Abstract
Background and purpose — Manipulation and cast immobilization is the primary management for diaphyseal forearm fractures in children, and re-displacement is the most common complication. We wanted (1) to analyze the incidence of re-displacement in a group of children treated with close reduction and casting; (2) to determine predictive factors such as demographics, mechanism of injury, affected bone, fracture pattern, degree of initial displacement and angulation, and reduction accuracy; and (3) to determine the prognostic effect of previously defined radiographic indices.
Patients and methods — We prospectively studied 269 consecutive children with closed and complete middle-third diaphyseal fractures treated with close reduction and casting from October 2014 to April 2015. Factors analyzed included demographics, initial fracture features, having a non-anatomical reduction, and the radiographic indices of cast quality.
Results — There were 189 fractures of both bones (70%) and 80 solitary fractures (30%). The overall re-displacement rate was 11%. According to multivariable analysis, independent predictors of re-displacement were initial angulation >10° (RR =5) and failure to achieve an anatomical reduction (RR =2). Statistically significant radiographic indices regarding increased rate of re-displacement included cast index ≥0.7 (RR =5), Canterbury index ≥1.1 (RR =3), and 3-point index ≥0.8 (RR =6).
Interpretation — Our results suggested that fractures with a higher degree of initial angulation and non-anatomical reduction more often result in re-displacement. Moreover, the casting quality examined with the radiographic indices played an important role in the success of a non-operative management.
Forearm shaft fractures of the radius and ulna account for one-third of all bone fractures in children (Schmittenbecher 2005, Naranje et al. 2016). The incidence of these fractures has increased noticeably in recent years (Mäyränpää et al. 2010, Sinikumpu et al. 2012).
These fractures may be challenging to manage (Garg et al. 2008), with a risk of complications and long-term morbidity (Landin 1997, Droll et al. 2007). The main purpose of treatment is achievement of reduction and restoration of the rotational range of motion, while minimizing complications (Fuller and Cullough 1982, Franklin et al. 2012). The majority of these fractures are successfully treated non-operatively by manipulative reduction and cast immobilization (Vopat et al. 2014, Sinikumpu and Serlo 2015).
Fracture re-displacement is the most frequently reported complication, which can lead to malunion—causing impairment of forearm rotation (Voto et al. 1990, Price and Knapp 2006, van Geenen and Besselaar 2007, Nagy et al. 2008, Mehman and Wall 2009). Refractures occur more frequently after forearm shaft fracture than after other fractures in children, with an incidence of approximately 6–10% (Lascombes et al. 2006, Sinikumpu and Serlo 2015).
Recognition of potential predictors of re-displacement can improve the effectiveness of cast immobilization and identify patients who need surgical intervention rather than closed management (Wilkins 2005, Price and Knapp 2006, van Geenen and Besselaar 2007, Nagy et al. 2008). Much of the recent literature on forearm fractures has focused on the rate of re-displacement after closed reduction and immobilization of distal metaphyseal radius fractures (Mani et al. 1993, Zamzam and Khoshhal 2005, Alemdaroglu et al. 2008), but there are limited data on the outcome of diaphyseal forearm fractures in children (Kay et al. 1986). In this prospective study, our purpose was therefore to identify the incidence of re-displacement after closed reduction and casting in diaphyseal forearm fractures in children. Another objective was to determine predictive factors of re-displacement based on initial fracture severity and quality of reduction. We also wanted to determine the prognostic effect of previously defined radiographic indices.
Patients and methods
Study setting and population
This prospective study involved a consecutive series of children who sustained a closed middle-third fracture of the forearm (International Classification of Diseases, 10th Revision, code S52.2, 3 and 4). Treatment was provided at the emergency department (ED) of a university-affiliated urban hospital that is a regional level-II trauma center with about 70,000 ED visits annually.
We included children under the age of 16 years who had complete angulated or displaced extraarticular forearm fractures (either of the radius or ulna, or both) with no history of previous forearm fractures. Patients were excluded if they had sustained an open fracture or if they had undergone a previous reduction and immobilization attempt before referral. Additional exclusion criteria were: (1) greenstick and bowing fractures (where one side of the bone is broken while the other is bent), (2) forearm fractures with concomitant dislocations (e.g. Monteggia or Galeazzi), (3) pathological fractures, (4) multiple trauma, (5) neuromuscular paralysis, and (6) central nervous system injuries. Fractures of the distal radius and/or ulna and fractures in the metaphysis-diaphysis junction (the meta-diaphyseal transition zone) were also excluded. A complete fracture was defined as a fracture that extended through the entire radial/ulnar cortex.
Management
Initial closed reduction and cast immobilization were performed in our ED. The fractures were reduced using a combination of sustained traction and manipulation. Following reduction, a short-arm fiberglass cast was applied and after the radiographic control, we finalized the long-arm fiberglass cast with cotton (used for padding) in all cases. Children underwent conscious sedation, which was provided by 2 experienced anesthetists. All fractures were treated by experienced board-certified pediatric orthopedic surgeons. 1 assistant and 1 cast technician assisted the surgeon during the intervention. Distal neurovascular examination of the affected extremity was performed before and after manipulation. A complete evaluation including subjective complaints of pain, deformity, and functional deficit was performed, and also objective assessment. Objective evaluation included radial and ulnar pulses and the motor and sensory function of the median, radial, and ulnar nerves. Range of motion of wrist, forearm, and elbow were determined, as well as grip strength.
The cast was padded and molded carefully to fit firmly without undue pressure. The wrist was immobilized in 10–15 degrees of flexion with 0–30 degrees of ulnar deviation. Flexion and ulnar deviation of the wrist minimize dislocation forces (Ekenstam et al. 1984). The forearm was cast in neutral rotation.
Diagnostic pre-intervention and immediate post-intervention forearm radiographs (true anteroposterior and lateral) were taken in all patients. Post-intervention radiographs were also evaluated and reviewed by another orthopedic surgeon for residual angulation, displacement, and shortening. If the findings were unsatisfactory, the procedure was repeated under general anesthesia.
The quality of initial reduction was assessed using the following criteria: anatomical reduction (no translation or angulation), good reduction (dorsal angulation of <10° or translation of ≤2 mm), fair reduction (angulation of 10–20° or translation of 2–5 mm, or any radial deviation <5° or a combination of dorsal angulation of 5–10° and translation of ≤2 mm). The level of reduction was considered to be poor if the degree of angulation was ≥20°. The classification system was developed by the authors for the purposes of this study. Re-manipulation at the ED was used only for fractures that had >20° of dorsal angulation, > 10° of radial deviation, or >4 mm of translation—or that had a combination of at least 2 of the following criteria: > 10° of dorsal angulation, > 5° of radial deviation, and ≥3 mm of translation. The injured arm was checked regularly for circulation 24 hours after the reduction.
Measurements
On arrival, baseline demographic and clinical data were collected prospectively, including age at time of injury, sex, mechanism of injury, extremity affected, and bone(s) fractured (single or both forearm bones). In addition, pre- and post-manipulation angulation and displacement, quality of reduction, quality of immobilization, need for re-manipulation at the ED, cast-related complications, and re-displacement rate were recorded. Cast index (Chess et al.1994), padding index, Canterbury index (Alemdaroglu et al. 2008, Bhatia and Housden 2006), the 3-point index (Alemdaroglu et al. 2008), and radiographic measures of the immobilization quality were also calculated after the initial closed reduction at the ED (see below for details). All measurements were taken by another orthopedic surgeon who was unaware of the patient’s intervention.
Following casting, the indices calculated were as follows (Figure 1): (1) cast index: the inside diameter of the cast on lateral view divided by the inside diameter of the cast on AP view at the fracture site (cutoff =0.7); (2) padding index: the dorsal fracture site gap divided by the maximum interosseous length on AP view (cutoff =0.3); (3) Canterbury index: cast index + padding index (cutoff =1.1); (4) 3-point index: [(distal radial gap + ulnar fracture site gap + proximal radial gap) divided by the contact between fracture fragments in transverse projection] + [(distal dorsal gap + volar fracture site gap + proximal dorsal gap) divided by the contact between fracture fragments in sagittal projection] (cutoff =0.8).
Figure 1.
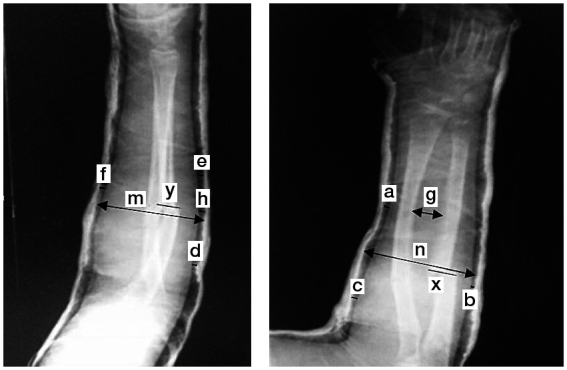
Cast index = m/n. Padding index = h/g. 3-point index = (a + b + c)/x + (d + e + f)/y. Canterbury index = padding index + cast index.
Follow-up
All patients were discharged after a 24-hour observation period in the ED, with weekly orthopedic follow-up arranged within 6–8 weeks of injury. Outcome assessment was performed by a board-certified pediatric orthopedic surgeon who was unaware of the patient data—and also when the study started and ended. At weekly follow-up visits, radiographs were assessed for loss of reduction, and the amounts of angulation or translation were also noted. Neurological assessment including sensory and motor function of the median, radial, and ulnar nerves was performed. Movement testing and muscle strength test of the wrist and hand were also done. We evaluated wrist flexion/extension, forearm pronation and supination, grip strength, and key and pinch grip strength.
Range of motion of the elbow and wrist, and forearm rotation were measured with a goniometer. The patients were asked if they experienced any subjective symptoms or limitation of function, and these responses were recorded. Casts were changed as necessary during weekly visits (due to breakage, skin irritation, self-removal, presence of foreign bodies, and other complaints).
Re-displacement was considered to have occurred if there was (1) increased angulation of >10°, (2) increased translation of >20%, or (3) increased angulation of >5° and increased translation of >10%. If re-displacement occurred at the first follow-up visit, the patients underwent close re-reduction under general anesthesia using fluoroscopy. Where there was inadequate re-reduction, open reduction and internal fixation were performed. All the other re-displacements observed after the initial visit were also managed operatively.
Statistics
We compared demographic and baseline clinical characteristics between re-displaced and non-re-displaced fractures using Student’s t-test or the Mann-Whitney U-test for quantitative variables; these are presented as mean (SD). Qualitative variables (percentages) and the differences in proportion were compared using the chi-squared test or Fisher’s exact test as appropriate. Possible predictors that correlated with re-displacement were identified using univariable and multivariable regression analysis with forward logistic regression techniques. Odds ratio (OR) associated with each factor measured and 95% confidence interval (CI) were calculated. For comparative purposes, we also used a method proposed by Zhang and Yu (1998) for obtaining a relative risk (RR) from a logistic regression model in cohort studies. The estimated RR and CI were calculated using the following formula. P0 is the incidence of the outcome in the unexposed group, "ORadj" is an odds ratio obtained from a logistic regression model, and "RR" is an estimated relative risk:
Discriminant function analysis with k-fold cross-validation was used in parallel to check the performance of the model. The concern of interest is how accurately the model will predict for an independent data series. We therefore chose to use a methodology called standard 10-fold cross-validation. Such an algorithm has been used to improve performance of prediction-modeling methods (Hastie et al. 2009, Lee et al. 2010, Greenland and Pearce 2015). The optimal number of folds (splits) of the data is debated, but 10 is a common choice and there is a trade-off with greater or less folds (Venables and Ripley 1999). Using this methodology, the dataset is randomly subdivided into 10 subsets. Each tenth of the data is systematically set aside for later testing, and the remaining nine-tenths are combined for model building. This process is repeated for each tenth of the data, which serves to act as new cases for prediction purposes, because they are excluded from model building when they are set aside. Predictive accuracy is measured for each tenth of the data that is set aside, and the median of the set-aside predictive accuracy values is used to assess the model validation (Harrell et al. 1982).
The diagnostic accuracy of the model and radiographic indices was assessed by measuring the areas under the receiver operating characteristic (ROC) curves. An area under the curve (AUC) equal to 1.0 is indicative of an ideal test, while AUC =0.5 characterizes a test of no diagnostic value.
All p-values were based on 2-tailed tests and a probability value of ≤0.05 was considered to be statistically significant. All statistical analyses were performed using R software.
Ethics
The study was reviewed and approved by our institutional review board and written informed consent was obtained from the patients or their parents before the investigation (date of issue: April 22, 2014; registration number: SB-209). The study was carried out according to the tenets of the Declaration of Helsinki (World Medical Association 2013).
Results
Study population
Between October 2014 and April 2015, 490 children with closed diaphyseal fractures were screened for eligibility. 269 patients fulfilled the inclusion criteria and were entered into the study. 221 patients were excluded for various reasons: incomplete and greenstick fractures (n = 82), a history of forearm shaft fractures (n = 48), multiple trauma (n = 38), previous reduction and immobilization (n = 35), neuromuscular paralysis (n = 6), and pathological fracture (n = 2). 10 patients’ family members refused to give their consent for participation in the study.
There were 210 boys (78%) boys and 59 girls treated (Table 1). The average age at injury was 10.0 (SD 4.2) years. There was no statistically significant difference between the patients aged ≥9 years compared to those aged <9 years regarding the rate of re-displacement (67% vs. 33%, p = 0.6). At presentation, 189 children (70%) had displaced fractures of both bones and 80 (30%) had one displaced fracture. 155 children (58%) had right-forearm fractures and 95 (35%) had left-forearm fractures. Average cast index was 6.2 (SD 3.5, range: 1–15).
Table 1.
Comparison of demographics and baseline fracture characteristics. Values are n (%)
| Re-displacementa | ||||
|---|---|---|---|---|
| Yes (n = 30) | No (n = 239) | p-value | ||
| Age | ||||
| ≥ 9 years | 20 (67) | 169 (71) | 0.6 | |
| < 9 years | 10 (33) | 70 (29) | ||
| Gender | ||||
| Girl | 12 (40) | 47 (20) | 0.01 | |
| Boy | 18 (60) | 192 (80) | ||
| Mechanism of injury | ||||
| Falling during running | 18 (60) | 94 (39) | 0.03 | |
| Falling from a height | 3 (10) | 66 (28) | 0.09 | |
| Motor accident | 4 (13) | 42 (18) | 0.6 | |
| Direct trauma | 5 (17) | 37 (15) | 0.9 | |
| Fracture side | ||||
| Right | 13 (43) | 142 (60) | 0.09 | |
| Left | 15 (50) | 80 (34) | 0.07 | |
| Both | 2 (7) | 17 (7) | 0.9 | |
| Fractured bone | ||||
| Radius | 14 (47) | 36 (15) | < 0.001 | |
| Ulna | 1 (3) | 29 (12) | 0.1 | |
| Both | 15 (50) | 174 (73) | 0.008 | |
| Reduction accuracy | ||||
| Anatomical | 6 (20) | 170 (71) | < 0.001 | |
| Non-anatomical | 24 (80) | 69 (29) | ||
Defined as (1) increased angulation of >10°, (2) increased translation of >20%, or (3) increased angulation of >5° and increased translation of >10%.
The most common etiology of the fractures was a fall during running (112 patients; 42%).
Outcome
None of the fractures were associated with neurological or vascular injury. Among all patients, anatomical reduction was achieved in 176 (65%). The quality of reduction was good in 63 patients (23%) and fair in 35 (13%). Of these, 28 children (10%) required a second manipulation and 17 children (6%) underwent a third reduction. The patients requiring additional reduction were older (11.1 (SD 3.9) years) than those with no need for re-reduction (9.8 (SD 4.2) years), but this difference did not reach statistical significance (p = 0.1).
At follow-up visits, re-displacement had occurred in 30 children (11%): 18 boys and 12 girls. Re-displacement mostly occurred within 3 weeks of the initial reduction (83%). Radiographs of the re-displaced fractures showed adequate cast fit.
Evaluation of range of motion at the latest follow-up showed normal elbow and wrist motion in all patients. A full range of forearm rotation was found in 248 cases (92%). The average loss of supination/pronation was 22 (17–55) degrees. Loss of forearm rotation mainly occurred in the patients who had re-displacement (p < 0.001).
At the latest follow-up, 254 of 269 children had no subjective symptoms. None of the patients had nerve dysfunction after treatment. No complex regional pain syndrome was encountered in any of them. No loss of reductions due to an unplanned cast change or removal occurred during the study period. According to univariable analysis, there was a statistically significant association between the rate of re-displacement and several baseline factors including sex (p = 0.01), falling during running (p = 0.03), the presence of fracture in the radial bone (p < 0.001), fracture with a spiral pattern (p = 0.054), initial angulation of >10° (p < 0.001), and initial displacement of >10 mm (p = 0.01). The rate of re-displacement was significantly higher in patients who had a primary non-anatomical reduction (24/93) compared to patients with an anatomical reduction (6/176, p < 0.001). The radiographic indices of the cast index with a cutoff point of 0.7 (p < 0.001), the padding index with a cutoff point of 0.3 (p < 0.001), the Canterbury index with a cutoff point of 1.1 (p < 0.001), and the 3-point index (p < 0.001) were all found to be associated with an increased rate of re-displacement (Table 2).
Table 2.
Comparison of radiographic features at injury. Values are n (%)
| Re-displacementa | ||||
|---|---|---|---|---|
| Yes (n = 30) | No (n = 239) | p-value | ||
| Fracture pattern | ||||
| Spiral | 22 (73) | 131 (55) | 0.05 | |
| Oblique | 5 (16) | 92 (38) | 0.02 | |
| Transverse | 3 (10) | 16 (7) | 0.5 | |
| Initial angulation >10° | ||||
| Yes | 12 (40) | 34 (14) | < 0.001 | |
| No | 18 (60) | 205 (86) | ||
| Initial displacement >10 mm | ||||
| Yes | 29 (97) | 184 (77) | 0.01 | |
| No | 1 (3) | 55 (23) | ||
| Radiological indices | ||||
| Cast index (≥ 0.7) | 25 (83) | 44 (18) | < 0.001 | |
| Padding index (≥ 0.3) | 25 (83) | 52 (22) | < 0.001 | |
| Canterbury index (≥ 1.1) | 21 (70) | 2 (0.8) | < 0.001 | |
| Three-point index (≥ 0.8) | 24 (80) | 57 (24) | < 0.001 | |
See footnote Table 1.
Results of multivariable stepwise regression analysis revealed that initial angulation of >10° (RR =4.5; p = 0.001) and failure to achieve an anatomical reduction (RR =2.2; p = 0.05) were independent predictive factors for re-displacement. Furthermore, a cast index of ≥0.7 (RR =4.8; p = 0.002), a Canterbury index of ≥1.1 (RR =3.4; p = 0.002), and a 3-point index of ≥0.8 (RR =6.2; p < 0.001) were found to predict re-displacement (Tables 3 and 4).
Table 3.
Multivariable regression analyses of possible predictive factors for the risk of re-displacement
| Odds ratio | 95% CI | p-value | |
|---|---|---|---|
| Baseline fracture variables | |||
| Gender | 1.2 | 0.2–4.0 | 0.6 |
| Mechanism of injury | |||
| Falling during running | 2.7 | 0.05–3.8 | 0.8 |
| Falling from a height | 4.4 | 0.01–6.1 | 0.5 |
| Both-bone fracture | 2.8 | 0.6–5.8 | 0.08 |
| Failure to achieve anatomical reduction | 3.9 | 1.1–7.7 | 0.05 |
| Radiographic features | |||
| Spiral pattern of fracture | 1.2 | 0.2–5.2 | 0.7 |
| Oblique pattern of fracture | 3.0 | 0.02–7.3 | 0.2 |
| Initial angulation >10° | 6.5 | 2.2–9.5 | 0.001 |
| Initial displacement >10 mm | 6.1 | 0.6–9.1 | 0.1 |
| Cast index (≥ 0.7) | 5.3 | 3.1–7.6 | 0.002 |
| Padding index (≥ 0.3) | 3.1 | 0.1–7.3 | 0.3 |
| Canterbury index (≥ 1.1) | 3.8 | 1.9–6.8 | 0.002 |
| 3-point index (≥ 0.8) | 7.4 | 2.1–10 | > 0.001 |
CI: 95% confidence interval.
Table 4.
Estimated relative risk (RR) of re-displacement by significant predictors
| Estimated RR | 95% CI | p-value | |
|---|---|---|---|
| Failure to achieve anatomical reduction | 2.2 | 1.1–2.8 | 0.05 |
| Initial angulation of >10° | 4.5 | 2.0–5.7 | 0.001 |
| Cast index (≥ 0.7) | 4.8 | 3.0–6.5 | 0.002 |
| Canterbury index (≥ 1.1) | 3.4 | 1.8–5.7 | 0.002 |
| Three point index (≥ 0.8) | 6.2 | 2.2–8.0 | > 0.001 |
CI: 95% confidence interval.
The ROC parameters for the model were: a sensitivity of 80% (CI: 77–92); a specificity of 96% (CI: 89–98); an AUC of 0.90 (CI: 0.82–0.98), and a precision of 71%. These results were confirmed with discriminant function analysis, which showed that the predictive factors discriminated the 2 groups of patients (re-displaced vs. non-re-displaced fractures) with an accuracy of 99% (Wilk’s lambda =0.34, chi square =289; p < 0.001). The accuracy was also confirmed using 10-fold cross-validation, and corresponded to 94%.
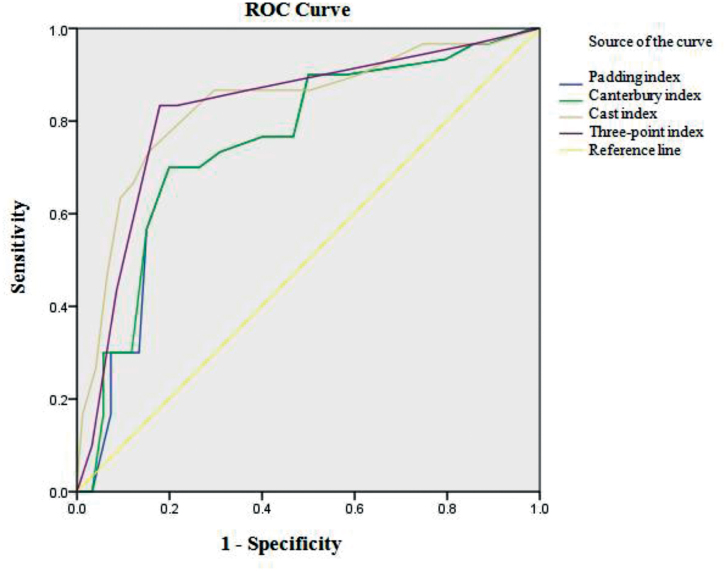
ROC curve analysis showed that the predictive value of the radiographic indices was high, with an area under the ROC curve of 0.83 for cast index, 0.75 for padding index, 0.76 for Canterbury index, and 0.82 for the 3-point index (p < 0.001) (Table 5 and Figure 2).
Table 5.
Area under the curve for ROC curve analysis of radiological indices
| AUC | 95% CI | p-value | |
|---|---|---|---|
| Cast index | 0.83 | 0.75–0.92 | < 0.001 |
| Padding index | 0.75 | 0.66–0.84 | < 0.001 |
| Canterbury index | 0.76 | 0.67–0.85 | < 0.001 |
| 3-point index | 0.82 | 0.74–0.91 | < 0.001 |
ROC: receiver operating characteristic; AUC: area under the curve; CI: 95% confidence interval.
Figure 2.
Area under the receiver operating characteristic (ROC) curves for the 4 radiographic indices.
Discussion
An acceptable restoration of forearm function is achieved in most children after non-operative treatment of diaphyseal forearm fractures. However, re-displacement is common after closed treatment (Voto et al. 1990, Price and Knapp 2006, van Geenen and Besselaar 2007, Nagy et al. 2008) and has been reported to occur in 7–20% of cases, with rates as high as 62% for older children (Rodríguez-Merchán 2005, Bochang et al. 2008, Madhuri et al. 2013).
In this study, we excluded fractures in the metaphysis-diaphysis junction, which do not behave in the same way as distal forearm or diaphyseal fractures. Meta-diaphyseal fractures are managed with different treatment principles, due to their different remodeling and healing potential compared to mid-diaphyseal fractures (Johari et al. 1999).
The present study is one of the few investigations to have assessed the rate of and predictive factors for re-displacement after closed treatment of middle-third forearm fractures in children. A number of studies have evaluated predictive factors for re-displacement of distal radius fractures (Mani et al. 1993, McLauchlan et al. 2002, Zamzam and Khoshhal 2005, Alemdaroglu et al. 2008), but much less data are available regarding predictors for re-displacement of diaphyseal fractures (Colaris et al. 2013, Iltar et al. 2013). Bowman et al. (2011) conducted a retrospective study of children with both-bone forearm shaft fractures who underwent closed treatment. They reported that 5% of children experienced re-displacement over 4 weeks of follow-up. Voto et al. (1990) found that the rate of re-displacement in non-operatively treated shaft fractures was 19%. A recent cohort study found that 22% of the cases in the non-operatively treated group had re-displacement during follow-up (Sinikumpu et al. 2013). In the present study, 10% of all children had re-displacement after closed fracture reduction and immobilization.
We also found that several factors were associated with an increased rate of re-displacement. Perfect anatomical reduction is one of the most widely accepted factors preventing re-displacement (Proctor et al. 1993, Zamzam and Khoshhal 2005). In line with earlier studies (Voto et al. 1990, Proctor et al. 1993, Haddad and Williams 1995,), our results suggest that a complete primary reduction is protective against re-displacement during the follow-up period. Similar observations were made by Yang et al. (2012). In our series, most of the fractures were reduced completely at the first attempt. A second reduction was necessary in 10%, and a third intervention in 6%. In agreement with our findings, a review of historical data involving 292 diaphyseal forearm fractures between 1976 and 1985 revealed that 11% and 2.4% of those fractures required second and third re-manipulation, respectively (Schmittenbecher 2005). 30% of our patients had a single bone fracture, which might reduce the need for initial re-manipulation and is protective against re-displacement. Both-bone forearm fracture is associated with a higher probability of incomplete reduction and re-displacement (Zamzam and Khoshhal 2005, Hang et al. 2011). It has already been reported that the re-manipulation rate and reduction accuracy depends on the experience and grade of the surgeon performing the reduction. Haddad and Williams (1995), who reviewed 86 cases of distal forearm fractures managed with closed reduction, suggested that perfect anatomic alignment—the most important prognostic factor for re-displacement—was more likely to be achieved when the reduction was conducted by an experienced surgeon. As in our patients, Fenton et al. (2012) reported that reduction performed by less experienced surgeons showed a positive correlation with re-angulation.
Complications from midshaft forearm fractures are rare. Delayed union or mal- or non-union occurs in less than 1% of midshaft fractures treated with closed reduction (Mehlman 2006). Non-union was not observed in our series. Our results are consistent with findings from other prospective reports (Zionts et al. 2005, Sinikumpu et al. 2013).
Another finding of our study was that re-displacement was more common in fractures with a greater initial degree of displacement. As also demonstrated by Bhatia and Housden (2006), Hang et al. (2011) found a 3% increase in the risk of re-displacement for each per cent of initial radial displacement in a review of 48 consecutive children.
Besides the above factors, the quality of casting was considered to be an important interventional factor that can influence re-displacement (Bhatia and Housden 2006). Numerous radiographic indices of cast molding have been proposed to be the most important predictors of re-displacement after closed reduction. Our findings confirmed that the cast and padding indices proposed by Chess et al. (1994), and also the Canterbury index described by Bhatia and Housden (2006), had a strong correlation with re-displacement. Previous investigators have stated that the casts must be molded perfectly to maintain the reduction (Bohm et al. 2006, Webb et al. 2006). Based on previous literature, the rate of loss of reduction was higher when the cast index was more than 0.7 (Bae 2008). There was a statistically significant difference in the cast-related indices between the re-displaced and non-re-displaced group in our investigation. Moreover, the area under the curve for all indices was more than 76%, indicating a strong correlation between increasing value of the indices and re-displacement. We also found a positive correlation between the 3-point index and the rate of displacement. A recent prospective study has shown that the 3-point index is the most predictive radiographic measure of late displacement after closed reduction and casting of distal radius fractures in children (Alemdaroglu et al. 2008). We found that measurement of these indices immediately after manipulation is a valuable approach and good practice before accepting any casting immobilization of forearm middle-third fractures.
To obtain optimal results after non-operative management, a neutral position of the forearm in the cast is recommended (Evans 1951, Carey et al. 1992, Kapandji 2001, Moore and Dalley 2006, Tubbs et al. 2007). Thus, in the present study, forearm fractures were cast in neutral supination/pronation, contributing to effective immobilization.
Several different malalignements occur when the forearm completely breaks by either indirect or direct forces. The bones shorten, angulate, and rotate within the boundaries of the adjacent periosteum, interosseous membrane, and muscle attachments (Noonan and Price 1998). The impact of rotational deformity on the wrist and forearm is still uncertain, although biomechanical investigations have determined the effects of this malposition of the forearm diaphysis on restriction of forearm supination/pronation and instability of the distal radio-ulnar joint (Tarr et al. 1984, Dumont et al. 2002, Kasten et al. 2003). This type of deformity is often quantified and described with difficulty from standard views of plain radiographs. Thus, there is a failure to assess the fracture rotation on a routine basis (Tarr et al. 1984, Noonan and Price 1998). To be successful in closed treatment and accurate prediction of a patient’s final outcome, it is essential to pay full attention to the rotational element of the deformity on admission (Prommersberger et al. 2004).
The association of malrotation and follow-up redisplacement was not referred specifically in this study. Further investigation is of importance for finding any correlation between deformity pattern of fractures and functional outcome and probable complications after management.
The present study had several limitations that should be considered. First, we had a relatively short duration of follow-up. However, all fractures were united after 6 weeks and we would expect that most complications would have been encountered during this time period. Secondly, outcome assessment in our analysis was limited to the rate of re-displacement as measured on radiographs, which may have been subject to measurement error.
In summary, acceptable clinical and radiographic outcomes often occur following remodeling irrespective of early re-displacement (Fuller and McCullough 1982). Also, secondary intervention—especially surgically—can be stressful and inconvenient to the patients and their families. It also delays mobilization and restoration of full function. Consequently, identification of the predictive factors for re-displacement would provide a basis for prevention of this complication after non-operative treatment.
This study has revealed that the ability to reach an optimal fracture reduction is of paramount importance in influencing the outcome. In other words, a poorer reduction and insufficient cast molding are more likely to cause re-displacement of mid-diaphyseal radial and ulnar fractures in children who have undergone closed reduction and casting. Moreover, initial alignment-related fracture parameters, such as the degree of fracture, can help predict the probability of re-displacement.
Conception and design: SA and KH; supervision: SA; acquisition, analysis, and interpretation of data: MP; drafting and revision of the manuscript: SA; critical review: SA and KH.
This study was supported by a grant (#SB-209) from Shahid Beheshti University of Medical Sciences (Tehran, Iran). We thank Hossein Dinpanah, Sadrollah Mahmoudi, Ensieh Ghaffari Shad, Alireza Maleki Rastekenari, Mehrdad Taghizadeh, and Mohammad Amiri for their valuable contributions to the study.
No competing interests declared.
References
- Alemdaroglu K B, Iltar S, Cimen O, Uysal M, Alagöz E, Atlihan D.. Risk factors in redisplacement of distal radial fractures in children. J Bone Joint Surg Am 2008; 90: 1224–30. [DOI] [PubMed] [Google Scholar]
- Bae D S. Paediatric distal radius and forearm fractures. J Hand Surg Am 2008; 33: 1911–23. [DOI] [PubMed] [Google Scholar]
- Bhatia M, Housden P H.. Re-displacement of paediatric forearm fractures: role of plaster molding and padding. Injury 2006; 37: 259–68. [DOI] [PubMed] [Google Scholar]
- Bochang C, Katz K, Weigl D, Jie Y, Zhigang W, Bar-On E.. Are frequent radiographs necessary in the management of closed forearm fractures in children? J Child Orthop 2008; 2: 217–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohm E R, Bubbar V, Yong Hing K, Dzus A.. Above and below-the-elbow plaster casts for distal forearm fractures in children. A randomized controlled trial. J Bone Joint Surg Am 2006; 88: 1–8. [DOI] [PubMed] [Google Scholar]
- Bowman E N, Mehlman C T, Lindsell C J, Tamai J.. Nonoperative treatment of bothbone forearm shaft fractures in children: predictors of early radiographic failure. J Pediatr Orthop 2011; 31: 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey P J, Alburger P D, Betz R R, Clancy M, Steel H H.. Both-bone forearm fractures in children. Orthopedics 1992; 15: 1015–19. [DOI] [PubMed] [Google Scholar]
- Chess D G, Hyndman J C, Leahey J L, Brown D C S, Sinclair A M.. Short arm plaster cast for distal pediatric forearm fractures. J Pediatr Orthop 1994; 14: 211–3. [DOI] [PubMed] [Google Scholar]
- Colaris J W, Allema J H, Reijman M, Biter L U, de Vries M R, van de Ven C P, Bloem R M, Verhaar J A.. Risk factors for the displacement of fractures of both bones of the forearm in children. Bone Joint J 2013; 95: 689–93. [DOI] [PubMed] [Google Scholar]
- Droll K P, Perna P, Potter J, Harniman E, Schemitsch E H, McKee M D.. Outcomes following plate fixation of fractures of both bones of the forearm in adults. J Bone Joint Surg Am 2007; 89: 2619–24. [DOI] [PubMed] [Google Scholar]
- Dumont C E, Thalmann R, Macy J C.. The effect of rotational malunion of the radius and the ulna on supination and pronation. J Bone Joint Surg Br 2002; 84: 1070–4. [DOI] [PubMed] [Google Scholar]
- Evans E M. Fractures of the radius and ulna. J Bone Joint Surg Br 1951; 33: 548–61. [DOI] [PubMed] [Google Scholar]
- Ekenstam F W, Palmer A K, Glisson R R.. The load on the radius and ulna in different positions of the wrist and forearm: a cadaver study. Acta Orthop Scand 1984; 55: 363–5. [DOI] [PubMed] [Google Scholar]
- Fenton P, Nightingale P, Hodson J, Luscombe J.. Factors in redisplacement of paediatric distal radius fractures. J Pediatr Orthop B 2012; 21: 127–30. [DOI] [PubMed] [Google Scholar]
- Franklin C C, Robinson J, Noonan K, Flynn JM.. Evidence-based medicine: management of pediatric forearm fractures. J Pediatr Orthop 2012; 32 (Suppl 2): S131–S134. [DOI] [PubMed] [Google Scholar]
- Fuller D J, McCullough C J.. Malunited fractures of the forearm in children. J Bone Joint Surg Br 1982; 64: 364–7. [DOI] [PubMed] [Google Scholar]
- Garg N K, Ballal M S, Malek I A, Webster R A, Bruce C E.. Use of elastic stable intramedullary nailing for treating unstable forearm fractures in children. J Trauma 2008; 65: 109–15. [DOI] [PubMed] [Google Scholar]
- Greenland S, Pearce N.. Statistical foundations for model-based adjustments. Annu Rev Public Health 2015; 36: 89–108. [DOI] [PubMed] [Google Scholar]
- Haddad F S, Williams R L.. Forearm fractures in children: avoiding redisplacement. Injury 1995; 26: 691–2. [DOI] [PubMed] [Google Scholar]
- Harrell F E Jr, Califf R M, Pryor D B, Lee K L, Rosati R A.. Evaluating the yield of medical tests. JAMA 1982; 247(18): 2543–6. [PubMed] [Google Scholar]
- Iltar S, Alemdaroglu KB, Say F, Aydogan NH.. The value of the three-point index in predicting redisplacement of diaphyseal fractures of the forearm in children. Bone Joint J 2013; 95: 563–7. [DOI] [PubMed] [Google Scholar]
- Hang J R, Hutchinson A F, Hau R C.. Risk factors associated with loss of position after closed reduction of distal radial fractures in children. J Pediatr Orthop 2011; 31: 501–6. [DOI] [PubMed] [Google Scholar]
- Hastie T, Tibshirani R, Friendman J.. The Elements of Statistical Learning: Data Mining, Inference and Prediction. New York: Springer; 2nd ed 2009. [Google Scholar]
- Johari A N, Orth D, Sinha M.. Remodeling of forearm fractures in children. J Pediatr Orthop B 1999; 8: 84–7. [PubMed] [Google Scholar]
- Kapandji A. Biomechanics of pronation and supination of the forearm. Hand Clin 2001; 17: 111–22. [PubMed] [Google Scholar]
- Kasten P, Krefft M, Hesselbach J, Weinberg A M.. How does torsional deformity of the radial shaft influence the rotation of the forearm? A biomechanical study. J Orthop Trauma 2003; 17: 57–60. [DOI] [PubMed] [Google Scholar]
- Kay S, Smith C, Oppenheim W L.. Both-bone midshaft forearm fractures in children. J Pediatr Orthop 1986; 6: 306–10. [DOI] [PubMed] [Google Scholar]
- Landin L A. Epidemiology of children’s fractures. J Pediatr Orthop B 1997; 6: 79–83. [DOI] [PubMed] [Google Scholar]
- Lascombes P, Haumont T, Journeau P.. Use and abuse of flexible intramedullary nailing in children and adolescents. J Pediatr Orthop 2006; 26: 827–34. [DOI] [PubMed] [Google Scholar]
- Lee B K, Lessler J, Stuart E A.. Improving propensity score weighting using machine learning. Stat Med 2010; 29: 337–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhuri V, Dutt V, Gahukamble A D, Tharyan P.. Conservative interventions for treating diaphyseal fractures of the forearm bones in children. Cochrane Database Syst Rev 2013; 4: CD008775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani G V, Hui P W, Cheng J C.. Translation of the radius as a predictor of outcome in distal radial fractures of children. J Bone Joint Surg Br 1993; 75: 808–11. [DOI] [PubMed] [Google Scholar]
- Mäyränpää M K, Mäkitie O, Kallio P E.. Decreasing incidence and changing pattern of childhood fractures: a population-based study. J Bone Miner Res 2010; 25: 2752–9. [DOI] [PubMed] [Google Scholar]
- McLauchlan G J, Cowan B, Annan I H, Robb JE .. Management of completely displaced metaphyseal fractures of the distal radius in children. A prospective, randomised controlled trial. J Bone Joint Surg Br 2002; 84: 413–7. [DOI] [PubMed] [Google Scholar]
- Mehman C T, Wall E J.. Injuries to the shafts of the radius and ulna In: Rockwood and Wilkins’ Fractures in Children: Text Plus Integrated Content Website (Eds. Beaty J H, Kasser J R). 7th ed. Lippincott Williams & Wilkins Co; Philadelphia: 2009: 347–402. [Google Scholar]
- Mehlman C T, Wall E J. Injuries to the shafts of the radius and ulna In: Rockwood and Wilkins’ Fractures in Children. (Eds. Beaty J H, Kasser J R). 6th ed. Lippincott Williams & Wilkins Co; Philadelphia: 2006: 400. [Google Scholar]
- Moore K, Dalley A.. Clinically oriented anatomy. Baltimore, MD: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- Nagy L, Jankauskas L, Dumont C E.. Correction of forearm malunion guided by the preoperative complaint. Clin Orthop Relat Res 2008; 466: 1419–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naranje S M, Erali R A, Warner W C, Sawyer J R, Kelly D M.. Epidemiology of pediatric fractures presenting to emergency departments in the United States. J Pediatr Orthop 2016; 36: e45–48. [DOI] [PubMed] [Google Scholar]
- Noonan K J, Price C T.. Forearm and distal radius fractures in children. J Am Acad Orthop Surg 1998; 6: 146–56. [DOI] [PubMed] [Google Scholar]
- Price C T, Knapp D R.. Osteotomy for malunited forearm shaft fractures in children. J Pediatr Orthop 2006; 26: 193–6. [DOI] [PubMed] [Google Scholar]
- Proctor M T, Moore D J, Paterson J M H.. Redisplacement after manipulation of distal radial fractures in children. J Bone Joint Surg Br 1993; 75: 453–4. [DOI] [PubMed] [Google Scholar]
- Prommersberger K J, Froehner S C, Schmitt R R, Lanz U B.. Rotational deformity in malunited fractures of the distal radius. J Hand Surg Am 2004; 29: 110–5. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Merchán E C. Pediatric fractures of the forearm. Clin Orthop Relat Res 2005; 432: 65–72. [PubMed] [Google Scholar]
- Schmittenbecher P P. State-of-the-art treatment of forearm shaft fractures. Injury 2005; 36 (Suppl 1): A25–A34. [DOI] [PubMed] [Google Scholar]
- Sinikumpu J J, Serlo W.. The shaft fractures of the radius and ulna in children: current concepts. J Pediatr Orthop B 2015; 24: 200–6. [DOI] [PubMed] [Google Scholar]
- Sinikumpu J J, Lautamo A, Pokka T, Serlo W.. The increasing incidence of paediatric diaphyseal both-bone forearm fractures and their internal fixation during the last decade. Injury 2012; 43: 362–6. [DOI] [PubMed] [Google Scholar]
- Sinikumpu J J, Lautamo A, Pokka T, Serlo W.. Complications and radiographic outcome of children’s both-bone diaphyseal forearm fractures after invasive and non-invasive treatment. Injury 2013; 44: 431–6. [DOI] [PubMed] [Google Scholar]
- Tarr R R, Garfinkel A I, Sarmiento A.. The effects of angular and rotational deformities of both bones of the forearm. An in vitro study. J Bone Joint Surg Am 1984; 66: 65–70. [PubMed] [Google Scholar]
- Tubbs R S, O’Neil J T, Key C D, Zarzour J G, Fulghum S B, Kim E J, Lyerly M J, Shoja M M, Salter G E, Oakes J W.. The oblique cord of the forearm in man. Clinical Anatomy 2007; 20: 411–5. [DOI] [PubMed] [Google Scholar]
- van Geenen R C, Besselaar P P.. Outcome after corrective osteotomy for malunited fractures of the forearm sustained in childhood. J Bone Joint Surg Br 2007; 89: 236–9. [DOI] [PubMed] [Google Scholar]
- Venables W N, Ripley B D.. Modern Applied Statistics with S-PLUS, 3rd ed Springer-Verlag; New York: 1999. [Google Scholar]
- Vopat M L, Kane P M, Christino M A, Truntzer J, McClure P, Katarincic J, Vopat B G.. Treatment of diaphyseal forearm fractures in children. Orthop Rev (Pavia). 2014; 6: 5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voto S J, Weiner D S, Leighley B.. Redisplacement after closed reduction of forearm fractures in children. J Pediatr Orthop 1990; 10: 79–84. [PubMed] [Google Scholar]
- Webb G R, Galpin R D, Armstrong D G.. Comparison of short and long arm plaster casts for displaced fractures in the distal third of the forearm in children. J Bone Joint Surg Am 2006; 88: 9–17. [DOI] [PubMed] [Google Scholar]
- Wilkins K E. Principles of fracture remodeling in children. Injury 2005; 36(Suppl): S3–S11. [DOI] [PubMed] [Google Scholar]
- World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013; 310: 2191–4. [DOI] [PubMed] [Google Scholar]
- Yang J J, Chang J H, Lin K Y, Lin L C, Kuo C L.. Redisplacement of diaphyseal fractures of the forearm after closed reduction in children: a retrospective analysis of risk factors. J Orthop Trauma 2012; 26: 110–6. [DOI] [PubMed] [Google Scholar]
- Zamzam M M, Khoshhal K I.. Displaced fracture of the distal radius in children: factors responsible for redisplacement after closed reduction. J Bone Joint Surg Br 2005; 87: 841–3. [DOI] [PubMed] [Google Scholar]
- Zhang J, Yu K F.. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 1998; 280: 1690–1. [DOI] [PubMed] [Google Scholar]
- Zionts L E, Zalavras C G, Gerhardt M B.. Closed treatment of displaced diaphyseal both-bone forearm fractures in older children and adolescents. J Pediatr Orthop 2005; 25: 507–12. [DOI] [PubMed] [Google Scholar]