Abstract
Background
Metallo-β-lactamase-production among Gram-negative bacteria, including Pseudomonas aeruginosa, has become a challenge for treatment of infections due to these resistant bacteria.
Objectives
The aim of the current study was to evaluate the metallo-β-lactamase-production and carriage of bla-VIM genes among carbapenem-resistant P. aeruginosa isolated from burn wound infections.
Patients and Methods
A cross-sectional study was conducted from September 2014 to July 2015. One hundred and fifty P. aeruginosa isolates were recovered from 600 patients with burn wound infections treated at Imam-Musa-Kazem Hospital in Isfahan city, Iran. Carbapenem-resistant P. aeruginosa isolates were screened by disk diffusion using CLSI guidelines. Metallo-β-lactamase-producing P. aeruginosa isolates were identified using an imipenem-EDTA double disk synergy test (EDTA-IMP DDST). For detection of MBL genes including bla-VIM-1 and bla-VIM-2, polymerase chain reaction (PCR) methods and sequencing were used.
Results
Among the 150 P. aeruginosa isolates, 144 (96%) were resistant to imipenem by the disk diffusion method, all of which were identified as metallo-β-lactamase-producing P. aeruginosa isolates by EDTA-IMP DDST. Twenty-seven (18%) and 8 (5.5%) MBL-producing P. aeruginosa isolates harbored bla-VIM-1 and bla-VIM-2 genes, respectively.
Conclusions
Our findings showed a high occurrence of metallo-β-lactamase production among P. aeruginosa isolates in burn patient infections in our region. Also, there are P. aeruginosa isolates carrying the bla-VIM-1 and bla-VIM-2 genes in Isfahan province.
Keywords: Burn Patients, Metallo-β-Lactamase, blaVIM-1, blaVIM-1, Pseudomonas aeruginosa
1. Background
Burn wounds are major public health problems all over the world. Infection is one of the most complicated issues in burn patients, because the skin, a barrier against microbes, has been destroyed and the immunity agents cannot reach the sites of infection. There is a correlation between the severity of infection and the extent of the burn (1). It has been estimated that about 75% of burn-related mortality involves infections (2, 3). The rate of nosocomial infections among patients hospitalized in burn units is high, and P. aeruginosa has been documented as the most prevalent isolated bacteria in these patients (4, 5). Pseudomonas aeruginosa is characterized by an innate resistance to multiple antimicrobial agent as well as by acquired multidrug resistance ability. Due to multi-drug resistant P. aeruginosa isolates, treating these patients’ burn wound infections is challenging (6). Carbapenems are a group of β-lactams that show resistance to hydrolysis by extended-spectrum β-lactamases (ESBLs), so are used as the drug of choice for treatment of infections caused by ESBL-positive Gram negative bacteria (7). Broad spectrum activity as well as these abilities have led to increased used of carbapenems, especially imipenem, for treatment of nosocomial infections (8). Unfortunately, the emergence of metallo-β-lactamase (MBL), the enzyme that inactivates carbapenems, has prevented the use of these antibiotics (9). In a study conducted at Ghotbeddin Burn hospital in Shiraz, 55 (20.4%) MBL-producing P. aeruginosa were identified among 270 P. aeruginosa isolated patients hospitalized in the burn unit (1). MBLs are categorized as Ambler class B (10). These β-lactamase groups of enzymes have resistance to all β-lactamase inhibitors, and due to the presence of zinc ion in their active sites, are inactivated by chelating agents such as ethylene diamine tetra acetic acid (EDTA) (9). Metallo-β-lactamase-production is encoded by either chromosomal genes or genes are inserted in mobile genetic elements, which especially facilitate the spread of these genes among Gram negative bacteria (9). Recently, several new MBL codes such as VIM have been documented in P. aeruginosa isolates (7). The three main clusters of VIM MBL have been reported, including VIM-1, VIM-2, and VIM-7 (7), and the VIM type metallo-β-lactamase genes are shown to encode by inserted cassettes in the mobile integrons (7, 11). The rate of the infections caused by P. aeruginosa in the burn unit of Imam-Musa-Kazem hospital in Isfahan was high; therefore rapid identification of MBL-producing P. aeruginosa strains is important.
2. Objectives
In the present study we determined the metallo-β-lactamase-production and carriage of bla-VIM genes among carbapenem-resistant P. aeruginosa isolates in burn patients in Isfahan Province.
3. Patients and Methods
3.1. Subjects
This cross-sectional study was conducted on 600 patients, who were hospitalized in the burn unit of Imam-Musa-Kazem university Hospital in Isfahan between September 2014 and July 2015.
3.2. Bacterial strains
For sampling, wound swabs were obtained from the burn patients and P. aeruginosa was identified by standard microbiological methods including Gram stains, culture on mediums such as blood agar, MacConkey agar, triple sugar iron agar (TSI), cetrimide agar (Merck, UK), oxidase (Sigma, USA), catalase, oxidative/fermentative (OF), indole, methyl red (MR), Voges-Proskauer (VP) tests (Merck, UK), and growth at 42°C.
3.3. Screening of Carbapenem-Resistant and MBL Producing Pseudomonas aeruginosa Isolates
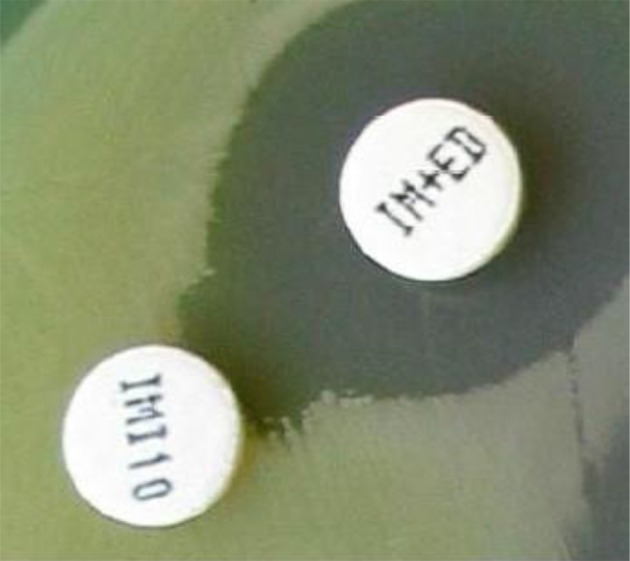
The disk diffusion method was used according to the clinical and laboratory standards institute (CLSI) guidelines for detection of carbapenem-resistant P. aeruginosa isolates, and the imipenem (10µg) and meropenem (10µg) disks were purchased from Mast, UK Company (12). A P. aeruginosa standard strain (ATCC 27853) was used for quality control in susceptibility testing. Phenotypic screening for MBL producers P. aeruginosa isolates was performed using an EDTA double disk synergy test (EDTA-IMP DDST) in which imipenem resistant isolates selected by the disk diffusion method were examined. Briefly, a colony of each P. aeruginosa strain was suspended in Mueller-Hinton (MH) broth to 106 CFU/mL. after being spread on an MH agar plate with a cotton swab. Two commercial disks containing 10µg imipenem with (10µg/750μg) and without EDTA were placed on the center of the plate at a distance of 25 mm from each other, and were incubated at 37°C overnight. After overnight incubation, the presence of a ≥ 7 mm synergistic inhibition zone around the IMP-EDTA disk in comparison to the IMP disk was interpreted as positive (13).
3.4. Polymerase Chain Reaction Detection of Genes for MBLs
DNA from each P. aeruginosa isolate was extracted by the boiling method. Templates of the DNA were stored at -20°C until PCR amplification was performed. MBL-producing P. aeruginosa strains which were identified by a DDST confirmatory test were amplified by the PCR method, using bla-VIM-1 and bla-VIM-2 specific primers (Table 1) for amplification of 261 bp and 801 bp fragments, respectively (14).
Table 1. Primers Used for Polymerase Chain Reaction and Sequencing.
| Primer | Target Gene | Sequence (5’→3’) | Amplification Product, bp |
|---|---|---|---|
| VIM-1 F | bla VIM-1 | AGT GGT GAG TAT CCG ACA G | 261 |
| VIM-1 R | bla VIM-1 | ATG AAA GTG CGT GGA GAC | |
| VIM-2 F | bla VIM-2 | ATG TTC AAA CTT TTG AGT AAG | 801 |
| VIM-2 R | bla VIM-2 | CTA CTC AAC GAC TGA GCG |
Reactions were carried out using a thermo cycler (Eppendorf master cycler®, MA) in the PCR conditions previously described (14). For confirmation, some PCR products were sent elsewhere for sequencing (Bioneer, South Korea). The nucleotide sequences were analyzed by the program Chromas Pro version1.7.5 Technelysium (www.technelysium.com.au), and were aligned with GenBank to check the identity of each sequence against those deposited in GenBank.
3.5. Ethical Considerations
Each patient’s demographic information was obtained by a survey questionnaire, and a written informed consent was received from all patients or their parents. The study design, including its ethical aspects, was reviewed and approved by the ethics committee of Kashan University of Medical Sciences (No. 4380).
3.6. Statistical Analysis
The statistical analysis of data was conducted using SPSS software version 16 (SPSS, Inc.). Differences by the χ2 test were considered statistically significant if the P value was less than 0.05 (P < 0.05).
4. Results
Of 600 burn patients, who had been hospitalized in the burn unit of Imam-Musa-Kazem university hospital in Isfahan, a total of 150 P. aeruginosa isolates were recovered. The patients with isolates included 69 (46.0%) females and 81 (54.0%) males, and their ages ranged between 1 and 72 years old (Table 2). Of the 150 P. aeruginosa isolates identified by standard biochemical tests, 144 (96.0%) and 144 (96.0%) showed resistance to imipenem and meropenem, respectively, by the disk diffusion method. An EDTA-IMP DDST phenotypic confirmatory test revealed that 100% of the imipenem resistant P. aeruginosa isolates were metallo-β-lactamase producing (Figure 1).
Table 2. Association Between Burn Patients’ Demographic Characteristics and blaVIM Genes Carriage Among Pseudomonas aeroginosa Isolates.
| Factor | bla VIM-1 | P Value | bla VIM-2 | P Value | Total, No. (%) | ||
|---|---|---|---|---|---|---|---|
| Positive, No. (%) | Negative, No. (%) | Positive, No. (%) | Negative, No. (%) | ||||
| Sex | |||||||
| Male | 20 (13.3) | 61 (40.7) | 0.374 | 4 (2.7) | 77 (51.3) | 0.449 | 81 (54.0) |
| Female | 7 (4.7) | 62 (41.3) | 4 (2.7) | 65 (43.3) | 69 (46.0) | ||
| Age group, y | |||||||
| Less than 30 | 5 (3.3) | 25 (16.7) | 0.812 | 1 (0.7) | 29 (19.3) | 1 | 30 (20.0) |
| 30 - 49 | 21 (14.0) | 90 (60.0) | 3 (2.0) | 108 (72.0) | 111 (74.0) | ||
| More than 50 | 1 (0.7) | 8 (5.3) | 4 (2.7) | 5 (3.3) | 9 (6.0) | ||
| Burn level (38.85 ± 7.58) | |||||||
| Equal and less than 35 | 7 (4.7) | 24 (16.0) | 0.041 | 3 (2.0) | 28 (18.7) | 0.032 | 31 (20.7) |
| More than 35 | 20 (13.3) | 99 (66.0) | 5 (3.3) | 114 (76.0) | 119 (79.3) | ||
| Burn cause | |||||||
| Fire and flame | 13 (8.7) | 49 (32.6) | 0.161 | 4 (2.7) | 58 (38.6) | 0.212 | 62 (41.3) |
| Chemical | 5 (3.3) | 14 (9.4) | 0 | 19 (12.7) | 19 (12.7) | ||
| Boiled water and hot liquids | 9 (6.0) | 60 (40.0) | 4 (2.7) | 65 (43.3) | 69 (46.0) | ||
Figure 1. Metallo-β-Lactamase Producing Pseudomonas aeruginosa Isolates Identified By a Imipenem-EDTA Double Disk Synergy Test (EDTA-IMP DDST) Phenotypic Confirmatory Test.
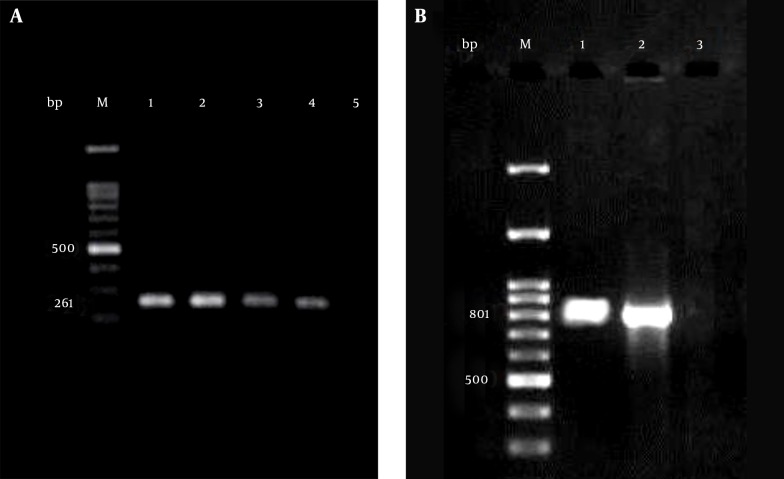
The finding of the PCR assays showed that of 144 MBL-producing P. aeruginosa isolates, 27 (18%) and 8 (5.5%) were positive for the bla-VIM-1 and bla-VIM-2 genes, respectively (Figure 2). The nucleotide sequences of the PCR products of the bla-VIM-1 and bla-VIM-2 genes were identical to those deposited in GenBank.
Figure 2. Amplification of blaVIM-1 and blaVIM-2 Genes in Imipenem Resistant MBL Positive Pseudomonas aeruginosa Isolates.
M lanes, 100-bp DNA ladder as the molecular size marker; A, lane 1, positive control; lanes 2 - 4, blaVIM-1 positive Pseudomonas aeruginosa isolates; lane 5, negative control; B, lane 1, positive control; lane 2, blaVIM-2 positive Pseudomonas aeruginosa isolate; lane 3, negative control.
5. Discussion
Pseudomonas aeruginosa is documented as one of the most frequently isolated bacteria in nosocomial infections, especially in burn units (3). Severe antimicrobial resistance in this bacterium has complicated the treatment of infections caused by drug-resistant P. aeruginosa strains among burn patients (15). Recently, the increase of carbapenem resistance in P. aeruginosa has been documented all over the world due to metallo-β-lactamase production, including the VIM type (16). This study revealed that the resistance rates to imipenem and meropenem were very high (96%). Farajzadeh Sheikh et al. (15) reported an imipenem resistance rate of 58.7% among P. aeruginosa isolates in Ahwaz, which was lower than our resistance rate. However, Anvarinejad and colleagues’ (1) study, also conducted in Ahwaz, was in agreement with our study: 98.1% of MBL-producing P. aeruginosa strains, which were isolated from hospitalized patients in a burn unit showed resistance to imipenem. Another report, in contrast with our study, found a resistance rate of 21% to imipenem among P. aeruginosa samples from burn patients in Kurdistan (3). As carbapenems, including imipenem, are the drugs of choice for treatment of infections caused by MDR-P. aeruginosa strains, this result in particularly alarming for burn patients with immune-compromised defenses. The high resistance rates to carbapenems may be relatively imprecise because they were not confirmed by confirmatory tests such as an E-test. The difference in resistance rates to imipenem in P. aeruginosa isolates from burn patients in Ahwaz, Isfahan, and Kurdistan could be due to frequent use of this antibiotic and the occurrence of high selective pressure in Ahwaz and Isfahan, and fewer patients’ access to antibiotics due to economic problems in Kurdistan.
MBL-producing P. aeruginosa have been isolated from hospitalized patients increasingly, and isolates that produce these families of enzymes have been documented as one cause of prolonged nosocomial infections (17). Here we showed that all imipenem-resistant P. aeruginosa isolates had the ability of MBL-production. In Kurdistan and Ahwaz 22% and 19.5% of P. aeruginosa isolates among burn patients were identified as MBL-producing isolates (3). In a study conducted by Farajzadeh Sheikh et al. (15) in Ahwaz among P. aeruginosa isolates in burn and non-burn patients, all of the imipenem-resistant isolates were MBL E-test positive, which correlates with our results, although it would be better if the MBL-production among our imipenem resistant P. aeruginosa isolates were confirmed by an MBL E-test as well. The EDTA-IMP DDST test and sodium mercaptoacetic acid (SMA)-IMP DDST revealed that 98.1% of imipenem-resistant P. aeruginosa and 78.9% of P. aeruginosa isolates were positive for production of metallo-β-lactamase enzymes in Brazil and Japan, respectively (8, 14). This finding shows that the prevalence of carbapenemases among nosocomial isolates of P. aeruginosa is high, especially in burn units.
It has been estimated that more than 40% of imipenem resistance in P. aeruginosa (IRPA) is due to MBL production, although enzyme types vary in different regions (18, 19). In the present study, 18% and 5.5% of MBL positive isolates were VIM-1 and VIM-2 MBL types, respectively. Similar to our finding, 19.3% of MBL-producing P. aeruginosa isolates in burn patients in Ahwaz were positive for bla-VIM genes (7). In a study in Brazil, 19% of IRPA isolates which were MBL-positive were shown to carry bla-VIM-2, whereas bla-VIM-1 was not detected among them (8). The fact that the bla-VIM-2 gene was detected in 48.8% of IRPA clinical isolates in Romania (20) shows that MBL type enzymes can vary geographically. Another interesting finding was that the remaining 109 IRPA isolates, which were MBL positive by a phenotypic test, proved to be negative for bla-VIM-1 and bla-VIM-2 genes. The presence of other types of MBL genes could be the reason.
Overall, the findings of the current study showed that the resistance rate to carbapenems, as the last-resort therapy in infections due to MDR P. aeruginosa strains among burn patients, is high. Therefore, in these patients a regular surveillance program for controlling infection and in vitro testing of antibiotics before empiric treatment could be helpful to control MDR-Pseudomonas aeruginosa infections in the burn units. Also, a high occurrence of MBL production among imipenem-resistant P. aeruginosa isolates in burn patients was seen. Further studies using more accurate confirmatory tests in our region are recommended.
Acknowledgments
We are grateful to Mr. Radan for collecting the samples and helping with technical assistance.
Footnotes
Authors’ Contributions:Farzaneh Firoozeh contributed to the study design, study management, supervision, and prepared and wrote the manuscript. Mohammad Pourbabaee performed the sampling, processing, and conventional and molecular procedures. Mohammad Zibaei provided advice, and read and arranged the final manuscript. Mahmood Saffari provided advice.
References
- 1.Anvarinejad M, Japoni A, Rafaatpour N, Mardaneh J, Abbasi P, Amin Shahidi M, et al. Burn Patients Infected With Metallo-Beta-Lactamase-Producing Pseudomonas aeruginosa: Multidrug-Resistant Strains. Arch Trauma Res. 2014;3(2):18182. doi: 10.5812/atr.18182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poole K. Multidrug efflux pumps and antimicrobial resistance in Pseudomonas aeruginosa and related organisms. J Mol Microbiol Biotechnol. 2001;3(2):255–64. [PubMed] [Google Scholar]
- 3.Kalantar E, Torabi V, Salimizand H, Soheili F, Ramezanzadeh R. Incidence and Susceptibility Pattern of Metallo-Beta-Lactamase Producers Among Pseudomonas aeruginosa Isolated From Burn Patients at Kurdistan Province. Jundishapur J Microbiol. 2012;5(3):507–10. doi: 10.5812/jjm.3664. [DOI] [Google Scholar]
- 4.Agnihotri N, Gupta V, Joshi RM. Aerobic bacterial isolates from burn wound infections and their antibiograms--a five-year study. Burns. 2004;30(3):241–3. doi: 10.1016/j.burns.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 5.Weldhagen GF, Poirel L, Nordmann P. Ambler class A extended-spectrum beta-lactamases in Pseudomonas aeruginosa: novel developments and clinical impact. Antimicrob Agents Chemother. 2003;47(8):2385–92. doi: 10.1128/AAC.47.8.2385-2392.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porras-Gomez M, Vega-Baudrit J, Nunez-Corrales S. Overview of Multidrug-Resistant Pseudomonas aeruginosa; and Novel Therapeutic Approaches. J Biomater Nanobiotechnol. 2012;03(04):519–27. [Google Scholar]
- 7.Khosravi AD, Mihani F. Detection of metallo-beta-lactamase-producing Pseudomonas aeruginosa strains isolated from burn patients in Ahwaz, Iran. Diagn Microbiol Infect Dis. 2008;60(1):125–8. doi: 10.1016/j.diagmicrobio.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Franco MR, Caiaffa-Filho HH, Burattini MN, Rossi F. Metallo-beta-lactamases among imipenem-resistant Pseudomonas aeruginosa in a Brazilian university hospital. Clinics (Sao Paulo). 2010;65(9):825–9. doi: 10.1590/S1807-59322010000900002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wirth FW, Picoli SU, Cantarelli VV, Goncalves AL, Brust FR, Santos LM, et al. Metallo-beta-lactamase-producing Pseudomonas aeruginosa in two hospitals from southern Brazil. Braz J Infect Dis. 2009;13(3):170–2. doi: 10.1590/s1413-86702009000300003. [DOI] [PubMed] [Google Scholar]
- 10.Doosti M, Ramazani A, Garshasbi M. Identification and characterization of metallo-beta-lactamases producing Pseudomonas aeruginosa clinical isolates in University Hospital from Zanjan Province, Iran. Iran Biomed J. 2013;17(3):129–33. doi: 10.6091/ibj.1107.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yatsuyanagi J, Saito S, Harata S, Suzuki N, Ito Y, Amano K, et al. Class 1 integron containing metallo-beta-lactamase gene blaVIM-2 in Pseudomonas aeruginosa clinical strains isolated in Japan. Antimicrob Agents Chemother. 2004;48(2):626–8. doi: 10.1128/AAC.48.2.626-628.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clinical and Laboratory Standards Institute. Performance Standards for antimicrobial susceptibility testing 21th information supplement. USA: CLSI; 2012. [Google Scholar]
- 13.Lee K, Lim YS, Yong D, Yum JH, Chong Y. Evaluation of the Hodge test and the imipenem-EDTA double-disk synergy test for differentiating metallo-beta-lactamase-producing isolates of Pseudomonas spp. and Acinetobacter spp. J Clin Microbiol. 2003;41(10):4623–9. doi: 10.1128/JCM.41.10.4623-4629.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shibata N, Doi Y, Yamane K, Yagi T, Kurokawa H, Shibayama K, et al. PCR typing of genetic determinants for metallo-beta-lactamases and integrases carried by gram-negative bacteria isolated in Japan, with focus on the class 3 integron. J Clin Microbiol. 2003;41(12):5407–13. doi: 10.1128/JCM.41.12.5407-5413.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farajzadeh Sheikh A, Rostami S, Jolodar A, Tabatabaiefar MA, Khorvash F, Saki A, et al. Detection of Metallo-Beta Lactamases Among Carbapenem-Resistant Pseudomonas aeruginosa. Jundishapur J Microbiol. 2014;7(11):12289. doi: 10.5812/jjm.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Touati M, Diene SM, Dekhil M, Djahoudi A, Racherache A, Rolain JM. Dissemination of a class I integron carrying VIM-2 carbapenemase in Pseudomonas aeruginosa clinical isolates from a hospital intensive care unit in Annaba, Algeria. Antimicrob Agents Chemother. 2013;57(5):2426–7. doi: 10.1128/AAC.00032-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alaghehbandan R, MacKay Rossignol A, Rastegar Lari A. Pediatric burn injuries in Tehran, Iran. Burns. 2001;27(2):115–8. doi: 10.1016/s0305-4179(00)00083-8. [DOI] [PubMed] [Google Scholar]
- 18.Livermore DM. Of Pseudomonas, porins, pumps and carbapenems. J Antimicrob Chemother. 2001;47(3):247–50. doi: 10.1093/jac/47.3.247. [DOI] [PubMed] [Google Scholar]
- 19.Livermore DM. Interplay of impermeability and chromosomal beta-lactamase activity in imipenem-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1992;36(9):2046–8. doi: 10.1128/aac.36.9.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mereuta AI, Badescu AC, Dorneanu OS, Iancu LS, Tuchilus CG. Spread of VIM-2 metallo-beta-lactamase in Pseudomonas aeruginosa and Acinetobacter baumannii clinical isolates from Iaşi, Romania. RRLM. 2013;21(4):423–30. [Google Scholar]