Abstract
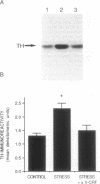
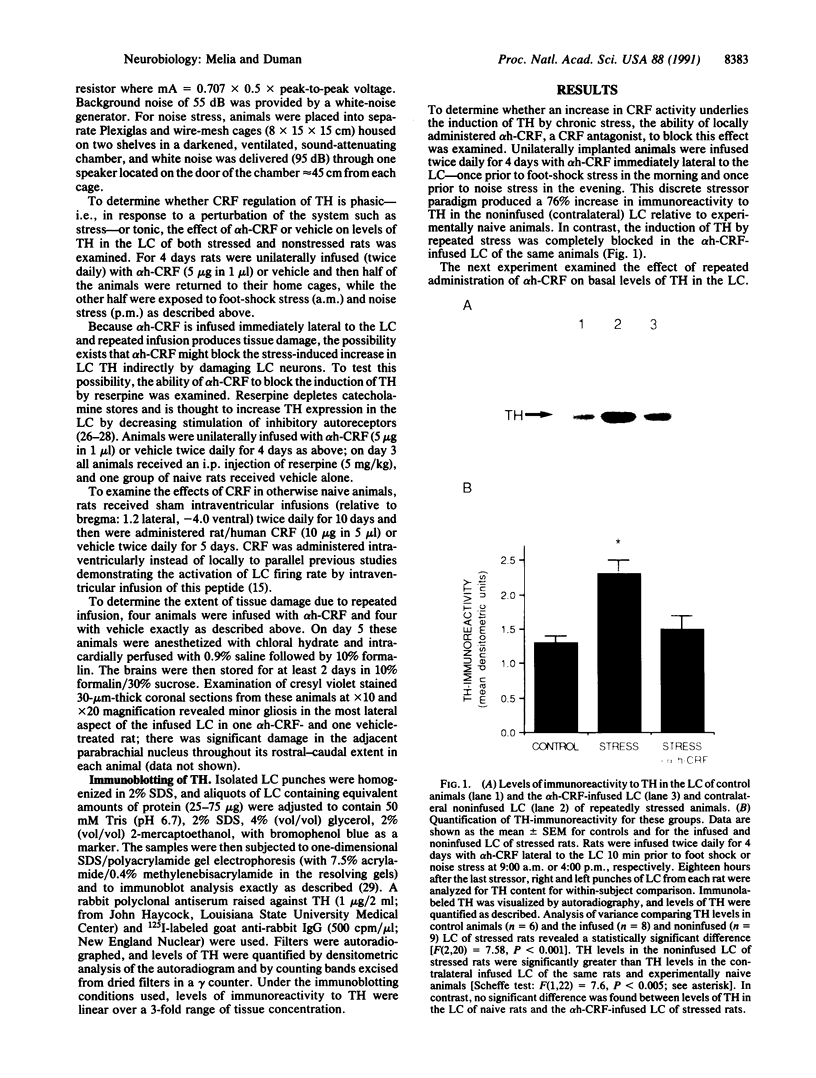
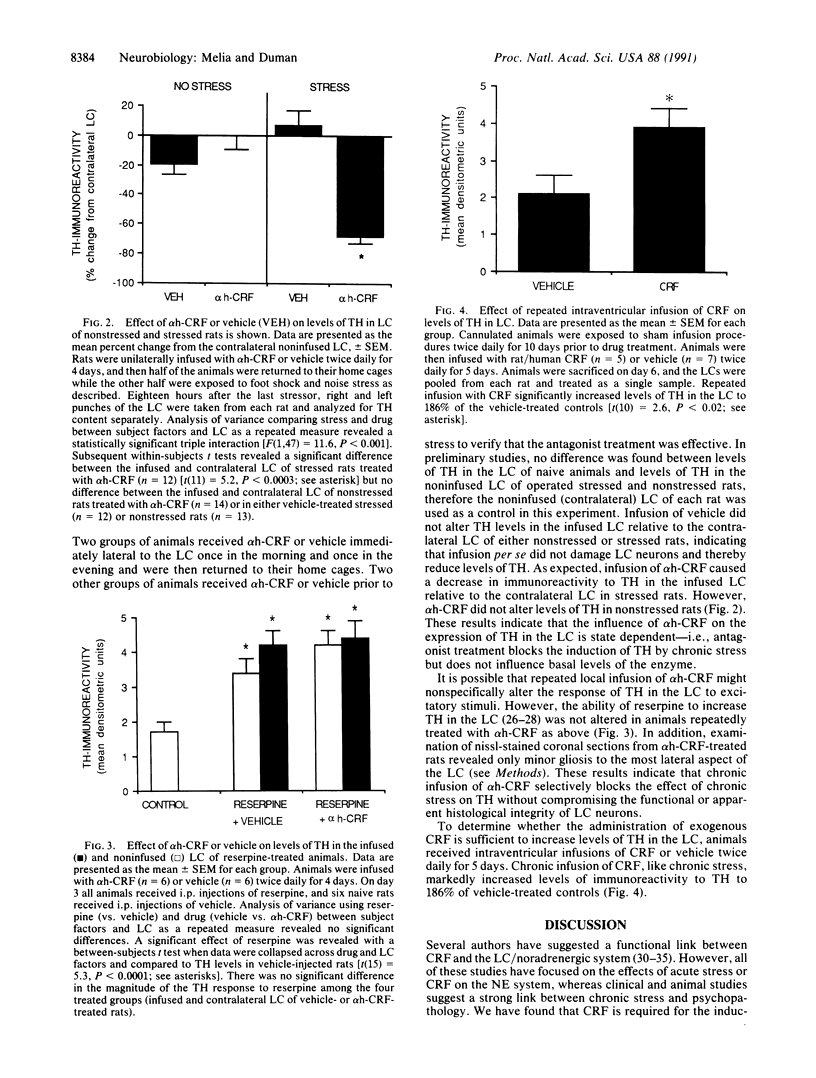
Corticotropin-releasing factor (CRF) and norepinephrine (NE) mediate many hormonal, autonomic, and behavioral effects of acute stress, and it is possible that an interaction between these neurotransmitters could underlie neuronal adaptations in response to chronic stress. To test this hypothesis, the influence of chronically administered CRF and a specific CRF antagonist, alpha-helical CRF, on the induction of tyrosine hydroxylase, the rate-limiting enzyme in NE biosynthesis, was examined in the rat locus coeruleus (LC). We now report that administration of alpha-helical CRF specifically blocks the induction of tyrosine hydroxylase in response to a repeated intermittent stress paradigm involving foot shock and noise stress but has no effect on steady-state levels of the enzyme in nonstressed animals or on the induction of the enzyme in response to reserpine treatment. In addition, repeated administration of CRF alone for 5 days, like chronic stress, increases levels of tyrosine hydroxylase in LC. The results demonstrate that endogenous CRF is necessary for the induction of tyrosine hydroxylase in response to this stress paradigm and that exogenously administered CRF is sufficient for the regulation of this enzyme in nonstressed rats. These findings may prove important in elucidating mechanisms by which chronic stress triggers and sustains the biochemical alterations associated with some stress-related psychiatric disorders.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aston-Jones G., Ennis M., Pieribone V. A., Nickell W. T., Shipley M. T. The brain nucleus locus coeruleus: restricted afferent control of a broad efferent network. Science. 1986 Nov 7;234(4777):734–737. doi: 10.1126/science.3775363. [DOI] [PubMed] [Google Scholar]
- Berridge C. W., Dunn A. J. Restraint-stress-induced changes in exploratory behavior appear to be mediated by norepinephrine-stimulated release of CRF. J Neurosci. 1989 Oct;9(10):3513–3521. doi: 10.1523/JNEUROSCI.09-10-03513.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge C. W., Dunn A. J. Restraint-stress-induced changes in exploratory behavior appear to be mediated by norepinephrine-stimulated release of CRF. J Neurosci. 1989 Oct;9(10):3513–3521. doi: 10.1523/JNEUROSCI.09-10-03513.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler P. D., Weiss J. M., Stout J. C., Nemeroff C. B. Corticotropin-releasing factor produces fear-enhancing and behavioral activating effects following infusion into the locus coeruleus. J Neurosci. 1990 Jan;10(1):176–183. doi: 10.1523/JNEUROSCI.10-01-00176.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler P. D., Weiss J. M., Stout J. C., Nemeroff C. B. Corticotropin-releasing factor produces fear-enhancing and behavioral activating effects following infusion into the locus coeruleus. J Neurosci. 1990 Jan;10(1):176–183. doi: 10.1523/JNEUROSCI.10-01-00176.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedarbaum J. M., Aghajanian G. K. Afferent projections to the rat locus coeruleus as determined by a retrograde tracing technique. J Comp Neurol. 1978 Mar 1;178(1):1–16. doi: 10.1002/cne.901780102. [DOI] [PubMed] [Google Scholar]
- Chappell P. B., Smith M. A., Kilts C. D., Bissette G., Ritchie J., Anderson C., Nemeroff C. B. Alterations in corticotropin-releasing factor-like immunoreactivity in discrete rat brain regions after acute and chronic stress. J Neurosci. 1986 Oct;6(10):2908–2914. doi: 10.1523/JNEUROSCI.06-10-02908.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole B. J., Koob G. F. Propranolol antagonizes the enhanced conditioned fear produced by corticotropin releasing factor. J Pharmacol Exp Ther. 1988 Dec;247(3):902–910. [PubMed] [Google Scholar]
- Cummings S., Elde R., Ells J., Lindall A. Corticotropin-releasing factor immunoreactivity is widely distributed within the central nervous system of the rat: an immunohistochemical study. J Neurosci. 1983 Jul;3(7):1355–1368. doi: 10.1523/JNEUROSCI.03-07-01355.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza E. B., Insel T. R., Perrin M. H., Rivier J., Vale W. W., Kuhar M. J. Corticotropin-releasing factor receptors are widely distributed within the rat central nervous system: an autoradiographic study. J Neurosci. 1985 Dec;5(12):3189–3203. doi: 10.1523/JNEUROSCI.05-12-03189.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn A. J., Berridge C. W. Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res Brain Res Rev. 1990 May-Aug;15(2):71–100. doi: 10.1016/0165-0173(90)90012-d. [DOI] [PubMed] [Google Scholar]
- Ennis M., Aston-Jones G. Activation of locus coeruleus from nucleus paragigantocellularis: a new excitatory amino acid pathway in brain. J Neurosci. 1988 Oct;8(10):3644–3657. doi: 10.1523/JNEUROSCI.08-10-03644.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote S. L., Bloom F. E., Aston-Jones G. Nucleus locus ceruleus: new evidence of anatomical and physiological specificity. Physiol Rev. 1983 Jul;63(3):844–914. doi: 10.1152/physrev.1983.63.3.844. [DOI] [PubMed] [Google Scholar]
- Gold P. W., Goodwin F. K., Chrousos G. P. Clinical and biochemical manifestations of depression. Relation to the neurobiology of stress (2) N Engl J Med. 1988 Aug 18;319(7):413–420. doi: 10.1056/NEJM198808183190706. [DOI] [PubMed] [Google Scholar]
- Gold P. W., Gwirtsman H., Avgerinos P. C., Nieman L. K., Gallucci W. T., Kaye W., Jimerson D., Ebert M., Rittmaster R., Loriaux D. L. Abnormal hypothalamic-pituitary-adrenal function in anorexia nervosa. Pathophysiologic mechanisms in underweight and weight-corrected patients. N Engl J Med. 1986 May 22;314(21):1335–1342. doi: 10.1056/NEJM198605223142102. [DOI] [PubMed] [Google Scholar]
- Gold P. W., Loriaux D. L., Roy A., Kling M. A., Calabrese J. R., Kellner C. H., Nieman L. K., Post R. M., Pickar D., Gallucci W. Responses to corticotropin-releasing hormone in the hypercortisolism of depression and Cushing's disease. Pathophysiologic and diagnostic implications. N Engl J Med. 1986 May 22;314(21):1329–1335. doi: 10.1056/NEJM198605223142101. [DOI] [PubMed] [Google Scholar]
- Holsboer F., Von Bardeleben U., Gerken A., Stalla G. K., Müller O. A. Blunted corticotropin and normal cortisol response to human corticotropin-releasing factor in depression. N Engl J Med. 1984 Oct 25;311(17):1127–1127. doi: 10.1056/NEJM198410253111718. [DOI] [PubMed] [Google Scholar]
- Holsboer F., von Bardeleben U., Buller R., Heuser I., Steiger A. Stimulation response to corticotropin-releasing hormone (CRH) in patients with depression, alcoholism and panic disorder. Horm Metab Res Suppl. 1987;16:80–88. [PubMed] [Google Scholar]
- Hotta M., Shibasaki T., Masuda A., Imaki T., Demura H., Ling N., Shizume K. The responses of plasma adrenocorticotropin and cortisol to corticotropin-releasing hormone (CRH) and cerebrospinal fluid immunoreactive CRH in anorexia nervosa patients. J Clin Endocrinol Metab. 1986 Feb;62(2):319–324. doi: 10.1210/jcem-62-2-319. [DOI] [PubMed] [Google Scholar]
- Kvetnanský R., Palkovits M., Mitro A., Torda T., Mikulaj L. Catecholamines in individual hypothalamic nuclei of acutely and repeatedly stressed rats. Neuroendocrinology. 1977;23(5):257–267. doi: 10.1159/000122673. [DOI] [PubMed] [Google Scholar]
- Labatut R., Buda M., Bérod A. Long-term changes in rat brain tyrosine hydroxylase following reserpine treatment: a quantitative immunochemical analysis. J Neurochem. 1988 May;50(5):1375–1380. doi: 10.1111/j.1471-4159.1988.tb03019.x. [DOI] [PubMed] [Google Scholar]
- Moore R. Y., Bloom F. E. Central catecholamine neuron systems: anatomy and physiology of the norepinephrine and epinephrine systems. Annu Rev Neurosci. 1979;2:113–168. doi: 10.1146/annurev.ne.02.030179.000553. [DOI] [PubMed] [Google Scholar]
- Nemeroff C. B., Owens M. J., Bissette G., Andorn A. C., Stanley M. Reduced corticotropin releasing factor binding sites in the frontal cortex of suicide victims. Arch Gen Psychiatry. 1988 Jun;45(6):577–579. doi: 10.1001/archpsyc.1988.01800300075009. [DOI] [PubMed] [Google Scholar]
- Nemeroff C. B., Widerlöv E., Bissette G., Walléus H., Karlsson I., Eklund K., Kilts C. D., Loosen P. T., Vale W. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science. 1984 Dec 14;226(4680):1342–1344. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- Nestler E. J., McMahon A., Sabban E. L., Tallman J. F., Duman R. S. Chronic antidepressant administration decreases the expression of tyrosine hydroxylase in the rat locus coeruleus. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7522–7526. doi: 10.1073/pnas.87.19.7522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavcovich L. A., Cancela L. M., Volosin M., Molina V. A., Ramirez O. A. Chronic stress-induced changes in locus coeruleus neuronal activity. Brain Res Bull. 1990 Feb;24(2):293–296. doi: 10.1016/0361-9230(90)90219-p. [DOI] [PubMed] [Google Scholar]
- Reis D. J., Joh T. H., Ross R. A., Pickel V. M. Reserpine selectively increases tyrosine hydroxylase and dopamine-beta-hydroxylase enzyme protein in central noradrenergic neurons. Brain Res. 1974 Dec 6;81(2):380–386. doi: 10.1016/0006-8993(74)90956-1. [DOI] [PubMed] [Google Scholar]
- Richard F., Faucon-Biguet N., Labatut R., Rollet D., Mallet J., Buda M. Modulation of tyrosine hydroxylase gene expression in rat brain and adrenals by exposure to cold. J Neurosci Res. 1988 May;20(1):32–37. doi: 10.1002/jnr.490200106. [DOI] [PubMed] [Google Scholar]
- Rivier C. L., Plotsky P. M. Mediation by corticotropin releasing factor (CRF) of adenohypophysial hormone secretion. Annu Rev Physiol. 1986;48:475–494. doi: 10.1146/annurev.ph.48.030186.002355. [DOI] [PubMed] [Google Scholar]
- Roy-Byrne P. P., Uhde T. W., Post R. M., Gallucci W., Chrousos G. P., Gold P. W. The corticotropin-releasing hormone stimulation test in patients with panic disorder. Am J Psychiatry. 1986 Jul;143(7):896–899. doi: 10.1176/ajp.143.7.896. [DOI] [PubMed] [Google Scholar]
- Roy A., Pickar D., Linnoila M., Chrousos G. P., Gold P. W. Cerebrospinal fluid corticotropin-releasing hormone in depression: relationship to noradrenergic function. Psychiatry Res. 1987 Mar;20(3):229–237. doi: 10.1016/0165-1781(87)90083-7. [DOI] [PubMed] [Google Scholar]
- Sakanaka M., Shibasaki T., Lederis K. Corticotropin releasing factor-like immunoreactivity in the rat brain as revealed by a modified cobalt-glucose oxidase-diaminobenzidine method. J Comp Neurol. 1987 Jun 8;260(2):256–298. doi: 10.1002/cne.902600209. [DOI] [PubMed] [Google Scholar]
- Segal D. S., Kuczenski R., Mandell A. J. Theoretical implications of drug-induced adaptive regulation for a biogenic amine hypothesis of affective disorder. Biol Psychiatry. 1974 Oct;9(2):147–159. [PubMed] [Google Scholar]
- Sherman J. E., Kalin N. H. ICV-CRH alters stress-induced freezing behavior without affecting pain sensitivity. Pharmacol Biochem Behav. 1988 Aug;30(4):801–807. doi: 10.1016/0091-3057(88)90103-7. [DOI] [PubMed] [Google Scholar]
- Svensson T. H. Peripheral, autonomic regulation of locus coeruleus noradrenergic neurons in brain: putative implications for psychiatry and psychopharmacology. Psychopharmacology (Berl) 1987;92(1):1–7. doi: 10.1007/BF00215471. [DOI] [PubMed] [Google Scholar]
- Takahashi L. K., Kalin N. H., Vanden Burgt J. A., Sherman J. E. Corticotropin-releasing factor modulates defensive-withdrawal and exploratory behavior in rats. Behav Neurosci. 1989 Jun;103(3):648–654. doi: 10.1037//0735-7044.103.3.648. [DOI] [PubMed] [Google Scholar]
- Thoenen H. Induction of tyrosine hydroxylase in peripheral and central adrenergic neurones by cold-exposure of rats. Nature. 1970 Nov 28;228(5274):861–862. doi: 10.1038/228861a0. [DOI] [PubMed] [Google Scholar]
- Vale W., Spiess J., Rivier C., Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981 Sep 18;213(4514):1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Valentino R. J., Foote S. L., Aston-Jones G. Corticotropin-releasing factor activates noradrenergic neurons of the locus coeruleus. Brain Res. 1983 Jul 4;270(2):363–367. doi: 10.1016/0006-8993(83)90615-7. [DOI] [PubMed] [Google Scholar]
- Valentino R. J., Wehby R. G. Corticotropin-releasing factor: evidence for a neurotransmitter role in the locus ceruleus during hemodynamic stress. Neuroendocrinology. 1988 Dec;48(6):674–677. doi: 10.1159/000125081. [DOI] [PubMed] [Google Scholar]
- Valentino R. J., Wehby R. G. Corticotropin-releasing factor: evidence for a neurotransmitter role in the locus ceruleus during hemodynamic stress. Neuroendocrinology. 1988 Dec;48(6):674–677. doi: 10.1159/000125081. [DOI] [PubMed] [Google Scholar]
- Zigmond R. E., Schon F., Iversen L. L. Increased tyrosine hydroxylase activity in the locus coeruleus of rat brain stem after reserpine treatment and cold stress. Brain Res. 1974 Apr 26;70(3):547–552. doi: 10.1016/0006-8993(74)90267-4. [DOI] [PubMed] [Google Scholar]
- Zigmond R. E., Schon F., Iversen L. L. Increased tyrosine hydroxylase activity in the locus coeruleus of rat brain stem after reserpine treatment and cold stress. Brain Res. 1974 Apr 26;70(3):547–552. doi: 10.1016/0006-8993(74)90267-4. [DOI] [PubMed] [Google Scholar]
- Zigmond R. E., Schwarzschild M. A., Rittenhouse A. R. Acute regulation of tyrosine hydroxylase by nerve activity and by neurotransmitters via phosphorylation. Annu Rev Neurosci. 1989;12:415–461. doi: 10.1146/annurev.ne.12.030189.002215. [DOI] [PubMed] [Google Scholar]