Abstract
Phenylalanyl-tRNA synthetases [L-phenylalanine:tRNAPhe ligase (AMP-forming), EC 6.1.1.20] from Escherichia coli, yeast cytoplasm, and mammalian cytoplasm have an unusual conserved alpha 2 beta 2 quaternary structure that is shared by only one other aminoacyl-tRNA synthetase. Both subunits are required for activity. We show here that a single mitochondrial polypeptide from Saccharomyces cerevisiae is an active phenylalanyl-tRNA synthetase. This protein (the MSF1 gene product) is active as a monomer. It has all three characteristic sequence motifs of the class II aminoacyl-tRNA synthetases, and its activity may result from the recruitment of additional sequences into an alpha-subunit-like structure.
Full text
PDF



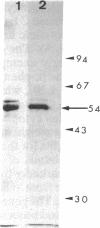
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltzinger M., Fasiolo F., Remy P. Yeast phenylalanyl-tRNA synthetase. Affinity and photoaffinity labelling of the stereospecific binding sites. Eur J Biochem. 1979 Jul;97(2):481–494. doi: 10.1111/j.1432-1033.1979.tb13136.x. [DOI] [PubMed] [Google Scholar]
- Brakhage A. A., Wozny M., Putzer H. Structure and nucleotide sequence of the Bacillus subtilis phenylalanyl-tRNA synthetase genes. Biochimie. 1990 Oct;72(10):725–734. doi: 10.1016/0300-9084(90)90157-c. [DOI] [PubMed] [Google Scholar]
- Cassio D., Waller J. P. Modification of methionyl-tRNA synthetase by proteolytic cleavage and properties of the trypsin-modified enzyme. Eur J Biochem. 1971 May 28;20(2):283–300. doi: 10.1111/j.1432-1033.1971.tb01393.x. [DOI] [PubMed] [Google Scholar]
- Cusack S., Berthet-Colominas C., Härtlein M., Nassar N., Leberman R. A second class of synthetase structure revealed by X-ray analysis of Escherichia coli seryl-tRNA synthetase at 2.5 A. Nature. 1990 Sep 20;347(6290):249–255. doi: 10.1038/347249a0. [DOI] [PubMed] [Google Scholar]
- Diatewa M., Boulanger Y., Stahl A. J. Comparison of yeast mitochondrial Phe-tRNA synthetase subunits to their cytoplasmic counterparts: isolation and determination of amino acid compositions. Biochem Biophys Res Commun. 1982 May 31;106(2):520–525. doi: 10.1016/0006-291x(82)91141-x. [DOI] [PubMed] [Google Scholar]
- Diatewa M., Stahl A. J. Purification and subunit structure of mitochondrial phenylalanyl-tRNA synthetase from yeast. Biochem Biophys Res Commun. 1980 May 14;94(1):189–198. doi: 10.1016/s0006-291x(80)80205-1. [DOI] [PubMed] [Google Scholar]
- Ducruix A., Hounwanou N., Reinbolt J., Boulanger Y., Blanquet S. Purification and reversible subunit dissociation of overproduced Escherichia coli phenylalanyl-tRNA synthetase. Biochim Biophys Acta. 1983 Nov 17;741(2):244–250. doi: 10.1016/0167-4781(83)90065-9. [DOI] [PubMed] [Google Scholar]
- Eriani G., Delarue M., Poch O., Gangloff J., Moras D. Partition of tRNA synthetases into two classes based on mutually exclusive sets of sequence motifs. Nature. 1990 Sep 13;347(6289):203–206. doi: 10.1038/347203a0. [DOI] [PubMed] [Google Scholar]
- Fasiolo F., Befort N., Boulander Y., Ebel J. P. Purification et quelques propriétés de la phenylalanyl-tRNA synthetase de levure de boulangerie. Biochim Biophys Acta. 1970 Oct 15;217(2):305–318. [PubMed] [Google Scholar]
- Fasiolo F., Fersht A. R. The aminoacyladenylate mechanism in the aminoacylation reaction of yeast phenylalanyl-tRNA synthetase. Eur J Biochem. 1978 Apr;85(1):85–88. doi: 10.1111/j.1432-1033.1978.tb12214.x. [DOI] [PubMed] [Google Scholar]
- Fasiolo F., Gibson B. W., Walter P., Chatton B., Biemann K., Boulanger Y. Cytoplasmic methionyl-tRNA synthetase from Bakers' yeast. A monomer with a post-translationally modified N terminus. J Biol Chem. 1985 Dec 15;260(29):15571–15576. [PubMed] [Google Scholar]
- Fayat G., Blanquet S., Dessen P., Batelier G., Waller J. P. The molecular weight and subunit composition of phenylalanyl-tRNA synthetase from Escherichia coli K-12. Biochimie. 1974;56(1):35–41. doi: 10.1016/s0300-9084(74)80353-6. [DOI] [PubMed] [Google Scholar]
- Fayat G., Mayaux J. F., Sacerdot C., Fromant M., Springer M., Grunberg-Manago M., Blanquet S. Escherichia coli phenylalanyl-tRNA synthetase operon region. Evidence for an attenuation mechanism. Identification of the gene for the ribosomal protein L20. J Mol Biol. 1983 Dec 15;171(3):239–261. doi: 10.1016/0022-2836(83)90092-x. [DOI] [PubMed] [Google Scholar]
- Gribskov M., Homyak M., Edenfield J., Eisenberg D. Profile scanning for three-dimensional structural patterns in protein sequences. Comput Appl Biosci. 1988 Mar;4(1):61–66. doi: 10.1093/bioinformatics/4.1.61. [DOI] [PubMed] [Google Scholar]
- Jasin M., Regan L., Schimmel P. Modular arrangement of functional domains along the sequence of an aminoacyl tRNA synthetase. Nature. 1983 Dec 1;306(5942):441–447. doi: 10.1038/306441a0. [DOI] [PubMed] [Google Scholar]
- Kern D., Giegé R., Ebel J. P. Purification and some properties of alanyl- and leucyl-tRNA synthetases from baker's yeast. Biochim Biophys Acta. 1981 Mar 26;653(1):83–90. doi: 10.1016/0005-2787(81)90106-4. [DOI] [PubMed] [Google Scholar]
- Koerner T. J., Myers A. M., Lee S., Tzagoloff A. Isolation and characterization of the yeast gene coding for the alpha subunit of mitochondrial phenylalanyl-tRNA synthetase. J Biol Chem. 1987 Mar 15;262(8):3690–3696. [PubMed] [Google Scholar]
- McClain W. H., Foss K. Nucleotides that contribute to the identity of Escherichia coli tRNA(Phe). J Mol Biol. 1988 Aug 20;202(4):697–709. doi: 10.1016/0022-2836(88)90551-7. [DOI] [PubMed] [Google Scholar]
- McDonald T., Breite L., Pangburn K. L., Hom S., Manser J., Nagel G. M. Overproduction, purification, and subunit structure of Escherichia coli glycyl transfer ribonucleic acid synthetase. Biochemistry. 1980 Apr 1;19(7):1402–1409. doi: 10.1021/bi00548a022. [DOI] [PubMed] [Google Scholar]
- Mechulam Y., Fayat G., Blanquet S. Sequence of the Escherichia coli pheST operon and identification of the himA gene. J Bacteriol. 1985 Aug;163(2):787–791. doi: 10.1128/jb.163.2.787-791.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pailliez J. P., Waller J. P. Phenylalanyl-tRNA synthetases from sheep liver and yeast. Correlation between net charge and binding to ribosomes. J Biol Chem. 1984 Dec 25;259(24):15491–15496. [PubMed] [Google Scholar]
- Regan L., Bowie J., Schimmel P. Polypeptide sequences essential for RNA recognition by an enzyme. Science. 1987 Mar 27;235(4796):1651–1653. doi: 10.1126/science.2435005. [DOI] [PubMed] [Google Scholar]
- Sampson J. R., DiRenzo A. B., Behlen L. S., Uhlenbeck O. C. Nucleotides in yeast tRNAPhe required for the specific recognition by its cognate synthetase. Science. 1989 Mar 10;243(4896):1363–1366. doi: 10.1126/science.2646717. [DOI] [PubMed] [Google Scholar]
- Sanni A., Mirande M., Ebel J. P., Boulanger Y., Waller J. P., Fasiolo F. Structure and expression of the genes encoding the alpha and beta subunits of yeast phenylalanyl-tRNA synthetase. J Biol Chem. 1988 Oct 25;263(30):15407–15415. [PubMed] [Google Scholar]
- Sanni A., Walter P., Ebel J. P., Fasiolo F. Construction of a FRS1-FRS2 operon encoding the structural genes for the alpha and beta subunits of yeast phenylalanyl-tRNA synthetase and its use in deletion analysis. Nucleic Acids Res. 1990 Apr 25;18(8):2087–2092. doi: 10.1093/nar/18.8.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmel P. R., Söll D. Aminoacyl-tRNA synthetases: general features and recognition of transfer RNAs. Annu Rev Biochem. 1979;48:601–648. doi: 10.1146/annurev.bi.48.070179.003125. [DOI] [PubMed] [Google Scholar]
- Schneller J. M., Schneller C., Martin R., Stahl A. J. Nuclear origin of specific yeast mitochondrial aminoacyl-tRNA synthetases. Nucleic Acids Res. 1976 May;3(5):1151–1165. doi: 10.1093/nar/3.5.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka W. K., Som K., Hardesty B. A. Comparison of free and ribosome-bound phenylalanyl-tRNA synthetase from rabbit reticulocytes. Arch Biochem Biophys. 1976 Jan;172(1):252–260. doi: 10.1016/0003-9861(76)90074-6. [DOI] [PubMed] [Google Scholar]
- Toth M. J., Schimmel P. Deletions in the large (beta) subunit of a hetero-oligomeric aminoacyl-tRNA synthetase. J Biol Chem. 1990 Jan 15;265(2):1000–1004. [PubMed] [Google Scholar]
- Tzagoloff A., Vambutas A., Akai A. Characterization of MSM1, the structural gene for yeast mitochondrial methionyl-tRNA synthetase. Eur J Biochem. 1989 Feb 1;179(2):365–371. doi: 10.1111/j.1432-1033.1989.tb14562.x. [DOI] [PubMed] [Google Scholar]