Abstract
Spleen is an unusual location for hydatid cyst. Here we report a case of primary splenic hydatid cyst in a 41-yr-old Iranian woman from Yasuj, southwest of Iran. The patient had been admitted to Shahid Beheshti Hospital because of abdominal pain. Abdominal sonography revealed a hypoechoic lesion of 150 X 130 mm in the spleen, suggestive of hydatid cyst. Splenectomy was performed for the patient and surgical interventions revealed a hydatid cyst occupying most of splenic parenchyma. She was discharged on the 5 day of her operation. Postoperative diagnosis and confirmation of hydatid cyst was done by histopathological, molecular and serological approaches. Histopathological evaluation revealed the classical laminated layer of hydatid cyst. DNA was extracted from a part of cyst and PCR amplified. Sequencing and analysis of PCR product revealed that the isolate has the most similarity with G1 strain of Echinococcus granulosus. Patient’s serum was positive for IgG anti-hydatid cyst antibodies, using antigen-B ELISA.
Keywords: Primary, Splenic, Hydatid cyst, Iran
Introduction
Cystic echinococcosis (CE) is the larval cystic stage of the tapeworm Echinococcus granulosus (1). The disease is endemic in the Central Asia, Mediterranean countries; Middle East, Africa and South America (1–2). Human become infected by accidentally ingesting of contaminated food and water with eggs of the tapeworm (1, 3). Ingestion of eggs of the adult parasite results in the development of one or several unilocular hydatid cysts in humans, mainly in the liver (70%) and lungs (20%). However, the larvae may pass through the liver and lung barriers and reach the other body sites. The unusual locations of hydatid cyst in human are included, but not limited to, the heart, orbit, brain, spleen, muscle, salivary gland, bone, urinary tract, and pancreas (4). Frequency of splenic hydatid cyst is low (about 0.5 to 6% of total incidence of hydatid cyst) even in endemic areas (4, 2).
Splenic hydatid cyst is usually asymptomatic and symptoms are few and nonspecific and patients may be asymptomatic for 5 up to 20 years before the diagnosis (5). Complications with splenic hydatid cyst are including, secondary infection, fistulization to adjacent tissues and cyst rapture to the peritoneal cavity, which in turn may cause a life-threatening systematic anaphylactic reaction.
Primary localization of hydatid cyst in unusual locations is a diagnostic challenge for clinicians. Diagnosis of splenic hydatid cyst is usually established during abdominal ultra-sound exam, performed for other clinical reasons.
The biological variants of E. granulosus have been designated as strains. Based on mitochondrial DNA (mtDNA) analysis, the E. granulosus complex has been split into E. granulosus sensu stricto (genotypes G1, G2 and G3), E. equinus (G4), E. ortleppi (G5) and the still controversial taxon E. canadensis (G6-G10)” (6). Most of human cases are related to G1 strain of E. granulosus.
Here we report a rare case of primary giant hydatid cyst. The case was evaluated by molecular, histopathological and serological approaches.
Case
A 41-yr-old woman admitted at department of surgery at Shahid Beheshti Hospital in Yasuj, southwest of Iran. She had been admitted because of abdominal pain. Informed consent was taken from the patient. Abdominal sonography evaluation revealed a large cyst in the spleen, measuring 150 X 130 mm. The cyst had a tick wall protruded from inferomedial aspect of spleen with patches of wall calcification. Preoperative diagnosis, based on cyst characteristics, was splenic hydatid cyst. Low level of hemoglobin (Hb=10.7 grams per deciliter) was noticed in her hematology tests. The rest of biochemistry, and hematological indices were normal. Splenectomy was performed for the patient and surgical interventions revealed a hydatid cyst occupying most of splenic parenchyma (Fig. 1–4).
Fig. 1:
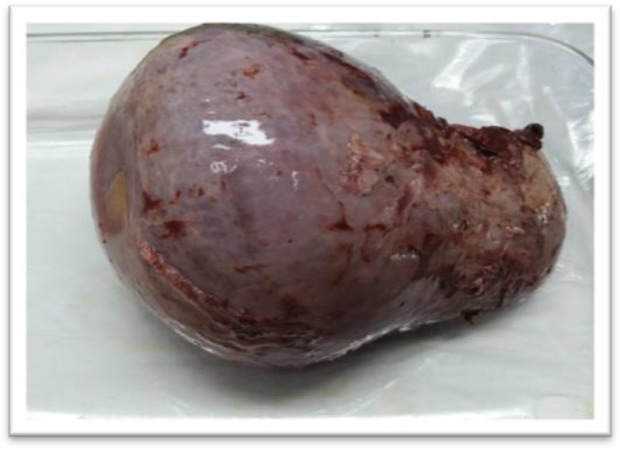
Gross picture of the spleen hydatid cyst (Original)
Fig. 4:
Isolated sections of the cyst showing the cyst layers (Original)
Fig. 2:
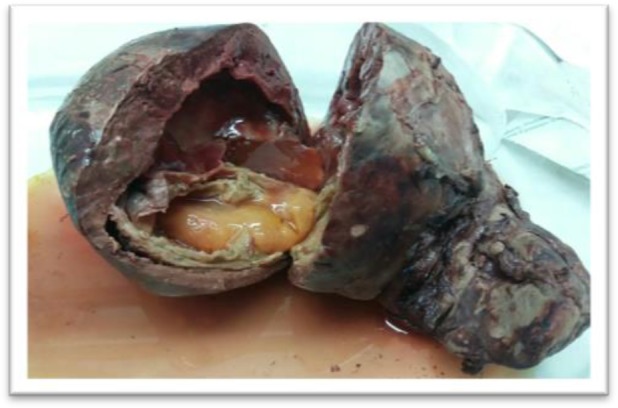
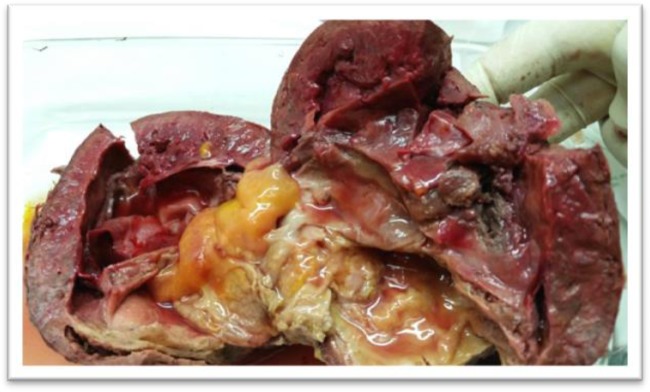
Cut section of the splenic hydatid cyst with huge secondary infection (Original)
Fig. 3:
Cut section of the splenic hydatid cyst showing calcified cyst layer (Original)
She was discharged on 5th postoperative day. Postoperative diagnosis and confirmation of hydatid cyst was done by histopathological, molecular and serological approaches.
Histopathological evaluation revealed the classical laminated layer of hydatid cyst. Patient serum was evaluated by AgB-ELISA, as described before, and was found to be positive by this sensitive serological assay (7).
The patient was housekeeper, used to have contact with animals including dogs and was from a known CE endemic area, where the hydatid cyst is quite common in both human and animals (2).
Molecular evaluation
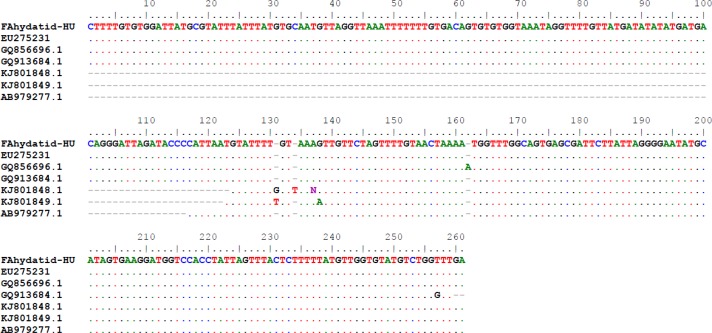
For the purpose of molecular characterization, a few parts of the cyst were stored in 70% ethanol until use. DNA was extracted from the sample. Extraction was done using 15 μL of proteinase K and 300 μL of lysis buffer (50 ml of Tris-HCl (100 Mm), pH = 8; 1 mM of EDTA, pH= 8; 1% Tween 20) followed by phenol/chloroform/ethanol/acetate extraction. Absolute ethanol was used to precipitate the DNA. Precipitated DNA was re-suspended in 100 μL of double-distilled water and stored at −20°C until use. PCR primer sets were used for amplifying a 280 bp fragment of mitochondrial 12S rRNA gene of E. granulosus, using G1 Forward: 5′– GCTTTTGTGTGGATTATGCG-3′ and G1 Reverse: 5′– TCAAACCAGACATACACCAA- 3′ primers (8). PCR reaction was carried out in a final volume of 25 μl, containing 2 μL of DNA template, 0.5 μL of each primer (10 Pmol), 12.5 μL of master mix and 9.5 μL of double-distilled water. PCR product was separated by electrophoresis in 1.5% agarose gel and stained with Gel-red. Thermocycler was programmed by one cycle of initial denaturation at 94 °C for 4min, followed by 35 cycle of denaturation at 94 °C for 94 seconds, annealing at 57 °C for 30 seconds, extension at 72 °C for 35 seconds and final extension at 72 °C for 4 min. After PCR amplification, an approximately 280 bp PCR product was amplified. PCR product was excised from the 1.5% agarose gel and purified with a DNA Gel Extraction Kit, according to the manufacturer’s instructions (Bioneer’s AccuPrep Gel Purification Kit). PCR product was sequenced and the sequence of the isolate was aligned and compared with those of available sequences of E. granulosus in the GenBank. The sequence of the isolate showed the most identity with accessible sequences of Echinococcus G1 genotypes, including EU275231.1 (from sheep from Iran), GQ856696.1 (from bovine from Iran), GQ913684.1 (from goat from Iran), KJ801848.1 (from sheep from Argentina), KJ801849.1 (from human from Yemen) and AB979277.1 (From human from Japan) (Fig 5).
Fig. 5:
Alignment of sequence of the 12S rRNA gene of E. granulosus isolated from the patient with some 12S rRNA gene of E. granulosus available in the GenBank. FAhydatid-HU: Sequence of the current splenic hydatid cyst; EU275231.1 (from sheep, Iran); GQ856696.1 (from bovine, Iran); GQ913684.1 (from goat, Iran); KJ801848.1 (from sheep, Argentina); KJ801849.1 (from human, Yemen); AB979277.1 (from human, Japan)
Discussion
Hydatid disease is an endemic zoonosis in many parts of Iran and is an important medical and veterinary concern in many countries. The most common locations for hydatid disease are the liver and lungs, but any organ or tissue can potentially be affected by this disease (4–5). Primary splenic involvement in hydatid cyst is uncommon, as the parasite embryo usually trapped in the liver (as first filter) and the lung (as second filter).
Clinical presentation of hydatid disease depends on the size, site and depth of the cyst. Splenic hydatid cyst is usually slowed growing and asymptomatic. The most common symptoms are abdominal pain and palpable mass in the left upper quadrant. Rapture of spleen cyst to thorax may cause severe spleno-thoracic fistula. Moreover, rapture of spleen cyst may cause systemic anaphylactic reaction leading to dyspnea, pruritus, edema or even death. The majority of splenic infestations are diagnosed following incidental findings at radiographic examination for unrelated complaints.
Hydatid cyst is diagnosed mainly by history, imaging, and serological methods (9–14). The performance of serological tests in the diagnosis of splenic hydatid cyst might not be satisfactory. In a series of 6 cases of splenic hydatid cyst, only 3 cases (concurrent cysts in spleen and liver) were positive by the serological methods and the remaining 3 cases, which had only splenic hydatid cyst, were negative (15). In two cases of splenic hydatid cyst in Greece, both have been positive by an immunoblotting test (16). In our case, serological evaluation revealed anti-hydatid cyst antibodies in patient serum, using antigen-B ELISA. Seropositivity of our case might be contributed to the use of a reliable serological test and the feature of the current splenic cyst, which was large and primary. Blood eosinophilia may present in less than 30% of patients with splenic hydatid cyst, make this blood parameter an unreliable laboratory finding. Blood eosinophilia was not present in our case. For treatment of splenic hydatid cyst, splenectomy is preferred in adults and is required when the cyst is particularly large or centrally located (17).
So far, ten different genotypes (G1-G10) have been identified for E. granulosus sensu lato by molecular approaches (6). The G1 genotype or sheep strain of E. granulosus sensu stricto is the most common genotype of E. granulosus in animals and humans worldwide (6, 18–19). The predominant strain of E. granulosus in human cases of hydatid cyst in Iran is E. granulosus sensu stricto (20). In keeping with this, genotype of our case was G1 sensu stricto of E. granulosus.
Conclusion
Although rare, hydatid cyst should be considered in differential diagnosis of all cystic masses in the spleen in any CE-endemic areas.
Acknowledgments
Technical assistance of Mr. Arefkhah is highly appreciated. The authors declare that there is no conflict of interest.
References
- 1. Eckert J, Deplazes P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin Microbiol Rev. 2004; 17 (1): 107– 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sarkari B, Sadjjadi SM, Beheshtian MM, Aghaee M, Sedaghat F. Human cystic echinococcosis in Yasuj District in Southwest of Iran: an epidemiological study of seroprevalence and surgical cases over a ten-year period. Zoonoses Public Health. 2010; 57 (2): 146– 150. [DOI] [PubMed] [Google Scholar]
- 3. McManus DP, Zhang W, Li J, Bartley PB. Echinococcosis. Lancet. 2003; 362 (9392): 1295– 1304. [DOI] [PubMed] [Google Scholar]
- 4. Geramizadeh B. Unusual locations of the hydatid cyst: a review from Iran. Iran J Med Sci. 2013; 38 (1): 2– 14. [PMC free article] [PubMed] [Google Scholar]
- 5. Sachar S, Goyal S, Goyal S, Sangwan S. Uncommon locations and presentations of hydatid cyst. Ann Med Health Sci Res. 2014; 4 (3): 447– 452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McManus DP. Current status of the genetics and molecular taxonomy of Echinococcus species. Parasitology. 2013; 140 (13): 1617– 1623. [DOI] [PubMed] [Google Scholar]
- 7. Sadjjadi SM, Abidi H, Sarkari B, Izadpanah A, Kazemian S. Evaluation of enzyme linked immunosorbent assay, utilizing native antigen B for serodiagnosis of human hydatidosis. Iran J Immunol. 2007; 4 (3): 167– 172. [PubMed] [Google Scholar]
- 8. Rostami Nejad M, Nazemalhosseini Mojarad E, Nochi Z, Fasihi Harandi M, Cheraghipour K, Mowlavi GR, Zali MR. Echinococcus granulosus strain differentiation in Iran based on sequence heterogeneity in the mitochondrial 12S rRNA gene. J Helminthol. 2008; 82 (4): 343– 347. [DOI] [PubMed] [Google Scholar]
- 9. Mohammadzadeh T, Sako Y, Sadjjadi SM, Sarkari B, Ito A. Comparison of the usefulness of hydatid cyst fluid, native antigen B and recombinant antigen B8/1 for serological diagnosis of cystic echinococcosis. Trans R Soc Trop Med Hyg. 2012; 106 (6): 371– 375. [DOI] [PubMed] [Google Scholar]
- 10. Rahimi H, Sadjjadi S, Sarkari B. Performance of antigen B isolated from different hosts and cyst locations in diagnosis of cystic echinococcosis. Iran J Parasitol. 2011; 6 (1): 12– 19. [PMC free article] [PubMed] [Google Scholar]
- 11. Sadjjadi SM, Sedaghat F, Hosseini SV, Sarkari B. Serum antigen and antibody detection in echinococcosis: application in serodiagnosis of human hydatidosis. Korean J Parasitol. 2009; 47 (2): 153– 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sedaghat F, Sadjjadi SM, Hosseini SV, Kazemian S, Sarkari B. Evaluation of a simple Dot-ELISA in comparison with countercurrent immunoelectrophoresis for diagnosis of human hydatidosis. Clin Lab. 2011; 57 (3–4): 201– 205. [PubMed] [Google Scholar]
- 13. Sarkari B, Rezaei Z. Immunodiagnosis of human hydatid disease: Where do we stand? World J Methodol. 2015; 5 (4): 185– 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sarkari B, Sadjjadi SM, Abidi H, Izadpanah A, Kazemian S, Rafati A. Application of western blotting using native antigen B for serodiagnosis of human cystic echinococcosis. Iran J Parasitol. 2007; 2 (3): 7– 12. [Google Scholar]
- 15. Fernandez-Ruiz M, Guerra-Vales JM, Enguita-Valls AB, Vila-Santos J, Garcia-Borda FJ, Morales-Gutierrez C. Splenic hydatid cyst, a rare location of extrahepatic echinococcosis: Report of six cases. Eur J Intern Med. 2008; 19 (7): e51– 53. [DOI] [PubMed] [Google Scholar]
- 16. Vezakis A, Dellaportas D, Polymeneas G, Tasoulis MK, Chondrogiannis C, Melemeni A, Polydorou A, Fragulidis GP. Two cases of primary splenic hydatid cyst in Greece. Korean J Parasitol. 2012; 50 (2): 147– 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ran B, Shao Y, Yimiti Y, Aji T, Shayiding P, Jiang T, Li H, Li J, Zhang W, Wen H. Spleen-preserving surgery is effective for the treatment of spleen cystic echinococcosis. Int J Infect Dis. 2014; 29: 181– 183. [DOI] [PubMed] [Google Scholar]
- 18. Sarkari B, Mansouri M, Khabisi SA, Mowlavi G. Molecular characterization and seroprevalence of Echinococcus granulosus in wild boars (Sus scrofa) in south-western Iran. Ann Parasitol. 2015; 61 (4): 269– 273. [DOI] [PubMed] [Google Scholar]
- 19. Mansouri M, Sarkari B, Mowlavi GR. Helminth parasites of wild boars, Sus scrofa, in Bushehr Province, Southwestern Iran. Iran J Parasitol. 2016; 11 (3): 377– 382. [PMC free article] [PubMed] [Google Scholar]
- 20. Nikmanesh B, Mirhendi H, Ghalavand Z, Alebouyeh M, Sharbatkhori M, Kia E, Mohebali M, Eghbali M, Rokni MB. Genotyping of Echinococcus granulosus isolates from human clinical samples based on sequencing of mitochondrial genes in Iran, Tehran. Iran J Parasitol. 2014; 9 (1): 20– 27. [PMC free article] [PubMed] [Google Scholar]