Abstract
The presence of enterococci in lake and seawater in an 18-month survey comparing molecular (PCR and quantitative PCR) and culture methods was evaluated, as well as the possibility that zooplankton could act as reservoirs for enterococci. Samples of both water and zooplankton were collected monthly from a Lake Garda site and an Adriatic Sea site. In lake water, the positive samples numbered 13 of 54 (24%) by culture and 32 of 54 (59%) when PCR was applied. In seawater, they numbered 0 of 51 by culture and 18 of 51 (35%) by PCR. Enterococci were found either totally bound to plankton or totally in water, depending on the presence or absence of plankton, respectively. These results clearly indicate that the PCR assay is a powerful tool for detecting fecal indicators and pathogens in the environment, thus providing a much more sensitive method than culture.
When bacteria of medical interest are released into the external environment, they find conditions that are often opposed to those encountered during infection, in that the environment (e.g., marine or freshwater) is often oligotrophic, cold, of high or very low osmolarity, and exposed to solar radiation (2, 6, 11). Starting in the early 1980s, Rita Colwell and her coworkers detected a survival strategy adopted by bacteria when exposed to environmental stresses to prevent rapid cell death. This strategy is known as the “viable but nonculturable” (VBNC) state (4-6, 14, 23, 27). This condition can be summarized as a dormant state into which bacteria enter and switch off most of the metabolic activities. In the VBNC state bacteria were shown to be unable to form colonies on conventional growth media, but cells maintained viability (6, 17, 18, 20, 32) and gene transcription, accompanied by specific mRNA production (9, 12, 20-22), and cell wall modifications (8, 17, 34, 35) took place. Furthermore, resuscitation, i.e., restoration of cell division, has been demonstrated in a part of the VBNC population on returning to suitable environmental conditions (7, 17, 18, 20, 29, 36). These bacterial forms are not detectable with the methods currently used to ascertain the microbiological quality of the environment. Thus, it could be concluded that the real bacterial-pathogen load in environments would be unidentified or underestimated (6, 14, 25, 28).
Another recently identified strategy which enables human pathogens to survive and persist when released in water is the ability to adhere to chitin-containing surfaces, such as those of zooplanktonic organisms (1, 3, 13, 15, 16, 26, 30, 37, 38). This strategy has been analyzed in detail for Vibrio cholerae and for other vibrios which are causative agents of human pathologies. It has been concluded that this may be the mode of persistence of vibrios in the environment in interepidemic periods (5, 14, 19, 31). In addition, vibrios adhering to zooplankton in both the culturable and nonculturable state were detected (19, 25). This strategy, however, could alter the evaluation of the microbiological load in waters, in that zooplankton, by binding bacteria, can withdraw and/or concentrate bacteria in a given volume of water.
Microbiological evaluation of surface waters usually involves the detection of specific pathogens and, most commonly, the detection of fecal indicators. Of all the indicators tested, enterococci are those which correlate best with the incidence of diarrheal diseases observed among swimmers (10). For this reason enterococci (mainly Enterococcus faecalis and Enterococcus faecium) are currently considered useful indicators of fecal contamination of waters. We have shown that enterococci can enter the VBNC state under exposure to oligotrophic environments (20, 22, 23). Enterococci in this state are no longer detectable in environmental samples if the culture method alone is applied (24).
In this study we describe the results of the monitoring of the microbiological quality of both freshwater and marine water by applying an approach consisting of detecting both culturable and nonculturable enterococci which are present in water and adherent to the plankton in order to evaluate to what extent the adhesion to plankton and the VBNC state may represent survival strategies and contribute to the formation of environmental reservoirs of these microorganisms.
Water sampling sites and conditions and microbiological procedures.
Samples of both freshwater and marine water were collected monthly between March 2001 and September 2002 from Lake Garda offshore from Lazise on the eastern side of the lake (Province of Verona, Italy) and from the Adriatic Sea offshore from Senigallia (Province of Ancona, Italy). Marine plankton organism sampling was carried out by dragging the water horizontally, at a depth of about 1 m, with a 200-μm-mesh plankton net, while for lake plankton organisms a 100-μm-mesh plankton net was used. Five to 10 m3 of water was dragged at each sampling. One-half of the dragged material was fixed with 2% (final concentration) formaldehyde for subsequent plankton characterization, while the other half was immediately transferred to the laboratory and processed within 6 h of collection for microbiological and molecular investigations. Ten liters of water was also collected and immediately filtered through a 100- or 200-μm net (lake and seawater, respectively) and then through a 64-μm net. This material was considered plankton ranging in size from 64 to 100 μm or 64 to 200 μm. At the same time as each plankton sampling, two additional liters of water was collected to evaluate the number of enterococci present in water.
To evaluate the load of culturable enterococci bound to zooplankton, 100 μl of the dragged material, after standing in a sonication bath for 30 s, was spread on m-Enterococcus agar (mEA; Becton Dickinson) and plates were incubated at 35°C for 3 days (39). To evaluate the enterococcal load present in water, the samples of fresh- or seawater were first filtered on a piece of the 64-μm net in order to remove the highly particulate matter (plankton included). One hundred milliliters of the resulting water was then filtered on a 0.22-μm-pore-size Millipore membrane (47 mm in diameter). Membranes were placed faceup on mEA plates, which were incubated at 35°C for 3 days (39).
To perform molecular detection of enterococci, DNA was extracted from either 1 ml of dragged material or 1 ml of 200× freshwater or seawater concentrated by filtration on 0.22-μm Millipore membrane, as previously described (24). The target sequence for PCR amplification was a 444-bp fragment located on the E. faecalis chromosome within the pbp5 gene, coding for penicillin binding protein 5 (PBP5), previously sequenced and demonstrated to be species specific (33). The fragment was amplified by PCR with two primers selected from within the gene: primer FWD (5′ CATGCGCAATTAATCGG 3′) and primer REV (5′ CATAGCCTGTCGCAAAAC 3′). To perform competitive PCR (cPCR), a deleted internal standard amplified by the same primers (24) was used. The sensitivity of the method was 50 cells per ml (24).
Zooplankton detection as a function of changes in physicochemical parameters in both freshwater and seawater.
Lake Garda is the biggest Italian, lake with a surface of 368 km2. The total volume is 49 km3, while the mean outflow rate of the Mincio river is 58 m3/s, so that total water replacement time is calculated to be 27 years. The resident population of the lakeside villages is 160,000 people, but this population is as much as 25 times greater in the warmest months (April to September) as a result of the booming tourist industry. The surface water temperature ranged from 7 to 26°C, and pH ranged from 7.9 to 9.1, while the only salt present was calcium carbonate at a concentration ranging from 150 to 180 mg/liter. The zooplankton (>100 μm) concentration varied according to temperature in that a peak was recorded during the summer (highest value in the summer of 2001, with 14,500 organisms/m3 of water, and 9,000 organisms/m3 of water in the summer of 2002), while during the winter (water temperature: 7°C) zooplankton practically disappeared (Table 1). The zooplankton comprised rotifers, cladocers, and copepods. Copepods, including Calanoides (Copipodiaptomus steueri) and Cyclopoides (Mesocyclops leuckarti) spp. were highly prevalent (>85%) among zooplankton families.
TABLE 1.
Occurrence of E. faecalis in lake water and plankton samples as determined by culture and molecular methods
| Mo | No. of plankton >100 μm (organisms/m3) | Level of E. faecalis by:
|
|||||
|---|---|---|---|---|---|---|---|
| Culture method
|
Quantitative PCRa
|
||||||
| On plankton >100 μm (CFU/m3) | On plankton 64 to 100 μm (CFU/100 ml) | In water (CFU/100 ml) | On plankton >100 μm (no./m3) | On plankton 64 to 100 μm (no./100 ml) | In water (no./100 ml) | ||
| April 2001 | 250 | 0 | 0 | 0 | 0 | 0 | 1.3 × 106 ± 4.9 × 105 |
| May | 300 | 0 | 3 | 300 | 0 | 0 | 2.3 × 107 ± 5.3 × 106 |
| June | 850 | 0 | 0 | 25 | 550 ± 140 | <50 | 9.2 × 106 ± 2.1 × 106 |
| July | 3,300 | 600 | 0 | 16 | 5.6 × 105 ± 1.4 × 105 | 1,200 ± 340 | 2.6 × 103 ± 1.2 × 103 |
| August | 10,000 | 750 | 0 | 0 | 5.2 × 108 ± 7.8 × 107 | 800 ± 260 | 0 |
| September | 14,500 | 0 | 0 | 0 | 7.3 × 108 ± 1.6 × 108 | 170 ± 120 | 0 |
| October | 1,150 | 0 | 0 | 0 | 8.9 × 104 ± 2.4 × 104 | 70 ± 45 | 0 |
| November | 500 | 0 | 0 | 0 | 0 | 0 | <50 |
| December | 200 | 0 | 0 | 0 | 0 | 0 | 2.5 × 106 ± 7.9 × 105 |
| January 2002 | 100 | 0 | 0 | 0 | 0 | 0 | 1.8 × 106 ± 7.4 × 105 |
| February | 250 | 0 | 0 | 0 | 0 | 0 | 1.1 × 106 ± 3.7 × 105 |
| March | 500 | 0 | 0 | 0 | 0 | 0 | 3.7 × 105 ± 1.3 × 105 |
| April | 1,400 | 0 | 0 | 0 | 0 | 0 | 5.3 × 104 ± 1.2 × 104 |
| May | 4,400 | 900 | 0 | 2 | 1,100 ± 270 | 0 | <50 |
| June | 9,000 | 500 | 0 | 5 | 6.1 × 106 ± 1.9 × 106 | 240 ± 170 | <50 |
| July | 7,500 | 3,000 | 0 | 60 (28)b | 6.4 × 104 ± 4.5 × 104 | 90 ± 75 | <50 |
| August | 6,000 | 0 | 1 | 0 | 4.4 × 106 ± 7.8 × 105 | 120 ± 90 | 0 |
| September | 4,500 | 0 | 0 | 0 | 8.6 × 104 ± 5.6 × 104 | 100 ± 80 | 0 |
E. faecalis-equivalent cell number (no.) was calculated as described by Lleò et al. (24).
Includes different enterococcal species: E. faecalis, E. faecium, and Enterococcus hirae. The E. faecalis count is in parentheses.
As far as the seawater was concerned, the water temperature changed seasonally, with a trend similar to that for Lake Garda, while zooplankton (>200 μm) counts per cubic meter of seawater were subject to substantial variability throughout the year, though this was not directly related to water temperature (Table 2). Copepods were highly dominant among the zooplankton organisms of the Adriatic Sea, and species such as Paracalanus parvus, Clausocalanus clause, Centropages typicus, Oithona elgolandica and Acartia clause were included. Cladocers such as Penilia avirostris, Evadne spp. and Podon spp. were detected, especially during the warm season.
TABLE 2.
Occurrence of E. faecalis in seawater and plankton samples as determined by culture and molecular methods
| Mo | No. of plankton >200 μm (organisms/m3) | Level of E. faecalis by:
|
|||||
|---|---|---|---|---|---|---|---|
| Culture method
|
Quantitative PCRa
|
||||||
| On plankton >200 μm (CFU/m3) | On plankton 64 to 200 μm (CFU/100 ml) | In water (CFU/100 ml) | On plankton >200 μm (no./m3) | On plankton 64 to 200 μm (no./100 ml) | In water (no./100 ml) | ||
| April 2001 | 1,300 | 0 | 0 | 0 | 0 | 0 | 0 |
| May | 1,000 | 0 | 0 | 0 | 420 ± 260 | 480 ± 370 | 0 |
| June | 2,100 | 0 | 0 | 0 | 0 | 0 | 0 |
| July | 300 | 0 | 0 | 0 | 8,400 ± 3,750 | 640 ± 290 | 4.2 × 104 ± 2.7 × 104 |
| August | 2,600 | 0 | 0 | 0 | 6.4 × 104 ± 2.9 × 104 | 360 ± 130 | 0 |
| September | 1,200 | 0 | 0 | 0 | 1,900 ± 640 | 120 ± 85 | 0 |
| October | 1,500 | 0 | 0 | 0 | 0 | 220 ± 90 | 0 |
| November | 1,700 | 0 | 0 | 0 | 1,450 ± 730 | 360 ± 125 | 0 |
| December | 2,000 | 0 | 0 | 0 | 0 | 0 | 0 |
| January 2002 | 550 | 0 | 0 | 0 | <50 | 0 | 0 |
| February | 60 | 0 | 0 | 0 | 0 | 0 | 0 |
| March | 500 | 0 | 0 | 0 | 0 | 0 | 0 |
| April | 900 | 0 | 0 | 0 | <50 | 0 | 0 |
| May | 3,300 | 0 | 0 | 0 | <50 | 0 | 0 |
| June | 300 | 0 | 0 | 0 | 0 | 0 | 0 |
| July | 3,700 | 0 | 0 | 0 | <50 | <50 | <50 |
| August | 3,250 | 0 | 0 | 0 | 0 | 0 | 0 |
E. faecalis-equivalent cell number (no.) was calculated as described by Lleò et al. (24).
Detection of enterococci adherent to zooplankton and in water by culture and molecular methods in both lake and seawater.
Table 1 shows the results of the search for enterococci by the cultural method in Lake Garda. Enterococci adherent to zooplankton measuring more than 100 μm or in water were sporadically detected by the culture method in late spring and early summer in both 2001 and 2002. With the exception of one sample (August 2002), plankton ranging in size from 64 to 100 μm did not yield bound enterococci. When, however, detection of enterococci was performed in the same samples by the molecular method (PCR) a much higher number proved positive (32 of 54 samples), thus suggesting that enterococci were present in Lake Garda all year round. cPCR (quantitative PCR) yielded surprising results: in samples collected in summer and early fall (June to October), when the highest numbers of zooplankton elements were detected, only very large numbers of enterococci adhering to zooplankton were detected, while no enterococci were detected in water. In samples collected during the cold months (December to April), in which zooplankton counts were lower than 500 plankton organisms/m3, enterococci were detected only in water.
Table 2 shows the results of enterococcus detection in seawater. The culture method yielded negative results for all samples whether the samples contained zooplankton or not. When the enterococcal presence was evaluated by the cPCR method several samples proved positive (18 of 51). In particular, positive results were detected in samples containing zooplankton that were collected during the months May to November 2001. In comparison with results for samples collected from Lake Garda, the number of enterococci was much lower. During the next few months and in spring to summer 2002 the number of positive samples and the bacterial counts were less than in the previous year; once again, however, the positive samples were those containing zooplankton. Enterococci were detected in only 3 out of 16 water samples, but the equivalent plankton samples always proved positive.
To rule out the possibility that these positive samples may simply have been an artifact due to binding of enterococcal free DNA (originating from the lysis of dead microorganisms) to zooplankton, we performed an in vitro experiment in which zooplankton collected from Lake Garda previously shown to be negative for enterococci by PCR was placed in contact with E. faecalis purified DNA in the presence of Lake Garda water. After extensive washing with lake water, any DNA still bound to the copepods was detected by PCR. No positive results were detected in the three separate experiments performed.
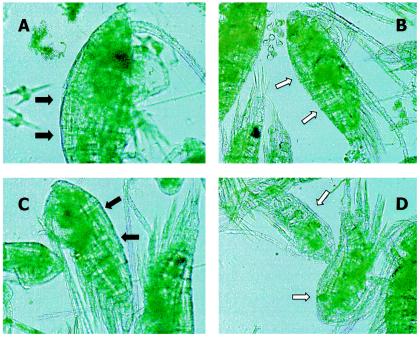
To confirm the unexpected results of the field research, in which enterococci were prevalently bound to plankton, we tried to detect whole adherent enterococci using anti-D antibodies bound to microbeads. A sample (100 μl) of formalin-fixed plankton was mixed with a drop of Phadebact Strep D reagent (Boule Diagnostics) and allowed to stand for a few seconds at room temperature with gentle agitation. Figure 1 shows that zooplankton organisms collected from Lake Garda that proved negative for enterococci as detected by PCR did not present microbeads on their surfaces (Fig. 1B and D), while the same zooplankton organisms that were exposed in vitro to enterococci resuspended in lake water presented bound microbeads (Fig. 1A). Figure 1C shows zooplankton organisms from a sample that was negative for enterococci by the culture method but positive by PCR that presented microbeads adhering to their surfaces.
FIG. 1.
Microscopic observation of lake zooplankton treated with anti-D antibody bound to latex microbeads (Phadebact Strep D reagent; Boule Diagnostic). Positive (A) and negative (B) controls consisted of zooplankton which was exposed, in vitro or not, to an E. faecalis culture. Black arrows (A and C) indicate the presence of latex microbeads as a black striation on the carapace; white arrows (B and D) indicate the carapace without adherent microbeads. (C) Sample collected from Lake Garda negative by culture but positive by PCR. (D) Sample collected from Lake Garda negative by both culture and PCR for enterococci.
Finally, to rule out the possibility of detection by PCR of DNA of inactivated but structurally intact enterococci, we performed, on some PCR-positive samples, a search for pbp5 mRNA, which was previously shown (22) to be a marker of the viability of nonculturable E. faecalis cells. All three water samples and five plankton samples positive by PCR contained pbp5 mRNA, as determined by reverse transcription-PCR (data not shown), thus indicating that at least part of the nonculturable enterococci detected by PCR were in the VBNC state.
The aim in this study was to compare culture and molecular (quantitative) methods of detecting E. faecalis in the water and/or adhering to zooplankton in order to define whether these survival strategies truly represent a matter for public concern. We show that molecular methods for the detection of enterococci resulted in a higher number of positive samples than the culture method. In particular, 13 of 54 (24%) samples of lake water were positive by the culture method, as against 32 of 54 (59%) when PCR was used. In seawater the respective figures were 0 of 51 (0%) by the culture method and 18 of 51 (35%) by PCR. Evaluation of E. faecalis cell numbers by cPCR yielded the highest values in lake water. This should not be surprising, in that lakes have a slow water replacement, which could result in serious environmental damage due to bacterial overload. Furthermore, the substantial discrepancy between the culture and molecular methods of detecting E. faecalis in both freshwater and seawater may be tentatively explained by the fact that this microorganism may persist in waters prevalently in the nonculturable state, like V. cholerae (4-6, 19, 25).
The most interesting result of this study was the observation that in Lake Garda E. faecalis is almost exclusively found either adhering to plankton or in water, and not both. This result was also confirmed by the results in seawater, although not to such an evident extent. One reason that can be adduced to explain this is the fact that plankton is always observed in coastal seawater all year round because of the continuous supply of warmer water and plankton from the open sea. Thus, in general, because plankton is observed all year round in the sea, positive plankton samples are much more prevalent than positive water samples.
To the best of our knowledge, this is the first description of a gram-positive bacterium that persists in the environment by adherence to plankton. Very recently, Whitman et al. (40) described the occurrence of Escherichia coli and enterococci in Cladophora (an alga) in nearshore water and beach sand of Lake Michigan.
Acknowledgments
We thank Orazio Ruzzenente (Laboratorio Analisi, Ospedale di Valeggio sul Mincio, Verona) for his invaluable help during the microscopic observation of plankton.
This study was supported by grants 01.00255.PF 49 and 01.00326.PF49(Target Project on Biotechnology) from the Consiglio Nazionale delle Ricerche and by Cofin2000 from the Ministero dell'Istruzione, dell'Università e della Ricerca, Italy.
REFERENCES
- 1.Amako, K., S. Shimodori, T. Imoto, S. Miake, and A. Umeda. 1987. Effects of chitin and its soluble derivatives on survival of Vibrio cholerae O1 at low temperatures. Appl. Environ. Microbiol. 53:603-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barcina, I., P. Lebaron, and J. Vives-Rego. 1997. Survival of allochthonous bacteria in aquatic systems: a biological approach. FEMS Microbiol. Ecol. 23:1-9. [Google Scholar]
- 3.Carli, A., L. Pane, L. Casareto, S. Bertone, and C. Pruzzo. 1993. Occurrence of Vibrio algynolyticus in Ligurian coast rock pools (Tyrrhenian Sea, Italy) and its association with the copepod Tigriopus fulvus (Fisher 1860). Appl. Environ. Microbiol. 59:1960-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carroll, J. W., M. C. Mateescu, K. Chava, R. R. Colwell, and A. K. Bej. 2001. Response and tolerance of toxigenic Vibrio cholerae O1 to cold temperatures. Antonie Leeuwenhoek 79:377-384. [DOI] [PubMed] [Google Scholar]
- 5.Colwell, R. R. 1996. Global climate and infectious disease: the cholera paradigm. Science 274:2025-2031. [DOI] [PubMed] [Google Scholar]
- 6.Colwell, R. R. 2000. Bacterial death revisited, p. 325-342. In R. R. Colwell, and D. J. Grimes (ed.), Nonculturable microorganisms in the environment. ASM Press, Washington, D.C.
- 7.Colwell, R. R., P. R. Brayton, A. Huq, B. Tall, P. Harrington, and M. Levine. 1996. Viable but non-culturable Vibrio cholerae O1 revert to a culturable state in the human intestine. World J. Microbiol. Biotechnol. 12:28-31. [DOI] [PubMed] [Google Scholar]
- 8.Costa, K., G. Bacher, G. Allmaier, M. G. Dominguez-Bello, L. Engstrand, P. Falk, M. A. De Pedro, and F. Garcia-del Portillo. 1999. The morphological transition of Helicobacter pylori cells from spiral to coccoid is preceded by a substantial modification of the cell wall. J. Bacteriol. 181:3710-3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher-Le Saux, M., D. Hervio-Heath, S. Loaec, R. R. Colwell, and M. Pommepuy. 2002. Detection of cytotoxin-hemolysin in nonculturable populations of environmental and clinical Vibrio vulnificus strains in artificial seawater. Appl. Environ. Microbiol. 68:5641-5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujioka, R. S. 1997. Indicators in marine recreational water quality, p. 176-183. In C. J. Hurst (ed.), Manual of environmental microbiology. ASM Press, Washington, D.C.
- 11.Gauthier, M. J. 2000. Environmental parameters associated with the viable but nonculturable state, p. 87-112. In R. R. Colwell, and D. J. Grimes (ed.), Nonculturable microorganisms in the environment. ASM Press, Washington, D.C.
- 12.Heim, S., M. M. Lleò, B. Bonato, C. A. Guzman, and P. Canepari. 2002. The viable but nonculturable state and starvation are different stress responses of Enterococcus faecalis, as determined by proteome analysis. J. Bacteriol. 184:6739-6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hood, M. A., and P. A. Winter. 1997. Attachment of Vibrio cholerae under various environmental conditions and to selected substrates. FEMS Microbiol. Ecol. 22:215-223. [Google Scholar]
- 14.Huq, A., I. N. G. Rivera, and R. R. Colwell. 2000. Epidemiological significance of viable but nonculturable microorganisms, p. 301-323. In R. R. Colwell, and D. J. Grimes (ed.), Nonculturable microorganisms in the environment. ASM Press, Washington, D.C.
- 15.Huq, A., P. A. West, E. B. Small, M. I. Huq, and R. R. Colwell. 1983. Ecological relationships between Vibrio cholerae and planktonic crustacean copepods. Appl. Environ. Microbiol. 45:275-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huq, A., P. A. West, E. B. Small, M. I. Huq, and R. R. Colwell. 1984. Influence of water temperature, salinity, and pH on survival and growth of toxigenic Vibrio cholerae serovar O1 associated with live copepods in laboratory microcosmos. Appl. Environ. Microbiol. 48:420-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jians, X., and T. Chai. 1996. Survival of Vibrio parahaemolyticus at low temperatures under starvation conditions and subsequent resuscitation of viable nonculturable cells. Appl. Environ. Microbiol. 62:1300-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaprelyants, A. S., and D. B. Kell. 1993. Dormancy in stationary-phase cultures of Micrococcus luteus: flow cytometry analysis of starvation and resuscitation. Appl. Environ. Microbiol. 59:3187-3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lipp, E. K., I. N. G. Rivera, A. I. Gil, E. M. Espeland, N. Choopun, V. R. Louis, E. Russek-Cohen, A. Huq, and R. R. Colwell. 2003. Direct detection of Vibrio cholerae in Peruvian coastal water and plankton by PCR. Appl. Environ. Microbiol. 69:3676-3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lleò, M. M., B. Bonato, M. C. Tafi, C. Signoretto, M. Boaretti, and P. Canepari. 2001. Resuscitation rate in different enterococcal species in the viable but non-culturable state. J. Appl. Microbiol. 91:1095-1102. [DOI] [PubMed] [Google Scholar]
- 21.Lleò, M. M., B. Bonato, C. Signoretto, and P. Canepari. 2003. Vancomycin resistance is maintained in enteroccci during their permanence in the viable but nonculturable state and after division is resumed. Antimicrob. Agents Chemother. 47:1154-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lleò, M. M., S. Pierobon, M. C. Tafi, C. Signoretto, and P. Canepari. 2000. mRNA detection by RT-PCR for monitoring viability over time in an Enterococcus faecalis viable but nonculturable population maintained in a laboratory microcosm. Appl. Environ. Microbiol. 66:4564-4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lleò, M. M., M. C. Tafi, and P. Canepari. 1998. Nonculturable Enterococcus faecalis cells are metabolically active and capable of resuming active growth. Syst. Appl. Microbiol. 21:333-339. [DOI] [PubMed] [Google Scholar]
- 24.Lleò, M. M., M. C. Tafi, C. Signoretto, C. Dal Cero, and P. Canepari. 1999. Competitive polymerase chain reaction for quantification of nonculturable Enterococcus faecalis cells in lake water. FEMS Microbiol. Ecol. 30:345-353. [DOI] [PubMed] [Google Scholar]
- 25.Louis, V. R., E. Russek-Cohen, N. Choopun, I. N. G. Rivera, B. Gangle, S. C. Jiang, A. Rubin, J. A. Patz, A. Huq, and R. R. Colwell. 2003. Predictability of Vibrio cholerae in Chesapeake Bay. Appl. Env. Microbiol. 69:2773-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montanari, M. P., C. Pruzzo, L. Pane, and R. R. Colwell. 1999. Vibrios associated with plankton in a coastal zone of the Adriatic Sea (Italy). FEMS Microbiol. Ecol. 29:241-247. [Google Scholar]
- 27.Oliver, J. D. 1993. Formation of viable but nonculturable cells, p. 239-272. In S. Kjelleberg (ed.), Starvation in bacteria. Plenum Press, New York, N.Y.
- 28.Oliver, J. D. 2000. The public health significance of viable but nonculturable bacteria, p. 277-300. In R. R. Colwell, and D. J. Grimes (ed.), Nonculturable microorganisms in the environment. ASM Press, Washington, D.C.
- 29.Oliver, J. D., and R. Bockian. 1995. In vivo resuscitation and virulence towards mice of viable but nonculturable cells of Vibrio vulnificus. Appl. Environ. Microbiol. 61:2620-2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pruzzo, C., A. Crippa, S. Bertone, L. Pane, and A. Carli. 1996. Attachment of Vibrio alginolyticus to chitin mediated by chitin binding proteins. Microbiology 142:2181-2186. [DOI] [PubMed] [Google Scholar]
- 31.Pruzzo, C., R. Tarsi, M. M. Lleò, C. Signoretto, M. Zampini, L. Pane, R. R. Colwell, and P. Canepari. 2003. Persistence of adhesive properties of Vibrio cholerae after long term exposure to sea water. Environ. Microbiol. 5:650-658. [DOI] [PubMed] [Google Scholar]
- 32.Rahman, I., M. Shahamat, P. A. Kirchman, E. Rissek-Cohen, and R. R. Colwell. 1994. Methionine uptake and cythopathogenicity of viable but nonculturable Shigella dysenteriae type 1. Appl. Environ. Microbiol. 60:3573-3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Signoretto, C., M. Boaretti, and P. Canepari. 1994. Cloning, sequencing and expression in Escherichia coli of the low-affinity penicillin-binding protein of Enterococcus faecalis. FEMS Microbiol. Lett. 123:99-106. [DOI] [PubMed] [Google Scholar]
- 34.Signoretto, C., M. M. Lleò, and P. Canepari. 2002. Modification of the peptidoglycan of Escherichia coli in viable but nonculturable state. Curr. Microbiol. 44:125-131. [DOI] [PubMed] [Google Scholar]
- 35.Signoretto, C., M. M. Lleò, M. C. Tafi, and P. Canepari. 2000. Cell wall chemical composition of Enterococcus faecalis in the viable but nonculturable state. Appl. Environ. Microbiol. 66:1953-1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steinert, M., L. Emody, R. Amann, and J. Hacker. 1997. Resuscitation of viable but nonculturable Legionella pneumophila Philadelphia JR 32 by Acanthamoeba castellanii. Appl. Environ. Microbiol. 63:2047-2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tamplin, M. L., A. L. Gauzens, A. Huq, D. A. Sack, and R. R. Colwell. 1990. Attachment of Vibrio cholerae serogroup O1 to zooplankton and phytoplankton of Bangladesh waters. Appl. Environ. Microbiol. 56:1977-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tarsi, R., and C. Pruzzo. 1999. Role of surface proteins in Vibrio cholerae attachment to chitin. Appl. Environ. Microbiol. 65:1348-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toranzos, G. A., and G. A. McFeters. 1997. Detection of indicator microorganisms in environmental freshwaters and drinking waters, p. 184-194. In C. J. Hurst (ed.), Manual of environmental microbiology. ASM Press, Washington, D.C.
- 40.Whitman, R. L., D. A. Shively, H. Pawlik, M. B. Nevers, and M. N. Byappanahalli. 2003. Occurrence of Escherichia coli and enterococci in Cladophora (Chlorophyta) in nearshore water and beach of Lake Michigan. Appl. Environ. Microbiol. 69:4714-4719. [DOI] [PMC free article] [PubMed] [Google Scholar]