Abstract
Photodynamic inactivation of Staphylococcus aureus planktonic and biofilm cells by a phtotosensitizer, merocyanine 540 (MC 540), was investigated. For the planktonic experiments, MC 540 binding efficiency to bacterial cells was found to increase with both increasing MC 540 concentration and increasing incubation time, but the binding became saturated following 10 min of incubation. The antimicrobial activity was enhanced with an increasing light dose, but an increase in the light dose could not further improve the antimicrobial activity if the maximum excitation level attainable was less than the necessary minimum threshold level. Complete inactivation was achieved when the excitation level of MC 540 was somewhere above the threshold level. The relationship between antimicrobial activity and the excitation level of MC 540 revealed that the more MC 540 was excited, the more S. aureus cells were killed. For the biofilm experiments, the antimicrobial activity was enhanced with an increase in the light dose. No viable cells were detected when organisms were exposed to 15 μg of MC 540 per ml and a light dose of 600 J/cm2 or to 20 μg of MC 540 per ml and a light dose of 450 J/cm2. A quantitative analysis of MC 540 bound to biofilms was also performed, and the images from confocal laser scanning microscopy provided direct evidence that revealed the difference between the MC 540 remaining in the biofilms prior to irradiation and the MC 540 remaining in the biofilms after irradiation. The results of both the planktonic and biofilm experiments suggest that the antimicrobial activity of photodynamic inactivation of S. aureus is closely related to the excitation level of MC 540.
The formation and persistence of biofilms result in elevated transfer resistance, material deterioration, and health risks (4). Many strategies for biofilm control have been proposed, including stopping biofilm growth, blocking biofilm attachment, killing biofilms, promoting detachment, and mechanical removal (5, 6, 22). Among these approaches, application of chemical additives or biocides to inhibit microbial growth or metabolism of biofilms is the most common and economical method. However, biofilms are infamous for their recalcitrance to antimicrobial agents. Many scientists have attempted to develop new antimicrobial treatment approaches that do not induce bacterial resistance to antimicrobial agents. Using analogues of quorum-sensing signal molecules to block cell-to-cell communication within biofilms was deemed promising, although much research is still required (10). An alternative approach involves the use of photodynamic therapy (PDT) against microbial biofilms.
In PDT the interaction between light and certain photoactive compounds, known as photosensitizers (PS), is used to inactivate cell functions (1). When a PS is irradiated with light of an appropriate wavelength and at a certain level, the molecule becomes excited and consequently experiences a series of molecular energy transfers. These energy transfers lead to the liberation of cytotoxic singlet oxygen from the photosensitizer, which is lethal to many different bacteria (14).
With the discovery of new PS and the development of light sources, PDT has been reported to inactivate bacteria (24, 29), fungi (3, 29), and viruses (2, 18). Inactivation of Helicobacter pylori by PDT was reported to occur in vitro (11) and ex vivo (13). Using the endogenous PS δ-aminolevulinic acid, van der Meulen et al. (25) reported a 4-log reduction in viable cells of planktonic Haemophilus parainfluenzae, while Szocs et al. (21) observed 2-log killing of Escherichia coli. In addition to endogenous photoactive compounds, exogenous PS, such as hematoporphyrins, have also been reported to be effective when they are used for PDT and directed against Bacillus subtilis and Streptococcus faecalis (19). Using poly-l-lysine chlorine6, Rovaldi et al. observed 6.2- to 7.5-log reductions in viable cells when the treatment was directed against 12 different microorganisms (17). Recently, Hamblin et al. reported that a large polycation was necessary for PS to penetrate the impermeable outer membrane of the gram-negative organism E. coli, while the gram-positive organism Staphylococcus aureus was more easily penetrated by small molecules (9).
Although photodynamic inactivation (PDI) of planktonic cultures has been studied for many years, little is known about using PDI against biofilm cultures. Wilson et al. first reported that cyanine photosensitizers exhibited activity against oral streptococci present in a biofilm (26). Using confocal laser scanning microscopy, Wood et al. (27) also provided a qualitative description of PDI of natural oral plaque biofilms. These authors found considerable damage to bacteria and evident changes in structure within biofilms after PDI treatment. Unfortunately, no quantitative results for the relationship between PS excitation and antimicrobial activity against biofilms were reported.
In this study, we examined PDI of S. aureus planktonic and biofilm cells using an exogenous PS, merocyanine 540 (MC 540). MC 540 has shown antimicrobial activity against some planktonic gram-positive and gram-negative bacteria (7); however, the relationship between the amount of MC 540 bound to cells and any associated antimicrobial activity remains unknown. The aim of this study was to provide a quantitative description of MC 540-mediated PDI of S. aureus planktonic and biofilm cells and to demonstrate that the antimicrobial activity of PDI is closely related to the level of PS excitation.
MATERIALS AND METHODS
Bacterial strain and culture medium.
S. aureus BCRC 10780, purchased from the Bioresource Collection and Research Center (Hsinchu, Taiwan), was used throughout this study. Tryptic soy broth (TSB) (Difco, Detroit, Mich.) was used as the liquid medium; 0.1× TSB was used for batch cultures, while 0.01× TSB was used for continuous biofilm cultures.
Photosensitizer.
A stock solution containing 1 mg of MC 540 (Sigma Chemical Co, St. Louis, Mo.) per ml was prepared in 10% (vol/vol) ethanol and filter sterilized (0.22-μm-pore-size Acrodisc; Gelman Sciences, Ann Arbor, Mich.) immediately prior to use. The dye was appropriately diluted with phosphate-buffered saline (PBS) to obtain the desired concentration.
Light source.
A noncoherent light source (LumaCare LC-051; MBG Technologies, Inc., London, United Kingdom) was used to activate MC 540. Green light with a wavelength of 510 to 570 nm was used, and the output power was set at 500 to 600 mW/cm2. The light dose was calculated by multiplying the output power by the irradiation time.
Biofilm preparation.
S. aureus biofilms were cultured on a rotating disk reactor modified from the design of Pitts et al. (15). The reactor was made with a 500-ml polypropylene container (Nalgene, Rochester, N.Y.) and consisted of a Teflon rotor and 24 removable 316L stainless steel disks inserted on the rotor. One milliliter of an S. aureus seed culture was inoculated into 150 ml of 0.1× TSB in the reactor and incubated at room temperature overnight. After incubation, the reactor was fed continuously with 0.01× TSB at a rate of 468 ml/h. The biofilms reached a steady state after 24 h, and the average biofilm density was 1.09 × 107 ± 0.37 × 107 CFU/cm2.
MC 540 incubation and PDI in planktonic cells.
S. aureus cells grown in TSB at 37°C overnight were harvested by centrifugation at 3,100 × g and 4°C for 10 min and resuspended in PBS to produce a cell suspension containing 108 CFU/ml. Suspensions containing 108 CFU of S. aureus per ml were treated with 0, 5, 10, 15, and 20 μg of MC 540 per ml in the dark for various periods of time and then centrifuged at 3,100 × g and 4°C for 10 min. Each pellet was then resuspended in PBS. The suspension was split into a number of equivalent portions, each of which was irradiated with green light for a different period of time. Following irradiation, each suspension was separated into two parts, one of which was used for plate counting on tryptic soy agar (Difco) and one of which was used to assay the remaining MC 540 attached to the bacterial cells.
Assay of MC 540 bound to planktonic cells.
Control experiments were performed prior to irradiation; these experiments included determination of the fluorescence intensity of MC 540 bound to cells in the various cell suspensions, which was determined with a fluorescence spectrometer (Fluorolog; Jobin Yvon, New York, N.Y.). The suspensions were excited at a wavelength of 512 nm, and the resultant fluorescent emissions were recorded in the wavelength range from 550 to 750 nm. The excitation level of MC 540 was determined from the difference between the value for MC 540 bound to cells before irradiation and the value for MC 540 bound to cells after irradiation. The relative excitation level of MC 540 was calculated by using a standard curve for fluorescence intensity versus the quantity of pure MC 540 present.
MC 540 incubation and PDI in biofilms.
The disks with biofilms were placed into a sterile 48-well microtiter plate and treated with 0, 5, 10, 15, and 20 μg of MC 540 per ml in the dark for 40 min and then moved to a new dish. The disks were irradiated with green light for different periods of time and divided into two parts after irradiation; one part was used for viable cell counting with tryptic soy agar, and the other part was used for an assay of the remaining MC 540 bound to the biofilm cells.
Assay of MC 540 bound to biofilms.
Biofilms that were subjected to and were not subjected to PDI treatment were placed in a solution containing 1 μg of allophycocyanin (APC), a fluorescent stain specific for protein (16), per ml. After a 10-min incubation, the fluorescence intensity of MC 540 bound to biofilms was determined with a confocal laser scanning microscope (TCS SP2; Leica, Wetzlar, Germany). The biofilms were excited at wavelengths of 514 nm for MC 540 and 633 nm for APC. The resultant fluorescent emissions were recorded simultaneously in the wavelength range from 550 to 600 nm for MC 540 and in the wavelength range from 655 to 665 nm for APC. The MC 540 and APC images were analyzed by using Image-Pro Plus 3.0 (Media Cybernetics, Silver Spring, Md.) to calculate the total intensity of MC 540 and the total area of APC. To reduce variation due to biofilm heterogeneity, the intensity of MC 540 bound to biofilms was normalized by using the biofilm total area determined by APC staining. The excitation level of MC 540 was obtained by calculating the difference between the intensity of MC 540 bound to biofilms before irradiation and the intensity of MC 540 bound to biofilms after irradiation.
RESULTS
Effect of incubation concentration and time upon MC 540 bound to planktonic cells.
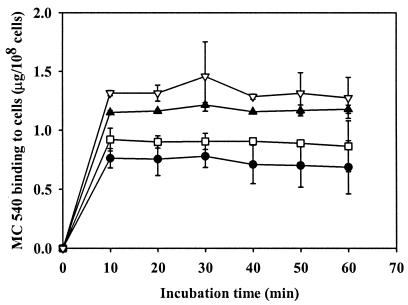
The amounts of MC 540 bound to S. aureus planktonic cells after incubation in the dark with a variety of concentrations of MC 540 for various time periods are shown in Fig. 1. No fluorescence from bacteria was detected without prior treatment of cells with MC 540; the relative amount of MC 540 bound to cells increased with time but reached a plateau after incubation for 10 min. The quantity of MC 540 bound to cells also increased with an increase in the MC 540 concentration.
FIG. 1.
MC 540 bound to S. aureus planktonic cells in response to treatment with 5 μg of MC 540 per ml (•), 10 μg of MC 540 per ml (□), 15 μg of MC 540 per ml (▴), and 20 μg of MC 540 per ml (▵) in the absence of light. The error bars indicate standard errors of the means from three separate experiments.
Effect of irradiation on antimicrobial activity and excitation level of MC 540 in planktonic cells.
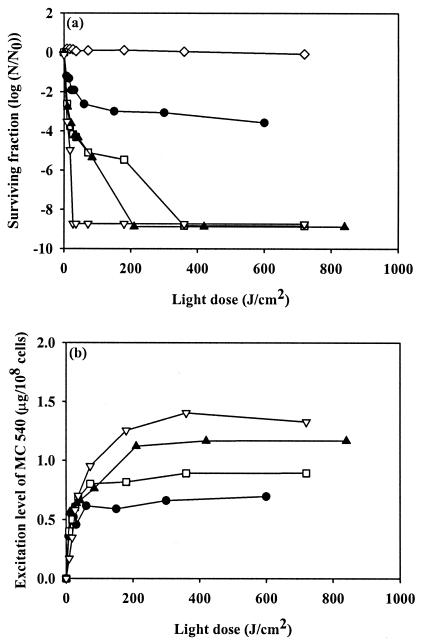
After a 10-min incubation of MC 540 with S. aureus, the cell suspension was irradiated with green light to activate the photosensitizer. Figure 2 shows the survival ratios and the excitation levels of MC 540 in response to different light doses. No antimicrobial activity was observed when the cells were irradiated without prior exposure to MC 540, regardless of the extent of irradiation. When cells were treated with 5 μg of MC 540 per ml, the survival of S. aureus cells decreased as the amount of irradiation increased, which resulted in a 3.0-log reduction in the number of viable cells present following exposure to 150 J/cm2. No further reduction in cell viability was observed, even if the light dose was increased to 600 J/cm2. A 5.5-log reduction in cell viability was observed when cells were treated with MC 540 at a concentration of 10 μg/ml and exposed to a light dose of 180 J/cm2, and no viable cell activity was detected when the light dose was increased to more than 360 J/cm2. All S. aureus cells became nonviable when they were exposed to MC 540 at a level of 15 μg/ml and when they were treated with a light dose of 210 J/cm2 or more. When MC 540 was added to bacterial cells at a concentration of 20 μg/ml, a subsequent light dose of 27 J/cm2 led to complete inactivation of irradiated S. aureus cells.
FIG. 2.
Antimicrobial activity of MC 540-mediated PDI of S. aureus planktonic cells (a) and excitation level of MC 540 (b) in response to treatment with no MC 540 (⋄), 5 μg of MC 540 per ml (•), 10 μg of MC 540 per ml (□), 15 μg of MC 540 per ml (▴), and 20 μg of MC 540 per ml (▵) for 10 min in the dark, followed by green light irradiation at various levels. N/N0 is the count measurement normalized by the initial count.
The excitation level of MC 540 was calculated from the difference between the degree of fluorescence of MC 540 attached to cells prior to irradiation and the degree of fluorescence of MC 540 attached to cells following irradiation. Most of the MC 540 bound to cells was excited by only 50 J of irradiation per cm2. When bacterial cells were exposed to MC 540 at levels of 5 and 10 μg/ml, the excitation level of MC 540 increased with the light dose and reached a plateau when the light dose was more than 50 J/cm2. Similar observations were also made for MC 540 concentrations of 15 and 20 μg/ml, and the corresponding maximum excitation level was reached at a light dose of less than 200 J/cm2.
Relationship between antimicrobial activity and excitation level of MC 540 in planktonic cells.
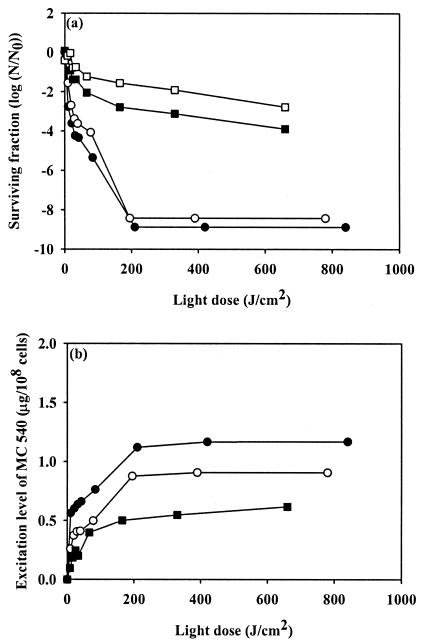
Figure 2 shows that an increase in the light dose could not further improve the antimicrobial activity of MC 540 if the maximum excitation level of MC 540 was less than 0.7 μg/108cells. By contrast, the antimicrobial activity data for MC 540 revealed that there was an increase in the activity with increasing light dose as long as the excitation level of MC 540 was greater than 0.82 μg/108cells; at this level, eventually complete killing of bacterial cells was achieved. Based on the observations described above, we hypothesized that a suprathreshold for the excitation level of MC 540 is required to activate PDT and to obtain complete antimicrobial activity. In order to verify this hypothesis, the following experiments were carried out. Initially, 108 CFU of S. aureus per ml was treated with 15 μg of MC 540 per ml in the dark for 10 min, and then the cells were centrifuged and the supernatant was removed. The pellets were diluted 1-, 2-, 5-, and 10-fold with PBS, and this was followed by irradiation for various periods of time. The bacterial cell survival ratio and corresponding excitation level of MC 540 after dilution are shown in Fig. 3. No viable cells were detected for the single dilution following exposure of cells to a light dose of 210 J/cm2, which resulted in a relative MC 540 excitation level of 1.12 ± 0.02 μg/108cells. Similar antimicrobial activity was observed for the twofold cellular dilution, when the excitation level was reduced to 0.87 ± 0.02 μg/108cells. For the fivefold dilution, the maximum excitation level was about 0.54 ± 0.01 μg/108cells, and only a 3.5-log reduction in the proportion of viable cells was observed. For the 10-fold dilution, we observed only a maximum of a 2.3-log reduction in cell viability, and the excitation level of MC 540 was below the detection limit due to insufficient cell numbers.
FIG. 3.
Antimicrobial activity of MC 540-mediated PDI of S. aureus (a) and excitation level of MC 540 (b) in response to treatment with 15 μg of MC 540 per ml for 10 min in the dark and 1-fold dilution (•), 2-fold dilution (○), 5-fold dilution (▪), and 10-fold dilution (□) with PBS, followed by green light irradiation at various intensities. N/N0 is the count measurement normalized by the initial count.
Effect of irradiation on antimicrobial activity of MC 540 against biofilms.
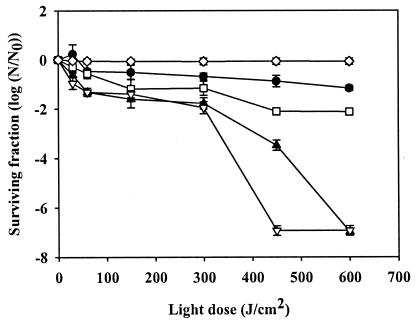
Figure 4 shows the survival data for MC 540-treated S. aureus biofilms in response to different light doses. No antimicrobial activity was found when the biofilms were irradiated without prior exposure to MC 540, regardless of the extent of irradiation. When the biofilms were treated with 5 μg of MC 540 per ml, the surviving fraction of S. aureus biofilm cells decreased gradually with increasing irradiation; there was a 1.2-log reduction in the number of viable cells after exposure to 600 J/cm2. A 2.1-log reduction in cell viability was observed when cells were treated with MC 540 at a concentration of 10 μg/ml and exposed to a light dose of 450 J/cm2; similar to biofilms treated with 5 μg/ml, no further reduction in cell viability was observed, even if the light dose was increased to 600 J/cm2. When biofilms were treated with 15 μg of MC 540 per ml, a 2-log reduction was observed after exposure to 300 J/cm2. The surviving fraction decreased dramatically as the level of irradiation reached 450 J/cm2, and no viable cells were detected when a light dose of 600 J/cm2 was used. All S. aureus cells were inactivated when they were exposed to 20 μg of MC 540 per ml and a light dose of 450 J/cm2 or more.
FIG. 4.
Antimicrobial activity of MC 540-mediated PDI of S. aureus biofilms. Biofilms were treated with no MC 540 (⋄), 5 μg of MC 540 per ml (•), 10 μg of MC 540 per ml (□), 15 μg of MC 540 per ml (▴), and 20 μg of MC 540 per ml (▵) for 40 min in the dark, followed by green light irradiation at various doses. The error bars indicate standard errors of the means from three separate experiments. N/N0 is the count measurement normalized by the initial count.
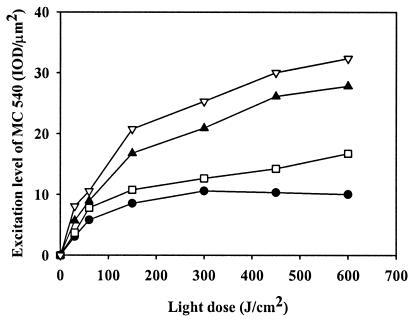
Effect of irradiation on excitation level of MC 540 in biofilms.
The biofilms binding MC 540 were an intense red color before irradiation. The area of MC 540 was identical to the area stained by APC prior to irradiation, indicating that there was universal binding of MC 540 to biofilms (data not shown). As the light dose increased, the total intensity of MC 540 bound to the biofilm decreased. Figure 5 shows the excitation level of MC 540 bound to S. aureus biofilms in response to different doses of irradiation. When biofilms were treated with 5 μg of MC 540 per ml, the excitation level of MC 540 increased with increasing light doses but reached a plateau after exposure to 300 J/cm2. When biofilms were treated with 10 μg of MC 540 per ml, an excitation level of 16.7 integrated optical density (IOD) units (units of fluorescent intensity recorded with a confocal laser scanning microscope)/μm2 was observed at a light dose of 600 J/cm2. When biofilms were treated with 15 μg of MC 540 per ml, the excitation level increased rapidly as the irradiation increased up to 150 J/cm2. When the irradiation exceeded 150 J/cm2, the excitation level increased slowly with increasing light doses and reached 27.8 IOD units/μm2 after the biofilm was treated with a 600-J/cm2 light dose. When biofilms were treated with 20 μg of MC 540 per ml, the same trend that was observed after treatment with 15 μg of MC 540 per ml was found.
FIG. 5.
Excitation level of MC 540 bound to biofilms. Biofilms were treated with 5 μg of MC 540 per ml (•), 10 μg of MC 540 per ml (□), 15 μg of MC 540 per ml (▴), and 20 μg of MC 540 per ml (▵) for 40 min in the dark, followed by green light irradiation at various doses.
Relationship between antimicrobial activity and excitation level of MC 540 in biofilms.
As shown in Fig. 4 and 5, the more MC 540 was excited, the more S. aureus biofilm cells were killed. Only a 2.1-log reduction in cell viability was observed when the excitation level of MC 540 was less than 25.3 IOD units/μm2. The antimicrobial activity of MC 540 increased when the excitation level reached 26.1 IOD units/μm2; at this level, there was a 3.5-log reduction in the number of viable cells. When the excitation level of MC 540 was greater than 26.1 IOD units/μm2, no viable cells were detected.
DISCUSSION
MC 540 has lethal photodamaging effects on a variety of enveloped viruses (20), as well as gram-positive and gram-negative bacteria (17). In our experiments with planktonic cultures and an assay of the efficiency of MC 540 binding to bacterial cells, the amount of MC 540 bound to cells reached saturation after a 10-min incubation period (Fig. 1). The efficiency of MC 540 bound to the bacterial cells might have been related to the composition and/or structural organization of the cellular membrane. For a liposome model, Yu and Hui proposed that MC 540 resides within cell membranes slightly above the domain of the glycerol backbone of the phospholipids and that its binding is very sensitive to the character of the lipid packing of the phospholipid bilayer (28). Gaffney et al. also reported that reducing the L1210 leukemia cell cholesterol content enhanced plasma membrane fluidity, the expression of dye-binding sites, and the susceptibility of the cells to MC 540-sensitized irradiation (8). Bacterial cell membranes differ from those of eukaryotic cells in that the former lack cholesterol. The precise details of the mechanism of binding of MC 540 to bacterial cells require further investigation. In this study, we investigated the relationship between the excitation level of MC 540 and the PDI efficacy against S. aureus planktonic and biofilm cells. In planktonic cultures, the amount of MC 540 bound to bacterial cells increased with both the incubation concentration and the duration of incubation, but the system appeared to be saturated after 10 min of incubation. These results are consistent with those of Tarshis et al., who reported that the amount of MC 540 bound to cells was dependent on the duration of incubation and the concentration (23). In biofilm cultures, complete inactivation of S. aureus biofilms was achieved by MC 540-mediated PDI. This might be attributed to a lack of a relationship between PDI and biofilm resistance mechanisms, such as diffusion limitation, biocide neutralization, and physiological adaptation. MC 540 is a negatively charged heterocyclic chromophore with a low molecular mass (570 Da). MC 540 had no difficulty diffusing into biofilms. MC 540 preferentially bound to biological membranes and liposomes (12), and it was less likely to be adsorbed or neutralized by exopolysaccharide. In addition, the binding of MC 540 to cells was not related to cell physiological adaptation.
We hypothesized that a certain minimum threshold for the level of excitation of MC 540 was required for PDI to result in optimal antimicrobial activity. This study revealed that at levels greater than a certain level of MC 540, an increase in the light dose does not further improve the antimicrobial activity if the maximum excitation level attainable is below the necessary minimum threshold level. The results from our dilution experiments also agreed with this hypothesis. A similar situation was also found in the biofilm experiments, as shown in Fig. 4 and 5.
To achieve the same PDI efficacy, a higher light dosage was required for biofilms than for planktonic cells. This phenomenon should not be attributed to incomplete penetration of MC 540 but might have been due to the stratification of light exposure within biofilms. In planktonic experiments, the cells were evenly exposed to light, and consequently, a lower dose of light was needed to obtain obvious PDI activity. By contrast, it took a higher dose of light to activate MC 540 within biofilms. The quantitative analyses of MC 540 bound to biofilms before and after photoactivation demonstrated that the antimicrobial activity of PDI was closely related to the level of PS excitation. The results of both planktonic and biofilm experiments suggest that MC 540-mediated PDI of S. aureus is a promising treatment. However, the use of PDI in the real world needs further development.
Acknowledgments
The financial support of the National Science Council of the Republic of China (grant NSC91-2320-B-002-198) is greatly appreciated.
REFERENCES
- 1.Ackroyd, R., C. Kelty, N. Brown, and M. Reed. 2001. The history of photodetection and photodynamic therapy. Photochem. Photobiol. 74:656-669. [DOI] [PubMed] [Google Scholar]
- 2.Ben-Hur, E., R. C. Hoeben, H. Van Ormondt, T. M. Dubbelman, and J. Van Steveninck. 1992. Photodynamic inactivation of retroviruses by phthalocyanines: the effects of sulphonation, metal ligand and fluoride. J. Photochem. Photobiol. B Biol. 13:145-152. [DOI] [PubMed] [Google Scholar]
- 3.Carre, V., O. Gaud, I. Sylvain, O. Bourdon, M. Spiro, J. Blais, R. Granet, P. Krausz, and M. Guilloton. 1999. Fungicidal properties of meso-arylglycosylporphyrins: influence of sugar substituents on photoinduced damage in the yeast Saccharomyces cerevisiae. J. Photochem. Photobiol. B Biol. 48:57-62. [DOI] [PubMed] [Google Scholar]
- 4.Costerton, J. W., Z. Lewandowski, D. E. Caldwell, D. R. Korber, and H. M. Lappin-Scott. 1995. Microbial biofilms. Annu. Rev. Microbiol. 49:711-745. [DOI] [PubMed] [Google Scholar]
- 5.Coudron, P. E., and C. W. Stratton. 1995. Utilization of time-kill kinetic methodologies for assessing the bactericidal activities of ampicillin and bismuth, alone and in combination, against Helicobacter pylori in stationary and logarithmic growth phases. Antimicrob. Agents Chemother. 39:66-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DiTizio, V., G. W. Ferguson, M. W. Mittelman, A. E. Khoury, A. W. Bruce, and F. DiCosmo. 1998. A liposomal hydrogel for the prevention of bacterial adhesion to catheters. Biomaterials 19:1877-1884. [DOI] [PubMed] [Google Scholar]
- 7.Dunne, W. M., Jr., and W. A. Slater. 1998. Antimicrobial activity of merocyanine 540: a photosensitizing dye. Diagn. Microbiol. Infect. Dis. 32:101-105. [DOI] [PubMed] [Google Scholar]
- 8.Gaffney, D. K., J. B. Feix, H. P. Schwarz, M. F. Struve, and F. Sieber. 1991. Cholesterol content but not plasma membrane fluidity influences the susceptibility of L1210 leukemia cells to merocyanine 540-sensitized irradiation. Photochem. Photobiol. 54:717-723. [DOI] [PubMed] [Google Scholar]
- 9.Hamblin, M. R., D. A. O'Donnell, N. Murthy, K. Rajagopalan, N. Michaud, M. E. Sherwood, and T. Hasan. 2002. Polycationic photosensitizer conjugates: effects of chain length and Gram classification on the photodynamic inactivation of bacteria. J. Antimicrob. Chemother. 49:941-951. [DOI] [PubMed] [Google Scholar]
- 10.Hentzer, M., K. Riedel, T. B. Rasmussen, A. Heydorn, J. B. Andersen, M. R. Parsek, S. A. Rice, L. Eberl, S. Molin, N. Hoiby, S. Kjelleberg, and M. Givskov. 2002. Inhibition of quorum sensing in Pseudomonas aeruginosa biofilm bacteria by a halogenated furanone compound. Microbiology 148:87-102. [DOI] [PubMed] [Google Scholar]
- 11.Marshall, B. 1983. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet 1:1273-1275. [PubMed] [Google Scholar]
- 12.Meagher, R. C., F. Sieber, and J. L. Spivak. 1983. Susceptibility to merocyanine 540-mediated photosensitization: a differentiation marker on murine hematopoietic progenitor cells. J. Cell Physiol. 116:118-124. [DOI] [PubMed] [Google Scholar]
- 13.Millson, C. E., M. Wilson, A. J. MacRobert, and S. G. Bown. 1996. Ex-vivo treatment of gastric Helicobacter infection by photodynamic therapy. J. Photochem. Photobiol. B Biol. 32:59-65. [DOI] [PubMed] [Google Scholar]
- 14.Pervaiz, S. 2001. Reactive oxygen-dependent production of novel photochemotherapeutic agents. FASEB J. 15:612-617. [DOI] [PubMed] [Google Scholar]
- 15.Pitts, B., A. Willse, G. A. McFeters, M. A. Hamilton, N. Zelver, and P. S. Stewart. 2001. A repeatable laboratory method for testing the efficacy of biocides against toilet bowl biofilms. J. Appl. Microbiol. 91:110-117. [DOI] [PubMed] [Google Scholar]
- 16.Rolinski, O. J., D. J. S. Birch, L. J. McCartney, and J. C. Pickup. 2001. Fluorescence nanotomography using resonance energy transfer: demonstration with a protein-sugar complex. Phys. Med. Biol. 46:N221-N226. [DOI] [PubMed] [Google Scholar]
- ‘17.Rovaldi, C. R., A. Pievsky, N. A. Sole, P. M. Friden, D. M. Rothstein, and P. Spacciapoli. 2000. Photoactive porphyrin derivative with broad-spectrum activity against oral pathogens in vitro. Antimicrob. Agents Chemother. 44:3364-3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rywkin, S., E. Ben-Hur, Z. Malik, A. M. Prince, Y. S. Li, M. E. Kenney, N. L. Oleinick, and B. Horowitz. 1994. New phthalocyanines for photodynamic virus inactivation in red blood cell concentrates. Photochem. Photobiol. 60:165-170. [DOI] [PubMed] [Google Scholar]
- 19.Shawar, R., and B. H. Cooper. 1990. Comparative kinetics of hematoporphyrin derivative uptake and susceptibility of Bacillus subtilis and Streptococcus faecalis to photodynamic action. Photochem. Photobiol. 52:825-830. [DOI] [PubMed] [Google Scholar]
- 20.Sieber, F., J. L. Spivak, and A. M. Sutcliffe. 1984. Selective killing of leukemic cells by merocyanine 540-mediated photosensitization. Proc. Natl. Acad. Sci. USA 81:7584-7587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szocs, K., F. Gabor, G. Csik, and J. Fidy. 1999. Delta-aminolaevulinic acid-induced porphyrin synthesis and photodynamic inactivation of Escherichia coli B. J. Photochem. Photobiol. B Biol. 50:8-17. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka, G., M. Shigeta, H. Komatsuzawa, M. Sugai, H. Suginaka, and T. Usui. 2000. Effect of clarithromycin on Pseudomonas aeruginosa biofilms. Chemotherapy 46:36-42. [DOI] [PubMed] [Google Scholar]
- 23.Tarshis, M., A. Katzenel, and S. Rottem. 1994. Use of merocyanine 540 and Hoechst 33258 for the selective killing of contaminating mycoplasmas in cell cultures. J. Immunol. Methods 168:245-252. [DOI] [PubMed] [Google Scholar]
- 24.Usacheva, M. N., M. C. Teichert, and M. A. Biel. 2001. Comparison of the methylene blue and toluidine blue photobactericidal efficacy against gram-positive and gram-negative microorganisms. Lasers Surg. Med. 29:165-173. [DOI] [PubMed] [Google Scholar]
- 25.van der Meulen, F. W., K. Ibrahim, H. J. Sterenborg, L. V. Alphen, A. Maikoe, and J. Dankert. 1997. Photodynamic destruction of Haemophilus parainfluenzae by endogenously produced porphyrins. J. Photochem. Photobiol. B Biol. 40:204-208. [DOI] [PubMed] [Google Scholar]
- 26.Wilson, M., T. Burns, and J. Pratten. 1996. Killing of Streptococcus sanguis in biofilms using a light-activated antimicrobial agent. J. Antimicrob. Chemother. 37:377-381. [DOI] [PubMed] [Google Scholar]
- 27.Wood, S., B. Nattress, J. Kirkham, R. Shore, S. Brookes, J. Griffiths, and C. Robinson. 1999. An in vitro study of the use of photodynamic therapy for the treatment of natural oral plaque biofilms formed in vivo. J. Photochem. Photobiol. B Biol. 50:1-7. [DOI] [PubMed] [Google Scholar]
- 28.Yu, H., and S. W. Hui. 1992. Merocyanine 540 as a probe to monitor the molecular packing of phosphatidylcholine: a monolayer epifluorescence microscopy and spectroscopy study. Biochim. Biophys. Acta 1107:245-254. [DOI] [PubMed] [Google Scholar]
- 29.Zeina, B., J. Greenman, W. M. Purcell, and B. Das. 2001. Killing of cutaneous microbial species by photodynamic therapy. Br. J. Dermatol. 144:274-278. [DOI] [PubMed] [Google Scholar]