal nematodoses in small ruminants.
Conclusion
Development of reliable methods for immunodiagnosis and the control of monospecific and poly-specific infection with H. contortus, O. columbianum and T. ovis could be a promising proposition. Such a method, if successfully developed, would have great impact on the productivity of small ruminant livestock in general and of goat in particular.
Acknowledgements
The authors thankfully acknowledge the financial assistance of the Indian Council of Agricultural Research, New Delhi in conducting this study under the research project entitled “All India Network Programme on Gastrointestinal Parasitism”. The authors declare that there is no conflict of interest.
References
- 1. Saha SB, Pramanik S, Mukherjee GS. Prevalence of gastrointestinal nematodes of goats in West Bengal. Ind J Anim Sci. 1996; 11 (1): 51– 52. [Google Scholar]
- 2. Jas R, Ghosh JD. Seasonal qualitative and quantitative variation in environmental contamination with gastrointestinal nematodes of goat. Environ Ecol. 2007; 25S (4): 1142– 1145. [Google Scholar]
- 3. Blanchard JL, Westcott RB. Enhancement of resistance of lambs to Haemonchus contortus by previous infection with Ostertagia circumcincta. Am J Vet Res. 1985; 46: 2136– 2140. [PubMed] [Google Scholar]
- 4. Adams DB, Anderson BH, Windon RG. Cross immunity between Haemonchus contortus and Trichostrongylus colubriformis in sheep. Int J Parasitol. 1989; 19: 717– 722. [DOI] [PubMed] [Google Scholar]
- 5. Hendrikx WNL. The nematode Haemonchus contortus: antigenic characterization and immune response in rabbits and sheepn. Tijdschr Diergeneeskd 1990; 115: 1092– 1101. [PubMed] [Google Scholar]
- 6. Gamble HR, Purcell JP, Fetterer RH. Biochemical characterization of cuticle polypeptides from the infective larvae of Haemonchus. Comp Biochem Physiol B. 1990; 96: 421– 429. [DOI] [PubMed] [Google Scholar]
- 7. Canals A, Gasbarre LC. Ostertagia Ostertagi: isolation and partial characterization of somatic and metabolic antigens. Int J Parasitol. 1990; 20 (8): 1047– 1054. [DOI] [PubMed] [Google Scholar]
- 8. Jasmer DP, McGuire TC. Protective immunity to a blood feeding nematode (Haemonchus contortus) induced by parasite gut antigens. Infec Immun. 1991; 59 (12): 4412– 4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jasmer DP, Perryman LE, Conder GA, Crow S, McGuire T. C. Protective immunity to Haemonchus contortus induced by immunoaffinity isolated antigens that share a phylogenetically conserved carbohydrate gut surface epitope. J Immunol. 1993; 151: 5450– 5460. [PubMed] [Google Scholar]
- 10. Jas R, Ghosh JD, DAS K. Diagnosis of Oesophagostomum columbianum infection in goat by indirect enzyme linked immunosorbent assay. Helminthologia. 2010a; 47 (2): 83– 87. [Google Scholar]
- 11. Klesius PH, Washburn SM, Haynes TB. Serum antibody response to soluble extract of the third larval stage of Ostertagia ostertagi in cattle. Vet Parasitol. 1986; 20: 307– 314. [DOI] [PubMed] [Google Scholar]
- 12. Lowry OH, Rosebrough NJ, Farr AB, Randall RJ. Protein measurement with the Folinphenol reagent. J Biol Chem. 1951; 193: 265– 75. [PubMed] [Google Scholar]
- 13. Hudson L, Hay FC. Practical Immunology. 3rd ed Blackwell scientific Publications, Oxford: London; 1989. [Google Scholar]
- 14. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970; 227: 680– 685. [DOI] [PubMed] [Google Scholar]
- 15. Towbin H, Stachelin T, Gorden G. Elecrophoretic transfer of proteins from polyacrilamide gels to nitrocellulose sheets: Procedure and some applications. Proc Natl Acad Sci U S A. 1979; 76: 4350– 4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Parkhouse RME, Almond NM, Cabrera Z, Harnett W. Nematode antigen in protection, diagnosis and pathology. Vet Immunol Immunopathol. 1987; 17: 313– 324. [DOI] [PubMed] [Google Scholar]
- 17. Schallig HDFH, Hornok S, Cornelissen JBWJ. Comparison of two enzyme immunoassays for the detection of Haemonchus contortus infections in sheep. Vet Parasitol. 1995; 57: 329– 338. [DOI] [PubMed] [Google Scholar]
- 18. Soulsby EJL. Helminths, arthropods and protozoa of domesticated animals. 7th ed The English Language Book Society and Bailliere, Tindall: London; 1982. [Google Scholar]
- 19. Jas R, Ghosh JD, DAS K. Polyclonal antibody based coproantigen detection immunoassay for diagnosis of Oesophagostomum columbianum infection in goats. Vet Parasitol. 2010b; 170: 262– 267. [DOI] [PubMed] [Google Scholar]


