Abstract
Background
Homocysteine (Hcy) has been considered as an independent risk factor for coronary artery disease (CAD). Folic acid and vitamin B12 are two vital regulators in Hcy metabolic process. We evaluated the correlations between serum Hcy, folic acid and vitamin B12 with the categories of CAD.
Methods
Serum Hcy, folic acid and vitamin B12 from 292 CAD patients, including 73 acute myocardial infarction (AMI), 116 unstable angina pectoris (UAP), 103 stable angina pectoris (SAP), and 100 controls with chest pain patients were measured, and the data were analyzed by SPSS software.
Results
Compared to SAP patients, patients with AMI and UAP had higher Hcy levels with approximately average elevated (4-5) μmol/L, while SAP patients were approximately higher 8 μmol/L than controls. However, the levels of folic acid and vitamin B12 had opposite results, which in AMI group was the lowest, while in controls was the highest. CAD categories were positively correlated with Hcy (r = 0.286, p < 0.001), and negatively correlated with folic acid (r = -0.297, p < 0.001) and vitamin B12 (r = -0.208, p < 0.001). There were significant trend toward increase in the prevalence of high Hcy, low folic acid and vitamin B12 from controls, to SAP, to UAP, and to AMI.
Conclusions
The present study provide the valuable evidence that high concentrations of Hcy and low levels of folic acid and vitamin B12 are significantly correlated with CAD categories.
Keywords: Homocysteine, Folic acid, Vitamin B12, Coronary artery disease, Atherosclerosis, Endothelial dysfunction
Background
Coronary artery disease (CAD) is seriously to harm people’s healthy disease in borth developed and developing countries, which was predominantly caused by atherosclerosis with endothelial dysfunction [1, 2]. Despite best efforts, available therapies protect only 30–40% of individuals at risk, and no therapeutic cure is anticipated for those who currently suffer from the disease [3]. The endothelium is a single layer of cells lining all blood vessels. It plays an important role in many physiological functions, including the control of blood cell trafficking, vasomotor tone, vessel permeability, and hemostatic balance. Endothelial cells produce a wide variety of substances in response to various physical and chemical stimuli, including vasodilator substances, and vasoconstrictor substances [4].
Researches have confirmed that endothelial dysfunction, as an impairment of endothelium-dependent relaxation of blood vessels, occur as the initial event in the pathogenesis of atherosclerosis, which considered to be the initiating factor and the key point of cardiovascular disease [5, 6]. Moreover, endothelial dysfunction also play the important role in all stages and categories of CAD from stable angina pectoris (SAP) to unstable angina pectoris (UAP), and to acute myocardial infarction (AMI) [7]. Early warning and immediate risk stratification of patients with different categories of CAD is frequently a challenging task in the current.
A large number of studies have confirmed that serum high homocysteine (Hcy) concentration (morm than 15 μmol/L), which is called hyperhomocysteinemia (HHcy), has been associated with endothelial dysfunction of atherosclerotic CAD owing to oxidative stress [8], endoplasmic reticulum stress [9], involved inflammation [10], increased level of asymmetric dimethylarginine (ADMA) [11] and so on [12–14]. Elevated ADMA can results in decreasing endothelium-derived nitric oxide concentration and bioavailability [15]. Nitric oxide as a most important mediator of endothelium-dependent relaxation, is a potent vasodilator, which plays a key role in normal vascular physiology in preserving the vessel wall in a quiescent state by inhibition of inflammation, thrombosis, and cellular proliferation [16]. Decreased nitric oxide bioavailability would result in the abnormal thrombosis, vasorelaxation, and atherosclerosis, thereby promoting the occurrence and development of CAD [17].
Folic acid and vitamin B12 play an important role in regulating the metabolic process of Hcy [18]. Current studies have shown that supplement folic acid, vitamin B12 in patients with HHcy could reduce Hcy levels [19]. Folic acid supplementation not only may be useful in reducing Hcy level in high risk patients with HHcy [20], but also can significantly improve endothelial dysfunction in patients with CAD [21]. On the other hand, folic acid deficiency or/and vitamin B12 deficiency would result in HHcy [22–24]. Vitamin B12 deficiency and HHcy are related to cardiovascular risk factors in patients with CAD [25]. However, the correlations of CAD categories with Hcy, folic acid, vitamin B12 have not been reported. Therefore, we evaluate the correlations between CAD categories and each of the metabolic parameters, anthropometric variables, life style habits and traditional cardiovascular risk factors in CAD patients and controls with chest pain patients.
Methods
This study included 292 CAD patients (203 male and 89 female) aged 36–85 (62.54 ± 14.52) years, and 100 controls with chest pain symptom (69 male and 31 female) aged 38–87 (60.93 ± 15.65) years, from the Sichuan Academy of Medical Sciences & Sichuan Provincial People’s Hospital. There were no statistically significant difference in age (means ± SD, t = 0.94, p = 0.348) and gender (male to female ratio, χ 2 = 0.01, p = 0.922) between CAD group and controlled group.
All enrolled CAD patients had been confirmed by coronary angiography and were diagnosed to be103 SAP, 116 UAP, 73 AMI according to 2007 ACC/AHA guidelines. 100 controls with chest pain patients in the same period were confirmed by coronary angiography too. Patients with the following diseases were excluded from this study: cancer, liver diseases, renal insufficiency, blood diseases, hyperthyroidism, thyroid dysfunction, systemic lupus erythematosus, malnutrition, pregnant woman, and supplemented folic acid and vitamin B12.
Participating subjects were explained their participation rights and written informed consent was obtained, and were asked about alcohol intake situation (yes or no, it was defined as yes at least once a week and drinking over 45° of alcohol more than 200 mL) and smoking habits (yes or no, non-smokers including never smoking and stop smoking more than 1 year).
The data was consecutively collected from October 2013 to September 2014. Fasting blood was sampled in the morning within 24 h that the patients had been admitted to hospital. The blood must be collected before the heparinization of coronary angiography. Moreover, AMI patient’s blood was collected before percutaneous coronary intervention and thrombolytic treatment. After blood was separated, a fresh serum were used with Hitachi 7600 Automatic Biochemistry Analyzer (Hitachi High-Tech Instruments Co., Ltd., Japan) for the determinations of Hcy, total cholesterol (TC), triglyceride (TG), high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), glucose (GLU) and uric acid (UA). Another fresh serum were used for the determinations of folic acid and vitamin B12 by ACCESS 2 Immunoassay System (Beckman Coulter, Inc., USA).
High Hcy, folic acid and vitamin B12 were defined as Hcy, folic acid and vitamin B12 greater than 15 μmol/L, 26.0 nmol/L and 675 pmol/L, respectively, while low Hcy, folic acid and vitamin B12 were defined as Hcy, folic acid and vitamin B12 less than 5 μmol/L, 6.8 nmol/L and 133 pmol/L, respectively, according to their references intervals were (5–15) μmol/L for Hcy, (6.8–26.0) nmol/L for folic acid and (133–675) pmol/L for vitamin B12, respectively. Systolic blood pressure (SBP) and diastolic blood pressure (DBP), body weight and height were measured with standard techniques. Body mass index (BMI) was calculated as body weight (kg) divided by the square of height (m).
Hypercholesterolemia and hypertriglyceridemia were defined as TC ≥ 6.22 mmol/L and TG ≥ 2.26 mmol/L, respectively, according to 2007 China Adult Dyslipidemia Prevention Guide. Diabetes mellitus was diagnosed when patients’ GLU ≥ 7.0 mmol/L. Hypertension was diagnosed when patients’ SBP ≥ 140 mmHg or DBP ≥ 90 mmHg. Overweight and obesity were defined as BMI (24.0–27.9) kg/m2, and ≥ 28 kg/m2, respectively, according to 2006 Guidelines for Prevention and Control of Overweight and Obesity in Chinese Adults.
Statistical analysis
The data were analyzed by using the statistical package for social science SPSS software version 16.0 (SPSS, Inc., Chicago, IL, USA). Continuous variables were expressed as mean ± SD because the data presented in this study showed a normal distribution. Means ± SD of two samples were compared by the Independent-Sample t-Test, and means ± SD of more than two samples were compared with the One-Way ANOVA. Categorical variables were expressed as percentage and compared by χ 2-test. The correlation coefficients of CAD categories with each of the metabolic parameters, anthropometric variables and life style habits were calculated by Spearman’s analysis because CAD is a grade variable, while the correlation study between Hcy and folic acid as well as vitamin B12 were performed on the measured data by using Pearson’s correlation a coefficient because Hcy, folic acid and vitamin B12 are continuous variables with normal distribution. A p-value < 0.05 was considered as significant.
Results
Comparison of principal characteristics between high Hcy, normal Hcy and low Hcy levels in CAD patients
Compared to normal and low Hcy groups, High Hcy group were characterized by smoking, Diabetes mellitus, hypercholesterolemia, hypertriglyceridemia, low folic acid, low vitamin B12, low HDL-C and high LDL-C (p < 0.05). There were no significant differences in the ratio of elder age, male, female, alcohol drinking, hypertension, overweight and obesity among three groups. The comparison of principal characteristics between high Hcy, normal Hcy and low Hcy levels in 292 CAD patients are reported in Table 1.
Table 1.
Comparison of principal characteristics between high Hcy, normal Hcy and low Hcy levels in 292 CAD patients
| High Hcy group (n = 231) | Normal Hcy group (n = 58) | Low Hcy group (n = 3) | χ 2 | p value | |
|---|---|---|---|---|---|
| Elder age ≥ 61 (%) | 61.47 | 51.72 | 33.33 | 2.67 | 0.264 |
| Male (%) | 71.00 | 63.79 | 66.67 | 1.15 | 0.564 |
| Female (%) | 29.00 | 36.21 | 33.33 | 1.15 | 0.564 |
| Smoking (%) | 29.87 | 13.79 | 33.33 | 6.19 | 0.045 |
| Alcohol drinking (%) | 39.39 | 34.48 | 66.67 | 1.47 | 0.479 |
| Hypertension (%) | 63.20 | 56.90 | 33.33 | 1.81 | 0.405 |
| Diabetes mellitus (%) | 30.30 | 13.79 | 33.33 | 6.46 | 0.039 |
| Hypercholesterolemia (%) | 46.32 | 25.86 | 0 | 10.15 | 0.006 |
| Hypertriglyceridemia (%) | 30.74 | 13.79 | 66.67 | 8.93 | 0.012 |
| Overweight and Obesity (%) | 40.26 | 44.83 | 33.33 | 0.48 | 0.789 |
| Low folic acid (%) | 51.08 | 6.90 | 0 | 39.39 | <0.001 |
| Low vitamin B12 (%) | 41.99 | 8.62 | 0 | 24.34 | <0.001 |
| Low HDL-C (%) | 38.96 | 20.69 | 0 | 8.44 | 0.015 |
| High LDL-C (%) | 39.83 | 22.41 | 0 | 7.81 | 0.020 |
More than half of the CAD patients (51.08%, 118/231) with high Hcy had low folic acid levels, 7 times higher than that (6.56%, 4/61) in CAD patients with normal-low Hcy concentrations (p < 0.001), and 41.99% (97/231) CAD patients with high Hcy had low vitamin B12 levels, 5 times higher than that (8.20%, 5/61) in CAD patients with normal-low Hcy concentrations (p < 0.001).
Comparison of Hcy, folic acid and vitamin B12 between CAD and controls
AMI patients had the highest serum concentrations of Hcy, and UAP patients were a little lower than AMI but were the second highest. and SAP patients had the third higher level of Hcy, which were significantly higher than controls (p < 0.001). Compared to SAP patients, patients with AMI and UAP had higher Hcy levels with approximately average elevated (4-5) μmol/L, while SAP patients were approximately higher 8 μmol/L than controls. However, the levels of folic acid and vitamin B12 had opposite results, which in AMI group had the lowest, while in controlled group had the highest. The comparison of Hcy, folic acid and vitamin B12 between AMI, UAP, SAP groups and controls are shown in Table 2.
Table 2.
Comparison of Hcy, folic acid and vitamin B12 between AMI, UAP, SAP patients and controlled group with chest pain patients
| Groups | Number | Hcy (μmol/L) | Folic acid (nmol/L) | Vitamin B12 (pmol/L) |
|---|---|---|---|---|
| Controls | 100 | 10.81 ± 4.62 | 12.86 ± 5.85 | 222.34 ± 62.58 |
| Stable angina pectoris | 103 | 18.63 ± 6.73a | 10.33 ± 4.95b | 167.52 ± 56.25b |
| Unstable angina pectoris | 116 | 22.62 ± 6.37ac | 9.21 ± 4.38bd | 148.65 ± 62.51bd |
| Acute myocardial infarction | 73 | 23.44 ± 5.78ac | 7.08 ± 3.43bde | 144.57 ± 52.24bd |
| F | 90.51 | 22.05 | 35.63 | |
| p value | <0.001 | <0.001 | <0.001 |
aSignificantly increased compared to controls, bSignificantly decreased compared to controls, cSignificantly increased compared to SAP group, dSignificantly decreased compared to SAP group, eSignificantly decreased compared to UAP group
Correlation coefficients of CAD categories with each of the variables and the correlations between Hcy with folic acid and vitamin B12
The correlation coefficients of CAD categories with each of the metabolic parameters, anthropometric variables and life style habits by Spearman’s analysis in 292 CAD patients are shown in Table 3. CAD categories were positively correlated with Hcy, TC, TG, LDL-C, age, SBP, DBP, BMI, gender and smoking, and negatively correlated with folic acid, vitamin B12 and HDL-C levels. On the contrary, CAD categories were not significantly correlated with GLU, UA and alcohol drinking. Among them, Hcy and folic acid showed the highest positively and negatively correlated with CAD categories, respectively.
Table 3.
Spearma’s correlation coefficients of CAD categories with each of the metabolic parameters, anthropometric variables and life style habits in 292 CAD patients
| Variable | CAD categories | p value |
|---|---|---|
| Homocysteine | 0.286 | <0.001 |
| Folic acid | -0.297 | <0.001 |
| Vitamin B12 | -0.208 | <0.001 |
| TC | 0.242 | <0.001 |
| TG | 0.141 | 0.016 |
| HDL-C | -0.153 | 0.009 |
| LDL-C | 0.187 | 0.001 |
| GLU | 0.088 | 0.132 |
| UA | 0.078 | 0.182 |
| Age | 0.151 | 0.010 |
| SBP | 0.135 | 0.021 |
| DBP | 0.125 | 0.032 |
| BMI | 0.148 | 0.011 |
| Gender | 0.128 | 0.028 |
| Smoking | 0.278 | <0.001 |
| Alcohol drinking | 0.072 | 0.218 |
Hcy homocysteine, TC total cholesterol, TG triglyceride, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, GLU glucose, UA uric acid, SBP systolic blood pressure, DBP diastolic blood pressure, BMI body mass index
Pearson’s correlation analysis showed that there were strongly moderate negative correlations between Hcy and folic acid (r = -0.666, p < 0.001) and vitamin B12 (r = -0.564, p < 0.001).
Prevalence of high Hcy, and low folic acid and vitamin B12 in CAD patients and controls with chest pain patient
Approximately four-fifths of CAD patients (79.11%, 231/292) had a prevalence of high Hcy. However, the levels of folic acid and vitamin B12 in CAD patients were reduced, the prevalence were 41.78% (122/292) for folic acid, and 34.93% (102/292) for vitamin B12, respectively. The prevalence of high Hcy, and low folic acid and vitamin B12 in 292 CAD patients and 100 controls with chest pain patient are shown in Table 4 and Fig. 1, respectively. There was a significant trend toward an increase in the prevalence of high Hcy from controls, to SAP, to UAP, and to AMI. The prevalence of high Hcy progressively increased from 5.00% in controls, to 66.02% in SAP group, to 81.90% in UAP group, and to 93.15% in AMI group (p < 0.001). Low folic acid and vitamin B12 also had significant trend toward rise in the prevalence from controls, to SAP, to UAP, and to AMI. The prevalence of low folic acid progressively increased from 13.00% in controls, to 32.04% in SAP group, to 39.66% in UAP group, and to 58.90% in AMI group, respectively (p < 0.001). Similarly, the prevalence of low vitamin B12 progressively increased from 15.00% in controls, to 24.27% in SAP group, to 34.48% in UAP group, and to 50.68% in AMI group, respectively (p < 0.001).
Table 4.
The prevalence of high Hcy, low folic acid and vitamin B12 in 292 CAD patients and 100 controls with chest pain patient (%)
| Number | High Hcy (n = 236) | Low folic acid (n = 135) | Low vitamin B12 (n = 117) | |
|---|---|---|---|---|
| Controls | 100 | 5.00 | 13.00 | 15.00 |
| Stable angina pectoris | 103 | 66.02 | 32.04 | 24.27 |
| Unstable angina pectoris | 116 | 81.90 | 39.66 | 34.48 |
| Acute myocardial infarction | 73 | 93.15 | 58.90 | 50.68 |
| χ 2 | 184.51 | 41.37 | 28.39 | |
| p value | <0.001 | <0.001 | <0.001 |
Fig. 1.
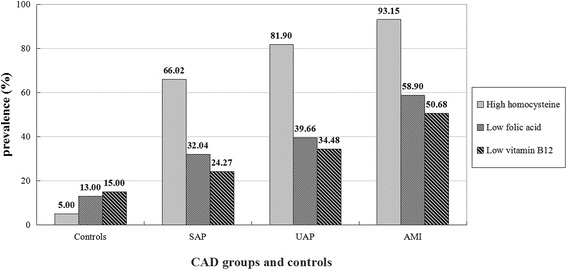
Prevalence of high Hcy, low folic acid and vitamin B12 in 292 CAD patients and 100 controls with chest pain patient aged 36–87 years
Discussion
CAD is a multifactorial disease, and these factors are called risk factors include a large number of traditional cardiovascular disease risk factors such as smoking, elder age, male, hypertension, lipid metabolism disorders (hyperlipidemia), glucose metabolism disorders (diabetes mellitus and insulin resistance), overweight and obesity, and some newly risk factors such as HHcy and inflammatory markers. Our previous study found that elevated serum myeloperoxidase activities are significantly associated with the prevalence of acute coronary syndrome (AMI and UAP) and high LDL-C levels in CAD patients, The interaction between multiple metabolic parameters, inflammatory markers and traditional cardiovascular risk factors promoted the occurrence and development of CAD [26]. This study revealed that high Hcy group in CAD patients were characterized by smoking, diabetes mellitus, hypercholesterolemia, hypertriglyceridemia, low HDL-C and high LDL-C. These findings suggest that Hcy and traditional cardiovascular risk factors may be synergistically prompt the occurrence and development of CAD.
Vascular endothelium has important regulatory functions in the cardiovascular system and a pivotal role in regulating blood flow, mediating vasodilatation, coagulation reactions, platelet activation, leukocyte adhesion, and vascular muscle function [27]. During the last two decades, extensive experimental evidence, both in vitro and in vivo, indicates that Hcy is an independent risk factor for cardiovascular disease and elevated serum Hcy level is associated with CAD events [28–30]. Homocysteine Studies Collaboration research revealed that elevated approximately 3 μmol/L Hcy will increase about 10% risk of cardiovascular events [31]. Humphrey et al. [32] analyzed has also demonstrated that increased 5 μmol/L Hcy concentration will increase approximately 20% risk of CAD events.
In present study, AMI patients had the highest serum concentrations of Hcy, and UAP patients were a little lower than AMI but were the second highest, and SAP patients had the third higher level of Hcy, which were significantly higher than controls. Compared to SAP patients, patients with AMI and UAP had higher Hcy levels with approximately average elevated (4-5) μmol/L, while SAP patients were approximately higher 8 μmol/L than controls. CAD categories were positively correlated with Hcy, TC, TG, LDL-C, age, SBP, DBP, BMI, gender and smoking. Among them, Hcy showed the highest positively correlated with CAD categories. The prevalence of high Hcy progressively increased from controls, to SAP, to UAP, and to AMI. The present provide the valuable evidence that high concentrations of Hcy are significantly correlated with CAD categories. The more serious patients with CAD suffer, the more higher concentration their Hcy have.
Folic acid and vitamin B12 as two vital regulators play an important role in regulating the metabolic process of Hcy [33, 34]. In biological cells, Hcy is derived from methionine after its utilization as a methyl group donor in biological methylation reactions. However, approximately 50% Hcy is produced to remethylate back to methionine by the transmethylation of methionine, while 50% Hcy metabolize via transsulfuration to cystathionine [35]. In this cycle, methionine is activated by condensation with adenosine triphosphate (ATP) to give the methyl donor, S-adenosylmethionine (SAM). SAM is transformed into S-adenosylhomocysteine (SAH) by donating its methyl group to the substrates of methylation reactions. Subsequently, SAH gives rise to Hcy in a reversible reaction that favors SAH over Hcy production [36]. Methyl-tetra-hydrofolic acid (MTHF) which derivate of folic acid provide methyl to remethylated of Hcy. Vitamin B12 is agon of methionine synthetase that catalyzed this reaction and participate transfusion of methyl [13]. Folic acid deficiency will prevent remethylation of Hcy because of raw material deficiency. Moreover, Folic acid deficiency will also influence the production of MTHF through to affect activity of methylene tetrahydrofolate reductase (MTHFR) [37, 38].
We found that besides HDL-C, CAD categories were significantly negative correlated with folic acid, vitamin B12. the levels of folic acid and vitamin B12 in AMI and in UAP patients were obviously lower compared to those in SAP and controls. The prevalence of low folic acid and vitamin B12 progressively increased from controls, to SAP, to UAP, and to AMI. Moreover, more than or close to half of the CAD patients with high Hcy had low folic acid or vitamin B12 levels, 7 times or 5 times higher than that in CAD patients with normal-low Hcy concentrations, respectively. Hcy was strongly moderate negative correlation with folic acid and vitamin B12. Our results confirmed that serum folic acid and vitamin B12 influence Hcy metabolism as cosubstrate and cofactor, respectively. Low serum levels of folic acid and vitamin B12 are also significantly correlated with CAD categories.
Study limitation
Since present study was just an investigation that the correlations between CAD categories and serum Hcy, folic acid, vitamin B12 and traditional cardiovascular risk factors. The larger sample number of multicenter study and longer prospective investigation are necessary to further observe serum Hcy changes and incidence of adverse cardiovascular events by supplementation of folic acid and vitamin B12 in CAD patients.
Conclusions
The present study confirmed that Hcy and traditional cardiovascular risk factors may be synergistically prompt the formation and development of atherosclerosis in CAD patients. High concentrations of Hcy and low levels of folic acid and vitamin B12 are significantly correlated with CAD categories.
Acknowledgements
The authors would like to appreciate the staff in the Department of Clinical Laboratory and Department of Cardiovascular at the Sichuan Academy of Medical Sciences & Sichuan Provincial People’s Hospital and Department of Clinical Laboratory, Chengdu Women’s and Children’s Central Hospital, Chongqing Medical University for their support and guidance.
Funding
This research was supported by Sichuan Provincial Science and Technology Department Research Foundation of China (No. 2013FZ0080) and Sichuan Provincial Health and Family Planning Research Project Foundation of China (No. 16PJ450), respectively.
Availability of data and materials
The raw date supporting the results and conclusions of the present study will be available from the corresponding author on reasonable request. We have shared the data which publiched on J Atheroscler Thromb: Elevated serum myeloperoxidase activities are significantly associated with the prevalence of ACS and high LDL-C levels in CHD patients, 2012, 19(5): 435–443.
Authors’ contributions
YM, CL and JL carried out the carried out the clinical case screening, data collection, and drafted the manuscript. DP and CH carried out the immunoassays, and participated in the design of the study. CL and YMa carried out the design of the study and coordination and helped to draft the manuscript. CH and DP carried out the detection of clinical biochemistry. JL, DP and CH carried out the sample measurements, information classification and performed the statistical analysis. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
All authors have read and approve to submit this original article to BMC Cardiovascular Disorders. Written consent for publication was obtained from either the patients or their relatives.
Ethics approval and consent to participate
The study was approved by the Medical Ethics Committee at Chengdu Women’s and Children’s Central Hospital, Chengdu, China [(2013)2, Medical Ethics Committee, CWCCH], and was carried out in accordance with the guidelines of the Declaration of Helsinki. Written informed consent was obtained from all participants in the study.
Abbreviations
- ADMA
Asymmetric dimethylarginine
- AMI
Acute myocardial infarction
- ATP
Adenosine triphosphate
- BMI
Body mass index
- CAD
Coronary artery disease
- DBP
Diastolic blood pressure
- GLU
Glucose
- Hcy
Homocysteine
- HDL-C
High density lipoprotein cholesterol
- HHcy
Hyperhomocysteinemia
- LDL-C
Low density lipoprotein cholesterol
- MTHF
Methyl-tetra-hydrofolic acid
- MTHFR
Methylene tetrahydrofolate reductase
- NO
Nitric oxide
- SAH
S-adenosylhomocysteine
- SAM
S-adenosylmethionine
- SAP
Stable angina pectoris
- SBP
Systolic blood pressure
- TC
Total cholesterol
- TG
Triglyceride
- THF
Ttrahydro-folic acid
- UAP
Unstable angina pectoris
Contributor Information
Yan Ma, Email: emily.xixi@hotmail.com.
Duanliang Peng, Email: 1161889509@qq.com.
Chenggui Liu, Phone: +8613808024436, Email: lablcg@126.com.
Chen Huang, Email: 1480618866@qq.com.
Jun Luo, Email: 165399244@qq.com.
References
- 1.Choi BJ, Matsuo Y, Aoki T, Kwon TG, Prasad A, Gulati R, et al. Coronary endothelial dysfunction is associated with inflammation and vasa vasorum proliferation in patients with early atherosclerosis. Arterioscler Thromb Vasc Biol. 2014;34:2473–2477. doi: 10.1161/ATVBAHA.114.304445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruggiero D, Paolillo S, Ratta GD, Mariniello A, Formisano T, Pellegrino AM, et al. Endothelial function as a marker of pre-clinical atherosclerosis: assessment techniques and clinical implications. Monaldi Arch Chest Dis. 2013;80:106–110. doi: 10.4081/monaldi.2013.71. [DOI] [PubMed] [Google Scholar]
- 3.Subbotin VM. Neovascularization of coronary tunica intima (DIT) is the cause of coronary atherosclerosis. Lipoproteins invade coronary intima via neovascularization from adventitial vasa vasorum, but not from the arterial lumen: a hypothesis. Theor Biol Med Model. 2012;9:1–22. doi: 10.1186/1742-4682-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aird WC. Endothelium as an organ system. Crit Care Med. 2004;32:S271–S279. doi: 10.1097/01.CCM.0000129669.21649.40. [DOI] [PubMed] [Google Scholar]
- 5.Pushpakumar S, Kundu S, Sen U. Endothelial dysfunction: the link between homocysteine and hydrogen sulfide. Curr Med Chem. 2014;21:3662–3672. doi: 10.2174/0929867321666140706142335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polovina MM, Potpara TS. Endothelial dysfunction in metabolic and vascular disorders. Postgrad Med. 2014;126(2):38–53. doi: 10.3810/pgm.2014.03.2739. [DOI] [PubMed] [Google Scholar]
- 7.Wen J, Wen Y, Zhiliang L, Lingling C, Longxing C, Ming W, et al. A decrease in the percentage of circulating mDC precursors in patients with coronary heart disease: a relation to the severity and extent of coronary artery lesions? Heart Vessels. 2013;28:135–142. doi: 10.1007/s00380-011-0218-1. [DOI] [PubMed] [Google Scholar]
- 8.Hoffman M. Hypothesis: hyperhomocysteinemia is an indicator of oxidant stress. Med Hypotheses. 2011;77:1088–1093. doi: 10.1016/j.mehy.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Wang XC, Sun WT, Yu CM, Pun SH, Underwood MJ, He GW, Yang Q. ER stress mediates homocysteine-induced endothelial dysfunction: Modulation of IKCa and SKCa channels. Atherosclerosis. 2015;242:191–198. doi: 10.1016/j.atherosclerosis.2015.07.021. [DOI] [PubMed] [Google Scholar]
- 10.Arzamastsev DD, Karpenko AA, Kostiuchenko GI. Inflammation of the vascular wall and hyperhomocysteinemia in patients with atherosclerosis obliterans of lower limb arteries. Angiol Sosud Khir. 2012;18:27–30. [PubMed] [Google Scholar]
- 11.Magné J, Huneau JF, Borderie D, Mathé V, Bos C, Mariotti F. Plasma asymmetric and symmetric dimethylarginine in a rat model of endothelial dysfunction induced by acute hyperhomocysteinemia. Amino Acids. 2015;47:1975–1982. doi: 10.1007/s00726-015-1959-4. [DOI] [PubMed] [Google Scholar]
- 12.Antoniades C, Antonopoulos AS, Tousoulis D, Marinou K, Stefanadis C. Homocysteine and coronary atherosclerosis: from folate fortification to the recent clinical trials. Eur Heart J. 2009;30:6–15. doi: 10.1093/eurheartj/ehn515. [DOI] [PubMed] [Google Scholar]
- 13.Lentz SR. Mechanisms of homocysteine-induced atherothrombosis. J Thromb Haemost. 2005;3:1646–1654. doi: 10.1111/j.1538-7836.2005.01364.x. [DOI] [PubMed] [Google Scholar]
- 14.Yang AN, Zhang HP, Sun Y, Yang XL, Wang N, Zhu G, Zhang H, Xu H, Ma SC, Zhang Y, Li GZ, Jia YX, Cao J, Jiang YD. High-methionine diets accelerate atherosclerosis by HHcy-mediated FABP4 gene demethylation pathway via DNMT1 in ApoE(-/-) mice. FEBS Lett. 2015;589:3998–4009. doi: 10.1016/j.febslet.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 15.Emeksiz HC, Serdaroglu A, Biberoglu G, Gulbahar O, Arhan E, Cansu A, Arga M, Hasanoglu A. Assessment of atherosclerosis risk due to the homocysteine-asymmetric dimethylarginine-nitric oxide cascade in children taking antiepileptic drugs. Seizure. 2013;22:124–127. doi: 10.1016/j.seizure.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 16.Karbach S, Wenzel P, Waisman A, Munzel T, Daiber A. eNOS uncoupling in cardiovascular diseases--the role of oxidative stress and inflammation. Curr Pharm Des. 2014;20:3579–3594. doi: 10.2174/13816128113196660748. [DOI] [PubMed] [Google Scholar]
- 17.Shu L, Park JL, Byun J, Pennathur S, Kollmeyer J, Shayman JA. Decreased nitric oxide bioavailability in a mouse model of Fabry disease. J Am Soc Nephrol. 2009;20(9):1975–1985. doi: 10.1681/ASN.2008111190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeng R, Xu CH, Xu YN, Wang YL, Wang M. The effect of folate fortification on folic acid-based homocysteine-lowering intervention and stroke risk: a meta-analysis. Public Health Nutr. 2015;18:1514–1521. doi: 10.1017/S1368980014002134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng D, Kong H, Pang W, Yang H, Lu H, Huang C, et al. B vitamin supplementation improves cognitive function in the middle aged and elderly with hyperhomocysteinemia. Nutr Neurosci. 2016;19(10):461–466. [DOI] [PubMed]
- 20.Guo H, Lee JD, Ueda T, Cheng J, Shan J, Wang J. Hyperhomocysteinaemia & folic acid supplementation in patients with high risk of coronary artery disease. Indian J Med Res. 2004;119(1):33–37. [PubMed] [Google Scholar]
- 21.Liu Y, Tian T, Zhang H, Gao L, Zhou X. The effect of homocysteine-lowering therapy with folic acid on flow-mediated vasodilation in patients with coronary artery disease: a meta-analysis of randomized controlled trials. Atherosclerosis. 2014;235:31–35. doi: 10.1016/j.atherosclerosis.2014.03.032. [DOI] [PubMed] [Google Scholar]
- 22.Obersby D, Chappell DC, Dunnett A, Tsiami AA. Plasma total homocysteine status of vegetarians compared with omnivores: a systematic review and meta-analysis. Br J Nutr. 2013;109:785–794. doi: 10.1017/S000711451200520X. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez-Gross M, Sola R, Albers U, Barrios L, Alder M, Castillo MJ, et al. B-vitamins and homocysteine in Spanish institutionalized elderly. Int J Vitam Nutr Res. 2007;77:22–33. doi: 10.1024/0300-9831.77.1.22. [DOI] [PubMed] [Google Scholar]
- 24.Green R, Miller JW. Vitamin B12 deficiency is the dominant nutritional cause of hyperhomocysteinemia in a folic acid-fortified population. Clin Chem Lab Med. 2005;43:1048–1051. doi: 10.1515/CCLM.2005.183. [DOI] [PubMed] [Google Scholar]
- 25.Mahalle N, Kulkarni MV, Garg MK, Naik SS. Vitamin B12 deficiency and hyperhomocysteinemia as correlates of cardiovascular risk factors in Indian subjects with coronary artery disease. J Cardiol. 2013;61:289–294. doi: 10.1016/j.jjcc.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 26.Liu C, Xie G, Huang W, Yang Y, Li P, Tu Z. Elevated serum myeloperoxidase activities are significantly associated with the prevalence of ACS and High LDL-C levels in CHD patients. J Atheroscler Thromb. 2012;19(5):435–443. doi: 10.5551/jat.9704. [DOI] [PubMed] [Google Scholar]
- 27.Eren E, Ellidag HY, Aydin O, Yılmaz N. Homocysteine, Paraoxonase-1 and Vascular Endothelial Dysfunction: Omnibus viis Romam Pervenitur. J Clin Diagn Res. 2014;8:CE01–CE04. doi: 10.7860/JCDR/2014/7827.4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akyürek Ö, Akbal E, Güneş F. Increase in the risk of ST elevation myocardial infarction is associated with homocysteine level. Arch Med Res. 2014;45:501–506. doi: 10.1016/j.arcmed.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Wu Y, Huang Y, Hu Y, Zhong J, He Z, Li W, et al. Hyperhomocysteinemia is an independent risk factor in young patients with coronary artery disease in southern China. Herz. 2013;38:779–784. doi: 10.1007/s00059-013-3761-y. [DOI] [PubMed] [Google Scholar]
- 30.Alam N, Khan HI, Chowdhury AW, Haque MS, Ali MS, Sabah KM, et al. Elevated serum homocysteine level has a positive correlation with serum cardiac troponin I in patients with acute myocardial infarction. Bangladesh Med Res Counc Bull. 2012;38:9–13. doi: 10.3329/bmrcb.v38i1.10445. [DOI] [PubMed] [Google Scholar]
- 31.Homocysteine Studies Collaboration Homocysteine and risk of ischemic heart disease and stroke: a meta-analysis. JAMA. 2002;288:2015–2022. doi: 10.1001/jama.288.16.2015. [DOI] [PubMed] [Google Scholar]
- 32.Humphrey LL, Fu R, Rogers K, Freeman M, Helfand M. Homocysteine level and coronary heart disease incidence: a systematic review and meta-analysis. Mayo Clin Proc. 2008;83:1203–1212. doi: 10.4065/83.11.1203. [DOI] [PubMed] [Google Scholar]
- 33.Abdollahi Z, Elmadfa I, Djazayeri A, Sadeghian S, Freisling H, Mazandarani FS, et al. Folate, vitamin B12 and homocysteine status in women of childbearing age: baseline data of folic acid wheat flour fortification in Iran. Ann Nutr Metab. 2008;53:143–150. doi: 10.1159/000170890. [DOI] [PubMed] [Google Scholar]
- 34.Chen KJ, Pan WH, Yang FL, Wei IL, Shaw NS, Lin BF. Association of B vitamins status and homocysteine levels in elderly Taiwanese. Asia Pac J Clin Nutr. 2005;14:250–255. [PubMed] [Google Scholar]
- 35.Tchantchou F. Homocysteine metabolism and various consequences of folate deficiency. J Alzheimers Dis. 2006;9:421–427. doi: 10.3233/jad-2006-9408. [DOI] [PubMed] [Google Scholar]
- 36.Troen AM, Lutgens E, Smith DE, Rosenberg IH, Selhub J. The atherogenic effect of excess methionine intake. Proc Natl Acad Sci U S A. 2003;100(25):15089–15094. doi: 10.1073/pnas.2436385100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tavares EF, Vieira-Filho JP, Andriolo A, Perez AB, Vergani N, Sañudo A, et al. Serum total homocysteine levels and the prevalence of folic acid deficiency and C677T mutation at the MTHFR gene in an indigenous population of Amazonia: the relationship of homocysteine with other cardiovascular risk factors. Ethn Dis. 2004;14:49–56. [PubMed] [Google Scholar]
- 38.Bozok Çetintaş V1, Gündüz C. Association between polymorphism of MTHFR c.677C>T and risk of cardiovascular disease in Turkish population: a meta-analysis for 2.780 cases and 3.022 controls. Mol Biol Rep. 2014;41:397–409. doi: 10.1007/s11033-013-2873-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw date supporting the results and conclusions of the present study will be available from the corresponding author on reasonable request. We have shared the data which publiched on J Atheroscler Thromb: Elevated serum myeloperoxidase activities are significantly associated with the prevalence of ACS and high LDL-C levels in CHD patients, 2012, 19(5): 435–443.


