Abstract
Typically, studies on indoor fungal growth in buildings focus on structures with known or suspected water damage, moisture, and/or indoor fungal growth problems. Reference information on types of culturable fungi and total fungal levels are generally not available for buildings without these problems. This study assessed 50 detached single-family homes in metropolitan Atlanta, Ga., to establish a baseline of “normal and typical” types and concentrations of airborne and dustborne fungi in urban homes which were predetermined not to have noteworthy moisture problems or indoor fungal growth. Each home was visually examined, and samples of indoor and outdoor air and of indoor settled dust were taken in winter and summer. The results showed that rankings by prevalence and abundance of the types of airborne and dustborne fungi did not differ from winter to summer, nor did these rankings differ when air samples taken indoors were compared with those taken outdoors. Water indicator fungi were essentially absent from both air and dust samples. The air and dust data sets were also examined specifically for the proportions of colonies from ecological groupings such as leaf surface fungi and soil fungi. In the analysis of dust for culturable fungal colonies, leaf surface fungi constituted a considerable portion (>20%) of the total colonies in at least 85% of the samples. Thus, replicate dust samples with less than 20% of colonies from leaf surface fungi are unlikely to be from buildings free of moisture or mold growth problems.
Environmental assessment of buildings for evidence of indoor fungal growth has increased dramatically in the past decade. When these assessments include collecting air or material samples from a building, interpretation of analytical results is often attempted by comparing concentrations of fungi in the samples to one of a number of proposed quantitative guidelines or standards (18). However, at present, no suitable numerical “standards” are generally accepted.
Conversely, it has long been recognized that qualitative determination of which fungi are recovered from environmental samples is more useful than determining the concentrations of fungi. Miller (14) recognized the value of comparing the proportions of fungal types in a sample in order to determine whether the sample came from a building with or without fungal colonization. Similar concepts underlie the currently recommended interpretive strategies (1, 3). Various building studies have interpreted samples from complaint buildings and from combinations of complaint and noncomplaint buildings in this way (15, 16). To date, as far as we are aware, no studies have characterized the prevalence and abundance of culturable fungi in homes specifically selected for the absence or minimal presence of water damage or indoor fungal growth.
This study establishes a reliable reference list of fungi and their relative abundances in nonproblem detached, single-family residences, that is, structures free of water damage and indoor fungal growth. This baseline will provide investigators with realistic reference data on the basis of which they can more effectively evaluate microbial growth and conditions in urban homes with suspected or known problems. This survey also sought to expand on the airborne fungus data from the U.S. Environmental Protection Agency's (EPA) Building Assessment, Survey and Evaluation (BASE) study, which was a cross-sectional study of 100 buildings to provide a baseline for indoor air quality in commercial office buildings not selected for moisture problems (13, 24).
MATERIALS AND METHODS
Selection of housing units.
Five thousand homeowners in central-city “zip code” areas in DeKalb and Fulton Counties, Georgia, were selected from a purchased mail list for areas overlying U.S. Census Bureau “inside-central-city” census tracts for the Atlanta metropolitan area. Residents were sent letters asking whether they would be interested in participating in this study if their homes met the inclusion criteria and whether they would be willing to have their homes surveyed for indoor fungal growth. In order to be eligible for the study, selected homes were required to meet the following four criteria: (i) single-family detached houses, (ii) built since 1945, (iii) with known water damage of no more than 2 ft2, and (iv) located within a central-city census tract. The first 50 eligible and willing respondents were selected for the survey.
Restricting the survey group homes to those that met the four criteria above helped reduce variability due to housing type and focused attention on single-family detached housing, the largest single type of dwelling in the Atlanta metropolitan area. If a home met these criteria but also had a small area of fungal growth (less than 1 ft2), it was still accepted. Any areas of water staining without visible fungal growth were noted.
A random subset of 50 residents who did not respond to the mail solicitation were contacted by telephone to obtain data on the types and ages of their homes, house sizes, and any history of water damage. These data were compared with those for the 50 homes selected for the survey group to assess the level of differences between the two groups of homes.
Sampling of homes.
Each home in the survey group was scheduled for sampling twice within a calendar year, once during the heating season (winter) and once during the cooling season (summer). A visual assessment of each home was conducted, and a brief questionnaire was administered during the first visit to determine whether there was visible evidence of water damage and to confirm that the home met all of the survey criteria.
Airborne fungi.
Indoor samples of airborne fungi were taken in three locations (main living area, kitchen, and master bedroom), by using a Surface Air System (SAS) High Flow model air sampler (Bioscience International, Rockville, Md.) to impact the air samples onto 2% malt extract agar (MEA) (Difco). The main living area was defined as the area where the family was likely to be 30 min after dinner. Outdoor air samples were taken approximately 50 ft in front of the main entrance of the house by the same procedure used for taking the indoor samples.
During each visit, two air samples were collected at each of the three inside locations and at the one outside location: one for 20 s and one for 60 s, yielding samples of approximately 60 and 180 liters, respectively. This sampling procedure was conducted once immediately upon arrival and repeated just prior to departure. A total of 32 air samples plus field and media blanks were taken to characterize each home, for a total of 1,600 samples in the entire data set of culturable airborne fungi.
Settled dust.
During each visit, composite settled-dust samples were collected in a clean vacuum dust collection bag (Johns Hopkins University Dermatology, Allergy, and Clinical Immunology Reference Laboratory) made of nonwoven synthetic fabric placed in the attachment end of a vacuum cleaner hose. The sizes of the areas from which samples were collected varied, but at least 1 m2 from each room was vacuumed if possible. Each sample included dust from the living room, kitchen, and master bedroom. The surface area vacuumed in each room was estimated visually. In the laboratory, collected dust was passed through a 50-mesh (pore size, 300 μm) sterile screen, sealed in a plastic bag, and stored at −20°C until analysis (typically less than 1 week). The entire data set of culturable dustborne fungi for all 50 homes totaled 100 samples. No outdoor dust samples were taken.
Laboratory analysis.
Air sample plates from the SAS air sampler were incubated at 25°C for at least 7 days after sampling and were examined twice, once when the colonies were young and again after approximately 1 week of incubation. During the first examination, all culturable fungi were counted and, for appropriate genera, identified to the species level, if possible (4, 11, 17, 23). For other genera requiring cultivation on diagnostic media, subcultures of representative isolates were made for identification. Media used were those described by Pitt (17) and Klich (11).
During the second examination, any fungi that had not been identified previously were identified. Analysis was completed when all subcultured fungi had been examined and, when possible, identified. Penicillium and Aspergillus isolates were identified to the species level, when possible. Species identifications were confirmed by subculturing representative isolates onto identification media as appropriate. A positive hole correction factor was applied to each sample prior to calculation of concentrations of fungi in each sample, expressed in CFU per cubic meter of air (21).
Dust samples were aseptically sieved (50 mesh) prior to weighing and plating. Replicate 2- to 5-mg samples of sieved dust were applied by direct plating (19) to each of two plates of MEA or DG-18 agar (10). Dust was directly “sprinkled” over the entire agar surface. Using this much dust helps to achieve a more uniform distribution, without exacerbating the risk of overgrowth. Culturable fungi were counted and identified as described for the air samples, except that the concentrations of fungi are expressed in CFU per gram.
Statistical analysis.
The frequency distribution of quantitative results was fit to a normal distribution by the Kolmogorov-Smirnov test. Data that were not normally distributed were log transformed prior to analysis of variance (ANOVA), which was performed to detect differences between group means, such as means for samples taken in different seasons or with different air volumes. Median values of groups were used for graphical presentation of group comparisons.
The rankings of particular fungal species or genera (types) were determined based on their prevalences and abundances among the samples in a data set. Rankings based on abundance of fungal types in the overall data set were also compared by Spearman's rank test. Taxa that occurred as fewer than three colonies overall from a particular location and season were considered too rare to be informative and were eliminated. Comparisons among rankings from more than two sites included only taxa that occurred in at least two sites.
The air and dust data sets were also examined specifically for the presence of fungi that would be indicative of indoor fungal growth. Three groups were identified: leaf surface fungi, soil fungi, and water indicator fungi. The group of water indicator fungi consisted of Chaetomium spp., Ulocladium spp., and Stachybotrys spp., since these are commonly encountered in the built environment on water-damaged building materials (5). Each dust sample was examined further to determine the percentage of fungal colonies that were leaf surface fungi. The distribution of the percentages was then examined graphically.
RESULTS
Housing characteristics: surveyed homes.
Overall results of the visual inspections and questionnaires conducted during the first visit showed that 16 of the 50 homes studied had some kind of water damage, ranging from small water spots to peeling paint. Only 1 of the 50 homeowners had indicated water damage during the initial phone interview, and this was reported to be less than 2 ft2. Six homes contained fungal growth of less than 1 ft2, and seven had some kind of wall dampness. While four homes had evidence of previous flooding, only two contained some standing water in the basement or crawl space. All 50 homes had forced-air heating and ventilation systems. Nearly all homes were air conditioned; 43 had central cooling systems, while 6 used window units. The air-conditioning status of one home was undetermined.
Within the three indoor areas in which samples were taken, three kitchens had some evidence of moisture on the walls or ceiling, and two had small areas (less than 1 ft2) of visible fungi. Four homes had some evidence of water damage on family room walls, but no visible fungi were observed in the family rooms. Although no visible fungi were observed in any of the bedrooms, four homes had some signs of water damage to the bedroom ceiling or walls, and two homes had some condensation on the single-pane windows.
Housing characteristics: nonrespondents' homes.
The majority of the 50 homes in the nonrespondent group were detached single-family houses; only 3 were not. The principal difference between the survey group and the nonrespondent group was the number of homes built prior to 1945. All of the homes in the survey group were built after 1945, but 21 of the homes in the nonrespondent group were not. Water damage was reported by occupants in 10 of the 50 nonrespondents' homes, compared with only 1 of the 50 participants' homes.
Airborne fungi.
By inspection of plotted data, the frequency distribution of the concentrations of culturable air fungi, expressed in CFU per cubic meter, appeared to be highly skewed. These values were compared to a normal distribution by the Kolmogorov-Smirnov test in order to assess the goodness of fit; the test indicated (P < 0.01) that the results did not fit a normal distribution. The CFU per cubic meter were then log transformed, plotted, and compared again to a normal distribution. Inspection of the frequency distribution indicated an acceptable fit of the log-transformed data to a normal distribution. Further statistical analyses were conducted on the log-transformed data.
To determine if the results of the two sample air volumes could be pooled, ANOVA was conducted on the log CFU-per-square-meter values for the entire data set of 1,600 air samples by season and by sample air volume. Seasonal effects were included in the model to control for expected differences between summer and winter (9). The results of the ANOVA indicated that significant differences were present between seasons (P < 0.01) and between sample volumes (P < 0.01). Seasonal effects were expected, because concentrations are typically higher in summer than in winter, as demonstrated in these results. Significant differences between sample volumes are likely to be an artifact of the sampler, related to the nonlinear response of sieve plate impactor samplers. Consequently, the results for the 60- and the 180-liter samples were inspected in order to select the group that had the optimal number of fungal colonies.
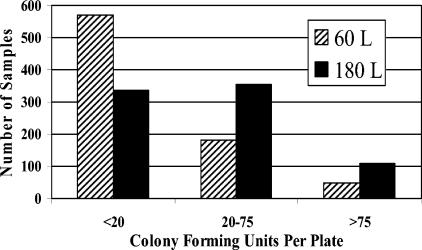
The colony loads in the 60- and 180-liter samples were compared, and samples were tallied in three categories: fewer than 20 colonies per plate, 20 to 75 colonies per plate, and more than 75 colonies per plate. We believe that the range of 20 to 75 colonies per plate is optimal for countable plates (Fig. 1).
FIG. 1.
Frequency distribution of air samples by colony loading per plate.
Among the 180-liter samples, 355 were optimal, whereas only 182 of the 60-liter samples were optimal (Fig. 1). Further, the 180-liter samples that were not considered optimal were more evenly divided among the too-few and too-many-colonies categories than the 60-liter samples that were not considered optimal, which were strongly skewed toward the too-few-colonies category. As a result, further analysis was conducted on the 180-liter samples only (all inclusive).
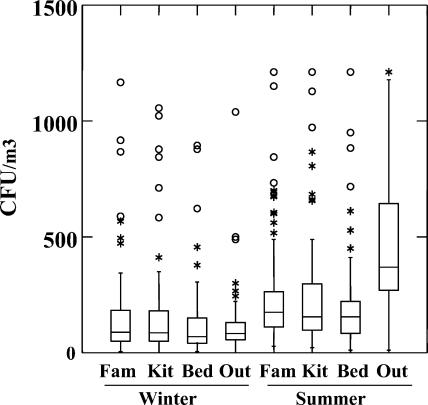
There were minimal differences in the concentration of culturable fungi among the different locations in winter (Fig. 2). Median concentrations for the indoor samples taken during the winter visit were 71, 92, and 89 CFU/m3 for the bedroom, family room, and kitchen, respectively, and the median concentration in the outdoor winter samples was 86 CFU/m3. The median concentrations in the indoor samples taken during the summer visit also were very similar to one another: 166, 189, and 166 CFU/m3 for the bedroom, family room, and kitchen, respectively. The median concentration of the outdoor summer samples was substantially higher, at 439 CFU/m3.
FIG. 2.
Box plots showing median concentrations (in CFU per cubic meter) and distribution of total airborne fungi by location and season in 180-liter air samples. The horizontal line in each box is the median value; the bottom and top of the box are the 25th and 75th percentiles, respectively; and the line extending above the box marks 1.5 times the median value. Circles, values greater than 3 times the median value; asterisks, values greater than 1.5 times the median value.
Results of the ANOVA of log CFU-per-cubic meter values by location and by season indicated significant differences (P < 0.01) among means both for season and for location. Seasonal differences were expected, as were differences by location when outdoor samples were included. Differences among locations persisted, however, when only indoor locations were compared, although they were barely significant (P = 0.0496) at the 0.05 level. Concentrations in bedrooms were lower than those in the other rooms in winter, and concentrations in family rooms were higher than those in the other rooms in summer. This result is surprising in view of the minimal overall differences among the median values cited in the preceding paragraph. It more likely reflects the statistical power of a large database than a meaningful biological difference.
The rankings of the most abundant types of airborne fungi were compared by season and by location. Both in winter (Spearman's rank statistic [rs] = 0.54; n = 38) and in summer (rs = 0.74; n = 57), rankings of fungi identified in the indoor samples (overall) and in the outdoor samples were significantly correlated (P < 0.01). Within seasons, individual locations indoors also were significantly correlated with outdoor abundance rankings (Table 1). Table 2 lists the 30 most abundant types of fungi in the airborne samples.
TABLE 1.
Spearman's rank coefficient and number of observations for pairwise abundance ranking comparisons by sample location and season
| Comparison | rs (n)a | |
|---|---|---|
| Winter | Summer | |
| Outdoors vs family room | 0.44 (36) | 0.68 (53) |
| Outdoors vs kitchen | 0.48 (34) | 0.72 (51) |
| Outdoors vs bedroom | 0.48 (34) | 0.72 (48) |
| Family room vs kitchen | 0.84 (43) | 0.79 (62) |
| Family room vs bedroom | 0.85 (44) | 0.83 (54) |
| Kitchen vs bedroom | 0.75 (40) | 0.73 (52) |
n, number of observations (i.e., number of taxa occurring in both locations in that season). rs values above 0.4251 in these comparisons indicate highly significant (P = 0.01) correlations of the rankings.
TABLE 2.
Top 30 most abundant types of airborne fungia in indoor and outdoor samples
| Fungus |
|---|
| Indoor (n = 600) |
| Cladosporium cladosporioides |
| Cladosporium spp. |
| Cladosporium sphaerospermum |
| Penicillium spp. |
| Penicillium sclerotiorum |
| Epicoccum nigrum |
| Penicillium brevicompactum |
| Penicillium decumbens |
| Yeast |
| Aspergillus niger |
| Nonsporulating fungi (hyaline) |
| Alternaria alternata |
| Penicillium pinophilum |
| Curvularia spp. |
| Penicillium corylophilum |
| Penicillium glabrum |
| Nonsporulating fungi |
| Arthrospore former |
| Nonsporulating fungi (pigmented) |
| Penicillium citrinum |
| Penicillium variabile |
| Aureobasidium pullulans |
| Penicillium crustosum |
| Alternaria sp. |
| Bipolaris sp. |
| Aspergillus fumigatus |
| Penicillium purpurogenum |
| Penicillium solitum |
| Penicillium chrysogenum |
| Aspergillus versicolor |
| Outdoor (n = 200) |
| Cladosporium cladosporioides |
| Cladosporium spp. |
| Cladosporium sphaerospermum |
| Penicillium chrysogenum |
| Penicillium spp. |
| Penicillium corylophilum |
| Penicillium brevicompactum |
| Aspergillus niger |
| Penicillium citrinum |
| Penicillium variabile |
| Nonsporulating fungi (hyaline) |
| Epicoccum nigrum |
| Penicillium commune |
| Penicillium decumbens |
| Penicillium glabrum |
| Curvularia spp. |
| Penicillium citreonigrum |
| Penicillium pinophilum |
| Yeast |
| Nonsporulating fungi |
| Penicillium sclerotiorum |
| Penicillium aurantiogriseum |
| Alternaria alternata |
| Arthrospore former |
| Aspergillus versicolor |
| Penicillium crustosum |
| Penicillium purpurogenum |
| Penicillium paxilli |
| Nonsporulating fungi (pigmented) |
| Penicillium rugulosum |
In 180-liter samples.
Water indicator fungi were essentially absent from the air samples. Two samples in the entire set of 800 samples contained one Ulocladium colony each. These two samples were from different homes and were taken during different seasons. Similarly, two samples, also from different homes, had Chaetomium; one of these was taken in winter and one in summer. Of these four samples, none were from outdoor air. Stachybotrys chartarum was not detected in any air sample, indoors or outdoors.
Dustborne fungi detected by direct plating.
The concentrations of culturable fungi in dust samples were skewed, with lower values more prevalent on both MEA and DG-18 agar. When the frequency distribution of the CFU-per-gram values was compared with a normal distribution, however, the difference was not significant by the Kolmogorov-Smirnov test for either the MEA or the DG-18 data. Even so, to maintain consistency with the presentation of the data for airborne fungi, descriptions of the dustborne fungal concentrations emphasize median values rather than average values. ANOVAs of dust results were performed on CFU-per-gram values rather than on log-transformed data.
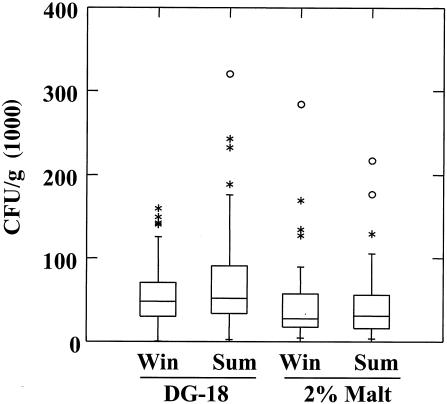
Median concentrations of culturable fungi were higher on DG-18 agar than on MEA and were higher in summer than in winter (Fig. 3). Median values were approximately 4.8 × 104 and 5.2 × 104 CFU/g for DG-18 in winter and summer, respectively, as opposed to 2.7 × 104 and 3.1 × 104 CFU/g for MEA in winter and summer, respectively. The increased concentration of fungi (7.7%) in summer samples was marginally significant (P = 0.06) for DG-18, and the increase on MEA (12.7%) was highly significant (P = 0.002).
FIG. 3.
Box plots showing median concentrations (in CFU per gram) and distribution of total culturable fungi detected by direct plating by medium and season. The horizontal line in each box is the median value; the bottom and top of the box are the 25th and 75th percentiles, respectively; and the line extending above the box marks 1.5 times the median value. Circles, values greater than 3 times the median value; asterisks, values greater than 1.5 times the median value.
The rankings of the most abundant types of fungi recovered from settled-dust samples were compared by season for each agar type used. Differences in the abundance rankings between winter and summer were not statistically significant for either 2% MEA (rs = 0.56; n = 50) or DG-18 agar (rs = 0.72; n = 49). For presentation, the seasonal data were pooled, and the 30 taxa that were most abundant overall are presented for each medium in Table 3.
TABLE 3.
Top 30 most abundant types of fungi in dustborne samples by direct plating on MEA or DG-18 agar
| Fungus |
|---|
| MEA |
| Cladosporium cladosporioides |
| Yeast |
| Cladosporium sphaerospermum |
| Cladosporium spp. |
| Penicillium spp. |
| Aureobasidium pullulans |
| Aspergillus niger |
| Epicoccum nigrum |
| Penicillium chrysogenum |
| Penicillium glabrum |
| Penicillium aurantiogriseum |
| Penicillium sclerotiorum |
| Penicillium citrinum |
| Penicillium purpurogenum |
| Alternaria alternata |
| Rhodotorula spp. |
| Aspergillus spp. |
| Curvularia spp. |
| Aspergillus versicolor |
| Penicillium spinulosum |
| Penicillium decumbens |
| Penicillium brevicompactum |
| Penicillium variabile |
| Penicillium citreonigrum |
| Penicillium corylophilum |
| Nonsporulating fungi |
| Nonsporulating fungi (pigmented) |
| Trichoderma harzianum |
| Unidentified |
| Pithomyces chartarum |
| Bipolaris spp. |
| DG-18 |
| Cladosporium spp. |
| Cladosporium cladosporioides |
| Penicillium spp. |
| Cladosporium sphaerospermum |
| Aspergillus niger |
| Penicillium chrysogenum |
| Penicillium brevicompactum |
| Aspergillus versicolor |
| Aspergillus spp. |
| Penicillium citrinum |
| Penicillium glabrum |
| Penicillium aurantiogriseum |
| Aspergillus ochraceus |
| Nonsporulating fungi |
| Rhodotorula spp. |
| Penicillium expansum |
| Penicillium variabile |
| Penicillium spinulosum |
| Aspergillus sydowii |
| Aspergillus unguis |
| Penicillium crustosum |
| Yeast |
| Eurotium amstelodami |
| Aureobasidium pullulans |
| Alternaria spp. |
| Syncephalastrum racemosum |
| Alternaria alternata |
| Unidentified |
| Penicillium solitum |
| Eurotium herbariorum |
| Nonsporulating fungi (hyaline) |
Water indicator fungi were essentially absent from the dust samples as well as from the air samples. A water indicator fungus was recovered from only a single sample of dust when analyzed on DG-18 agar, which is a low-water-activity agar. When samples were plated on MEA, however, only 5 of the 100 dust samples had any water indicator fungi. Ulocladium was recovered from one home sampled during the winter and two other homes sampled during the summer. Chaetomium globosum was found in one sample taken during the summer at one other home. A sample taken during the winter in a fifth home had both C. globosum and Stachybotrys chartarum. The S. chartarum recovered from this one dust sample represented the only recovery of this species in the entire survey, and this was a single colony (n = 24,310 total colonies).
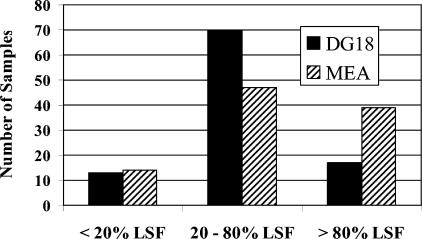
The percentage of all colonies in each sample that were leaf surface fungi was also examined. As expected, the majority of samples had a sizable percentage of colonies that were leaf surface fungi. In fact, in more than 85% of the dust samples, at least 20% of the colonies were leaf surface fungi (Fig. 4). Thus, in about 15% of the samples, there was only a minimal presence of leaf surface fungi. Further, in only four homes did the winter settled-dust sample and the summer settled-dust sample have less than 20% leaf surface fungi. This means that only about one sample in seven from homes without fungus problems would be expected to have less than 20% leaf surface fungi. By extension, only about 1 home in 12 would be expected to have two samples with a low percentage of leaf surface fungi.
FIG. 4.
Frequencies of dust samples (direct plated) with various proportions of leaf surface fungi (LSF). Fewer than one in five samples had very low proportions (<20%) of LSF.
DISCUSSION
Studies of airborne or dustborne fungi in buildings generally focus on problem or complaint buildings or on buildings occupied by study populations. Although some studies include comparison buildings for reference, buildings are not usually selected for the absence of water damage, moisture, and/or indoor fungal-growth-related problems. Li and Kendrick (12), Chao et al. (2), and Stark et al. (22) characterized buildings to assess exposures of a population. Miller et al. (15) and Morey et al. (16) compared building conditions as revealed by air sampling and by destructive inspection (for example, dismantling building walls to inspect for fungal colonization). Shelton et al. (20) characterized buildings based on data from commercially analyzed samples, presumably from complaint buildings that were under investigation. Gots et al. (6) summarized a variety of reports that included measures of airborne fungi taken in building investigations including noncomplaint, reference buildings. This review notes that the suggested numerical guidelines for “acceptable” levels of “total airborne fungi” vary and that widespread agreement on their relevance is lacking. Irrespective of that variety and disagreement, which they note, the authors fail to consider any ecological groupings of fungi and continue to emphasize total airborne fungi by concluding that since levels in outdoor air frequently exceed 500 CFU/m3, that numerical standard is too low.
By comparison, the BASE study attempted a random sample of buildings and thus sought numbers of both problem and nonproblem buildings proportional to their overall prevalence (13, 24). The analysis by Macher et al. (13) used ecological groupings and found that all their groups occurred more frequently in summer than in winter and more frequently outdoors than indoors, except for their group of “water-requiring” fungi. Their results did not detect a significant seasonal difference among these fungi outdoors. It is noteworthy that our “water indicator” group of Chaetomium, Ulocladium, and Stachybotrys has fewer sources in addition to water-damaged building materials (is a more restrictive list) than do the additional taxa in the water-requiring group of Macher et al. (Fusarium, Sporobolomyces, and Botrytis spp., Aspergillus fumigatus, yeasts, and zygomycetes).
We are not aware of any published empirical data that have been collected exclusively from buildings without (nontrivial) indoor fungal growth, water damage, or moisture problems. The present study prospectively screened buildings specifically to ensure that no significant water damage or visible fungal colonization was present. Thus, the relative abundances of fungal types and their concentrations (the composition) reported from this set of buildings represent the normal mycoflora (fungi associated with nonproblem houses) and describe typical background levels for the Atlanta, Ga., area and, by extension, for the southeastern United States.
The results of the present study also confirm that the composition of indoor airborne fungi from houses preselected to avoid any with water damage, moisture, and/or indoor fungal growth problems resembles the composition of outdoor airborne fungi, which was verified by comparing both prevalence rankings and abundance rankings. This is a significant finding, as it is widely recognized that composition of indoor airborne fungi should reflect the diversity of outdoor airborne fungi, but at lower concentrations.
Note that the rankings generated in this study are based on a detailed list of taxa that was developed by identifying the fungi recovered to the species level where possible. This greatly expands the number of taxa available for ranking and strengthens the comparison. For example, whereas Shelton et al. (20), Stark et al. (22), and Chao et al. (2) list Penicillium only to the genus level, as is often done in culture-based studies, the relative abundances of 11 to 17 species of Penicillium are presented in the rankings in the present study.
Conversely, in studies that have sampled indoor airborne fungi in buildings with water damage, substantial changes in the composition of the indoor airborne mycoflora have been noted. Specifically, Miller et al. (15) sampled air in an apartment building with suspected water intrusion. The amount of fungal colonization in the walls of the building was then objectively determined by destructive examination of the walls of 58 apartments. Concentrations of airborne fungi did not differ between the 15 most heavily colonized and the 15 least colonized apartments. However, the airborne mycoflora compositions from the least affected apartments were only 10% as likely as those from heavily affected apartments to differ significantly from outdoor air.
Another multiunit residential building investigation, by Morey et al. (16), sampled air prior to destructive characterization of fungal colonization in walls. This study also showed that atypical indoor air samples (composition differing from the outdoor mix of fungal types) were more common in “leaky” rooms with greater amounts of colonized materials in the walls. Total concentrations of fungi in the leaky and the nonleaky rooms were comparable, based on pooled data from 353 air samples on DG-18 agar. The rank order was significantly different (P < 0.05) from the outdoor samples only in the leaky rooms, however.
Seasonal differences in the present study were limited. Total concentrations of both airborne and dustborne fungi were higher in summer than in winter, presumably due to warmer temperatures and higher humidity. The most dramatic change between summer and winter was the increase in total outdoor concentrations. This is likely due to the increase in vegetation in the summer, since many of the outdoor fungi are phylloplane species. Noteworthy, however, is the overall similarity in species composition demonstrated in these results, which the total concentration by itself cannot assess, regardless of season. The other significant seasonal difference was the independence of rankings between indoor winter air samples and outdoor summer air samples. This difference underscores the need for contemporaneous sampling of outdoor air to be used as a reference for comparison to indoor air sampling results.
We also sampled settled dust in addition to air. Sampling settled dust has long been proven useful in characterizing the microbial status of buildings (8). More recently, Chao et al. (2) reported that the type of culturable fungi detected in settled dust from nonfloor surfaces was a significant predictor of nonspecific symptoms. Culturable fungal propagules accumulate in dust and are likely less variable than airborne fungi, although the varying survival rates of different species will contribute variability to the dustborne burden of fungal propagules. We are not aware of empirical data on the survivability of propagules for many of these species.
Dustborne fungi were cultivated on two media which have very different water activities and hence are expected to favor the recovery of different fungal types. Consequently, no direct comparison of the fungi recovered was attempted; however, seasonal differences were considered for fungi recovered on each medium. Not surprisingly, total concentrations were higher for samples on both media in the summer than in winter. No significant differences were noted in the rankings either by prevalence or by abundance. These findings support the postulate that dustborne fungal populations are reasonably constant, which is a useful factor for interpreting sampling from a building.
Grant et al. (7) described the shift in microbial composition of water-damaged building materials in terms of the minimum required water activity of the fungi colonizing a material. Although widely cited as the source of the terms primary, secondary, and tertiary colonizers, these authors perhaps more importantly laid the basis for detecting indoor fungal-growth problems by comparing the composition rather than the concentration of indoor fungi. Miller (14) presented summary data from buildings with and without “microbial air problems.” In addition to concentration data, two broad categories of fungi were used to interpret the data: leaf surface fungi and soil fungi. Whereas the concentrations of fungi did not differ substantially between buildings with and without problems, there was an obvious shift in the relative proportions of soil versus leaf surface fungi (the composition). Samson et al. (19) have more recently presented this concept by referring to the typical fungal composition of indoor surfaces, tabletop molds.
In the present study, the ratio of leaf surface to soil fungi was analyzed in the dust sample results. Leaf surface fungi are types such as Cladosporium, Alternaria, Epicoccum, and Curvularia spp., and soil fungi include Penicillium, Aspergillus, Emericella, and Paecilomyces spp. When the culturable fungi from dust samples were separated into these groups, leaf surface fungi constituted at least one-fifth of the culturable fungi in more than 85% of the samples. The 15% of the samples that were strongly dominated (>80%) by soil fungi occurred about evenly in summer and winter and typically did not occur in samples from the same house in both seasons. Thus, dust samples that are strongly dominated by soil fungi are atypical among samples taken from houses selected as having no fungal growth and no substantial water or moisture problems. Only three samples were strongly dominated by soil fungi in both analyses, that is, by cultivation on both 2% MEA and DG-18 agar. Thus, we consider a confirmed low proportion of soil fungi in a dust sample to be a useful indicator that the sample is derived from a house without substantial mold growth, water damage, or moisture problems.
To summarize, we present empirical data from prospectively selected buildings supporting the concept that a shift in the composition of culturable fungi is a hallmark of fungal colonization of building materials as a result of water damage or moisture problems. Many studies have observed and documented this change in water-damaged buildings or in comparisons of building groups. To our knowledge, the converse situation has not been fully characterized. That is, buildings have not been selected solely on the basis of the absence of moisture problems, water damage, and indoor fungal growth for a characterization of their fungal ecology. We have prospectively selected such buildings, sampled each in two seasons, and conclude that leaf surface fungi dominate the composition of culturable fungi in samples from nonproblem buildings and that the composition of samples, especially dust samples, is a useful indicator of fungal growth in a building.
This study corroborates general interpretive guidelines that emphasize using the ratio of indoor to outdoor molds as well as the ratio of leaf surface fungi to water indicator fungi and/or soil molds in order to make an accurate assessment. Thus, investigators can compare their results from a home with a suspected problem to these data from homes known not to have problems and be relatively assured they are coming to the right conclusion. As noted, this study describes typical background indoor and outdoor fungal levels for the Atlanta, Ga., area and by extension for the southeastern United States.
Acknowledgments
This project was funded in part by a grant from the Healthy Homes Initiative of the U.S. Department of Housing and Urban Development.
We acknowledge the helpful technical advice of Peter Ashley of the Healthy Homes Initiative. We appreciate the logistical skill of Pam Hogue and the aid of Tim Tront with database management and of Barbara Bergan with statistical analysis.
REFERENCES
- 1.ACGIH Bioaerosols. 1999. Bioaerosols assessment and control. American Conference of Governmental Industrial Hygienists, Cincinnati, Ohio.
- 2.Chao, H. J., J. Schwartz, D. K. Milton, and H. A. Burge. 2003. The work environment and workers' health in four large office buildings. Environ. Health Perspect. 111:1242-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dillon, H. K., P. A. Heinsohn, and J. D. Miller. 1996. Field guide for the determination of biological contaminants in environmental samples. American Industrial Hygiene Association, Fairfax, Va.
- 4.Domsch, K. H., W. Gams, and T. H. Anderson. 1980. Compendium of soil fungi. IHW-Verlag,, Eching, Germany.
- 5.Flannigan, B., and J. D. Miller. 2001. Microbial growth in indoor environments, p. 35-67. In B. Flannigan, R. A. Samson, and J. D. Miller (ed.), Microorganisms in home and indoor work environments. Taylor & Francis, New York, N.Y.
- 6.Gots, R. E., N. J. Layton, and S. W. Pirages. 2003. Indoor health: background levels of fungi. Am. Ind. Hyg. Assoc. J. 64:427-438. [DOI] [PubMed] [Google Scholar]
- 7.Grant, C., C. A. Hunter, B. Flannigan, and A. F. Bravery. 1989. The moisture requirements of moulds isolated from domestic dwellings. Int. Biodeterior. 25:259-284. [Google Scholar]
- 8.Gravesen, S. 1978. Identification and prevalence of culturable mesophilic microfungi in house dust from 100 Danish homes. Allergy 33:268-272. [DOI] [PubMed] [Google Scholar]
- 9.Haselwandter, K., M. R. Ebner, and A. Frank. 1989. Seasonal fluctuations of airborne fungal allergens. Mycol. Res. 28:170-176. [Google Scholar]
- 10.Hocking, A. D., and J. I. Pitt. 1980. Dichloran-glycerol medium for enumeration of xerophilic fungi from low-moisture foods. Appl. Environ. Microbiol. 39:488-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klich, M. A. 2001. Identification of common Aspegillus species. United States Department of Agriculture Agricultural Research Service, Southern Regional Research Center, New Orleans, La.
- 12.Li, D. W., and B. Kendrick. 1995. A year-round comparison of fungal spores in indoor and outdoor air. Mycologia 87:190-195. [Google Scholar]
- 13.Macher, J. M., F. C. Tsai, L. E. Burton, K-S. Liu, and J. M. Waldman. 2001. Prevalence of culturable airborne fungi in 100 U.S. office buildings in the Building Assessment Survey and Evaluation (BASE) Study, p. 1-9. In Moisture, microbes, and health effects: indoor air quality and moisture in buildings. Proceedings of IAQ 2001. American Society of Heating, Refrigeration, and Air-Conditioning Engineers, Inc., Atlanta, Ga.
- 14.Miller, J. D. 1993. Fungi and the building engineer, p. 147-162. In M. Geshwiler (ed.), Environments for people. Proceedings of IAQ '92. American Society of Heating, Refrigeration, and Air-Conditioning Engineers, Inc., Atlanta, Ga.
- 15.Miller, J. D., P. D. Haisley, and J. H. Reinhardt. 2000. Air sampling results in relation to extent of fungal colonization of building materials in some water-damaged buildings. Indoor Air 10:146-151. [DOI] [PubMed] [Google Scholar]
- 16.Morey, P. R., M. C. Hull, and M. Andrew. 2003. El Nino water leaks identify rooms with concealed mould growth and degraded indoor air quality. Int. Biodeterior. Biodegrad. 52:197-202. [Google Scholar]
- 17.Pitt, J. I. 2000. Laboratory guide to common Penicillium species, 3rd ed. CSIRO Food Research Laboratory, North Ryde, Australia.
- 18.Rao, C. Y., H. A. Burge, and J. C. Chang. 1996. Review of quantitative standards and guidelines for fungi in indoor air. J. Air Waste Manag. Assoc. 46:899-908. [DOI] [PubMed] [Google Scholar]
- 19.Samson, R. A., J. Houbraken, R. C. Summerbell, B. Flannigan, and J. D. Miller. 2001. Common and important species of fungi and actinomycetes in indoor environments p. 287-490. In B. Flannigan, R. A. Samson, and J. D. Miller. (ed.) Microorganisms in home and indoor work environments. Taylor & Francis, New York, N.Y.
- 20.Shelton, B. G., K. H. Kirkland, W. D. Flanders, and G. K. Morris. 2002. Profiles of airborne fungi in buildings and outdoor environments in the United States. Appl. Environ. Microbiol. 68:1743-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Somerville, M. C., and J. C. Rivers. 1994. An alternative approach for the correction of bioaerosol data collected with multiple jet impactors. Am. Ind. Hyg. Assoc. J. 55:127-131. [Google Scholar]
- 22.Stark, P. C., H. A. Burge, L. M. Ryan, D. K. Milton, and D. R. Gold. 2003. Fungal levels in the home and lower respiratory tract illnesses in the first year of life. Am. J. Respir. Crit. Care Med. 168:232-237. [DOI] [PubMed] [Google Scholar]
- 23.Wang, C. J. K., and R. A. Zabel. 1990. Identification manual for fungi from utility poles in the eastern United States. American Type Culture Collection. Rockville, Md.
- 24.Womble, S., E. Ronca, and J. Girman. 1996. Developing baseline information on buildings and indoor air quality (BASE '95), p. 109-117. In Paths to better building environments. Proceedings of IAQ '96. American Society of Heating, Refrigeration, and Air-Conditioning Engineers, Inc., Atlanta, Ga.