Abstract
A nationwide survey was carried out in Korea to assess the prevalence of Shigella strains producing extended-spectrum β-lactamases (ESBLs). From 1991 to 2002, 5,911 clinical strains were isolated and screened for resistance to extended-spectrum cephalosporins. Twenty of the Shigella isolates were ESBL positive, based on the synergistic effects between clavulanate and selected β-lactams (ceftazidime and cefotaxime). Nucleotide sequence analysis of these isolates revealed that they harbored blaTEM-19 (eight isolates), blaTEM-15 (five isolates), blaTEM-52 (six isolates), blaTEM-17 (one isolate), blaTEM-20 (one isolate), and blaCTX-M-14 (three isolates). All the ESBL-encoding genes in this study were carried in conjugable plasmids. Thus, TEM-19, TEM-15, TEM-52, and CTX-M-14 β-lactamases can be considered common Korean ESBL types in Shigella sonnei and are probably transmitted through interspecies spread between medical facilities and the community in Korea. This is the first report of the presence of TEM-17, TEM-19, and TEM-20 in Korea and in S. sonnei.
Recently, shigellosis has been a major problematic infectious disease in Korea. After a large outbreak in 1998, shigellosis has been prevalent throughout the country (20). The predominant pathogenic species responsible for shigellosis in Korea are Shigella sonnei and Shigella flexneri. Because most strains of these species showed multidrug resistance to antibiotics (12, 13), shigellosis has become a serious threat to public health. Extended-spectrum cephalosporins and quinolones are generally used for the treatment of shigellosis in Korea. However, these antibiotics were ineffective for several incurable cases, and the development of resistance to extended-spectrum cephalosporins in Shigella spp. was strongly suggested. Production of CTX-M-14-type extended-spectrum β-lactamase (ESBL) was reported for one S. sonnei strain isolated from one of these incurable cases (22). ESBLs have been found in gram-negative organisms worldwide and are implicated as the major enzymes responsible for resistance to β-lactam antibiotics, such as ceftriaxone, cefotaxime (CTX), and aztreonam (4, 5, 9, 29). Although Shigella is known to be resistant to various antibiotics, only a few cases of ESBL production, i.e., production of CTX-M-14 (22), SHV-11 (1), and OXA (27), have been reported. For this reason, in this study we investigated and evaluated the spreading pathway of ESBL-producing clinical Shigella isolates collected post-1991 in Korea.
(This report was presented at the 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy, 27 to 30 September 2002.)
MATERIALS AND METHODS
Bacterial strains.
369 strains of S. flexneri and 5,542 strains of S. sonnei were collected from 1991 to 2002 from the national public health network in Korea. All the collected S. sonnei and S. flexneri isolates were tested for ESBL production by the disk diffusion method on the basis of a synergistic effect between clavulanate and selected β-lactams (ceftazidime [CAZ] and CTX) (18, 19).
Susceptibility tests.
ESBL production was determined by National Committee for Clinical Laboratory Standards ESBL phenotypic confirmatory tests with CAZ and CTX for disk diffusion methods. In brief, Mueller-Hinton agar plates (Difco brand; Becton Dickinson BioSciences, Sparks, Md.) and disks containing 30 μg of CAZ or CTX, with and without 10 μg of clavulanic acid (CA), were used for testing. All the antibiotic disks were purchased from Becton Dickinson. A 5-mm increase in the zone diameter for CAZ or CTX tested in combination with CA versus its zone when tested alone was considered indicative of ESBL production (19). Escherichia coli ATCC 25922 and Klebsiella pneumoniae ATCC 700603 were used as quality reference strains.
The MICs for each ESBL-producing strains and its transconjugants were determined by the E-test (Biodisk, Solna, Sweden) method for the following antibiotics: ampicillin, aztreonam, gentamicin, ceftazidime, imipenem, ceftriaxone, ampicillin-sulbactam, amoxicillin-clavulanic acid, cefotaxime, cefoxitin, and cephalothin. The E-test was performed in accordance with the manufacturer's instructions. E. coli ATCC 25922 was used as a quality reference strain.
Analytical IEF.
Crude β-lactamase preparations were obtained by the sonication method (23). Isoelectric focusing (IEF) was performed by the method of Matthew et al. (16) with an LKB Phast system and PhastGel (pH 3 to 9; Amersham Pharmacia Biotech, Uppsala, Sweden). Enzyme activity was detected by overlaying the gel with filter paper containing nitrocefin (0.5 mg/ml). β-Lactamases were identified by comparison to reference enzymes run in tracks adjacent to the test samples. TEM-52, SHV-1, SHV-12, CMY-1, and CTX-M-14 β-lactamases were used as reference enzymes (22, 23).
Transfer of resistance, plasmid analysis, and Southern blotting.
Plasmid transfer of cefotaxime or ceftazidime resistance markers was performed by a broth culture conjugation method (26). E. coli J53 Azir was used as a recipient. The mating time was 4 h. Transconjugants were selected on MacConkey agar containing 100 μg of sodium azide (Sigma, St. Louis, Mo.)/ml and 10 μg of cefotaxime/ml (10). To confirm the presence of plasmids and to estimate their sizes, plasmids from clinical isolates and transconjugants were extracted using a Plasmid Midi kit (QIAGEN, Chatsworth, Calif.) and electrophoresed. The plasmid DNAs were transferred from the agarose gel to a nylon membrane (Amersham Pharmacia) by the method of Southern (28) and were hybridized with peroxidase-labeled blaTEM or blaCTX-M gene fragments with the ECL direct nucleic acid labeling and detection systems (Amersham Pharmacia)
β-Lactamase type-specific PCR and DNA sequencing.
Plasmids from clinical isolates and their transconjugants were used as templates in PCR. The primer used for amplification of TEM-related genes and the cycling conditions used have been described previously: 5′-ATA AAA TTC TTG AAG ACG AAA-3′ and 5′-GAC AGT TAC CAA TGC TTA ATC-3′ for amplification of a 1,076-bp sequence of blaTEM (15). For the CTX-M β-lactamase-specific PCR, primers CTX1 (5′-ATG GTG ACA AAG AGA GTG CAA-3′) and CTX2 (5′-TTA GAC CCC TTC GGC GAT-3), corresponding to nucleotides 1 to 21 and 859 to 876 of the blaCTX-M gene, respectively, were used. The cycling conditions used for the PCR with these primers were 30 cycles of 94°C for 1 min, 56°C for 1 min, and 72°C for 1 min, with a final extension of 72°C for 10 min. The amplified PCR products were cloned into the pDrive vector (QIAGEN) and transformed into E. coli DH5α. The transformed plasmids were purified with a Qiaprep spin miniprep kit (QIAGEN), and the inserted nucleotide sequences were determined with an ABI3700 sequencer (Applied Biosystems). The nucleotide sequences of both DNA strands and three independently amplified products were determined.
PFGE.
The genetic relatedness of ESBL-producing S. sonnei was investigated by pulsed-field gel electrophoresis (PFGE) by using the method described by Gautom with modifications (7). Briefly, for the preparation of plugs, bacterial cells were suspended in cell suspension TE buffer (100 mM Tris and 100 mM EDTA, pH 7.5) and mixed with an equal volume of 1.2% SeaKem Gold Agarose (Biowhittaker). The plugs were lysed with ES buffer (0.5 M EDTA, pH 9.0, 1% sodium lauroyl sarcosine) containing proteinase K. The lysed plugs were digested with 30 U of XbaI (New England Biolabs, Boston, Mass.), and PFGE was performed with 1% agarose gel in 0.5× Tris-borate-EDTA buffer at 14°C by using a CHEF mapper apparatus (Bio-Rad, Richmond, Calif.) at 6 V/cm with a linearly ramped switching time of 2.16 to 54.17 s for 18 h. PFGE banding pattern analysis was performed with Fingerprinting II Informatix software (Bio-Rad). Analysis of banding patterns was performed with the Dice coefficient by using a 1.2% tolerance for the band migration distance. Clustering of the patterns was performed by the unweighted pair group method with arithmetic averages.
RESULTS
Isolate selection.
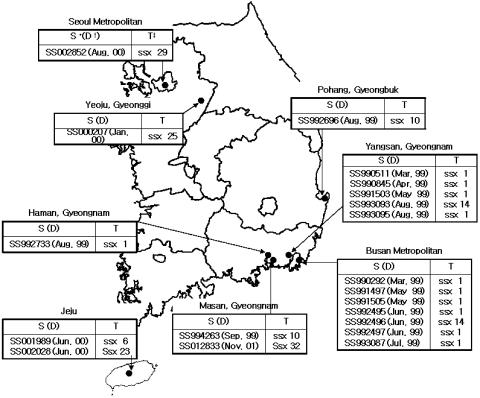
Twenty strains of S. sonnei were phenotypically confirmed to be ESBL producers because they showed a 5- to 16-mm difference in zone diameter for CAZ or CTX tested in combination with CA compared to the zone of inhibition when CAZ or CTX was tested alone (data not shown). Among the 20 S. sonnei isolates, 15 strains were isolated in 1999 in the Gyeongnam, Gyeongbuk, and Busan prefectures; four strains were isolated in 2000 at Jeju, Gyeonggi, and Seoul; and one strain was isolated in 2001 at Gyeongnam (Fig. 1). ESBL-positive S. sonnei was isolated from 12 male and 8 female patients, and among these 20 patients, 15 patients were under 10 years of age, 3 patients were 11 to 18 years of age, and 2 patients were over 65. The 20 S. sonnei strains were investigated further.
FIG. 1.
Strains tested in this study and their PFGE types. S, strain number; D, date of isolation; T, PFGE type.
Transfer of resistance, and plasmid analysis.
Plasmid transfer of the ESBL phenotype to E. coli J53 Azir was successful for all 20 of the isolates. The MICs for each S. sonnei isolate presumed to produce ESBL and for the transconjugants were determined (Table 1). All 20 strains tested showed decreased susceptibility to extended-spectrum cephalosporins and were more resistant to cefotaxime than to ceftazidime. All of the ESBL-producing strains remained susceptible to imipenem. All strains, except for SS991503, were susceptible to cefoxitin. For the transconjugants, the β-lactam MICs were similar to those for each wild-type isolate, and the presence of sulbactam greatly reduced the ampicillin MICs. All the isolates showed decreased susceptibility to ciprofloxacin (MIC, 0.2 to 1 μg/ml).
TABLE 1.
MICs for β-lactams, ESBL type, and pI values of tested strains and their transconjugants
| Strain | MIC (μg/ml)
|
ESBL type | pI | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ampicillin | Aztreonam | Ceftazidime | Imipenem | Cefotaxime | Cefoxitin | Cephalothin | Amoxicillin-clavulanate | Ceftriaxone | Ampicillin-sulbactam | |||
| SS990292 | >256 | 1.5 | 0.5 | 0.125 | >32 | 1 | >256 | 4 | 32 | 8 | 5.4, 8.0 | |
| Transconjugant | >256 | 1 | 0.5 | 0.19 | 24 | 1.5 | >256 | 8 | 24 | 12 | TEM-19, CTX-M14 | 5.4, 8.0 |
| SS990511 | >256 | 1.5 | 4 | 0.19 | 32 | 1.5 | 256 | 6 | 6 | 8 | 5.4, 5.9 | |
| Transconjugant | >256 | 3 | 12 | 0.19 | 16 | 1.5 | 128 | 4 | 6 | 1.5 | TEM-52 | 5.4, 5.9 |
| SS990845 | >256 | 2 | 6 | 0.125 | >32 | 1 | >256 | 8 | 24 | 32 | 5.4, 5.9 | |
| Transconjugant | >256 | 0.75 | 3 | 0.125 | >32 | 0.19 | 256 | 8 | 3 | 15 | TEM-19 | 5.4, 5.9 |
| SS991497 | >256 | 1 | 4 | 0.19 | >32 | 1.5 | 256 | 6 | 4 | 24 | 5.4, 5.9 | |
| Transconjugant | >256 | 2 | 12 | 0.25 | >32 | 12 | 256 | 12 | 16 | 128 | TEM-15 | 5.4, 5.9 |
| SS991503 | >256 | 1.5 | 6 | 2 | >32 | 32 | >256 | 6 | 4 | 24 | 5.4, 5.9 | |
| Transconjugant | >256 | 3 | 8 | 0.5 | 16 | 8 | >256 | 8 | 4 | 48 | TEM-52 | 5.4, 5.9 |
| SS991505 | >256 | 2 | 12 | 0.125 | >32 | 1 | 256 | 3 | 12 | 4 | 5.4, 5.9 | |
| Transconjugant | >256 | 3 | 16 | 0.125 | >32 | 1.5 | >256 | 6 | 8 | 6 | TEM-19 | 5.4 |
| SS992495 | >256 | 1 | 2 | 0.19 | 6 | 1 | 128 | 4 | 2 | 12 | 5.4, 5.9 | |
| Transconjugant | >256 | 1 | 4 | 0.064 | 6 | 3 | 256 | 3 | 2 | 12 | TEM-19 | 5.4, 5.9 |
| SS992496 | >256 | 2 | 4 | 0.125 | 12 | 1 | >256 | 6 | 3 | 24 | 5.4, 5.9 | |
| Transconjugant | >256 | 1 | 4 | 0.19 | 12 | 2 | >256 | 12 | 6 | 24 | TEM-19 | 5.4, 5.9 |
| SS992497 | >256 | 1 | 6 | 0.19 | 8 | 1.5 | >256 | 8 | 16 | 32 | 5.4, 5.9 | |
| Transconjugant | >256 | 1 | 6 | 0.19 | 8 | 1.5 | 64 | 8 | 4 | 24 | TEM-15 | 5.4, 5.9 |
| SS992696 | >256 | 1.5 | 12 | 0.125 | 24 | 16 | 256 | 6 | 8 | 24 | 5.4, 5.9 | |
| Transconjugant | >256 | 0.75 | 12 | 0.19 | 8 | 16 | >256 | 16 | 8 | 16 | TEM-52 | 5.4, 5.9 |
| SS992733 | >256 | 1 | 4 | 0.19 | >32 | >256 | >256 | 32 | 6 | 5.4, 5.9 | ||
| Transconjugant | >256 | 1.5 | 16 | 0.125 | >32 | 1.5 | >256 | 6 | 4 | 12 | TEM-52 | 5.4, 5.9 |
| SS993087 | >256 | 0.75 | 2 | 0.094 | 4 | 1 | 256 | 4 | 4 | 4 | 5.4, 5.9 | |
| Transconjugant | >256 | 1.5 | 6 | 0.125 | 16 | 2 | >256 | 5 | 12 | 32 | TEM-19 | 5.4, 5.9 |
| SS993093 | >256 | 1.5 | 8 | 0.064 | >32 | 0.5 | >256 | 2 | 16 | 1 | 5.4, 5.9 | |
| Transconjugant | >256 | 1.5 | 16 | 0.19 | 32 | 0.5 | 256 | 6 | 32 | 2 | TEM-19 | 5.4, 5.9 |
| SS993095 | >256 | 1 | 2 | 0.094 | 6 | 1.5 | 256 | 1.5 | 2 | 4 | 5.4, 5.9 | |
| Transconjugant | >256 | 0.75 | 2 | 0.094 | 6 | 1.5 | 256 | 6 | 2 | 8 | TEM-15 | 5.4, 5.9 |
| SS994263 | >256 | 0.5 | 2 | 0.094 | 6 | 0.5 | 24 | 4 | 2 | 12 | 5.4, 5.9 | |
| Transconjugant | >256 | 0.5 | 3 | 0.125 | 4 | 1 | 32 | 4 | 2 | 16 | TEM-19 | 5.4, 5.9 |
| SS000207 | >256 | 1.5 | 16 | 0.125 | 32 | 1.5 | 256 | 6 | 16 | 16 | 5.4, 5.9 | |
| Transconjugant | >256 | 3 | 16 | 0.19 | 32 | 1.5 | 256 | 6 | 12 | 16 | TEM-17, 20, 52 | 5.4, 5.9 |
| SS001989 | >256 | 4 | 1.5 | 0.125 | >32 | 2 | >256 | 8 | 32 | 16 | 8.0 | |
| Transconjugant | >256 | 3 | 0.5 | 0.125 | 32 | 1.5 | 256 | 6 | 16 | 16 | CTX-M14 | 8.0 |
| SS002028 | >256 | 1 | 0.5 | 0.125 | 32 | 1.5 | 256 | 4 | 256 | 12 | 5.4, 5.9, 8.0 | |
| Transconjugant | >256 | 0.5 | 0.25 | 0.19 | 32 | 1.5 | 256 | 4 | 24 | 6 | TEM-52, CTX-M14 | 5.9, 8.0 |
| SS002582 | >256 | 3 | 4 | 0.25 | 32 | 2 | >256 | 12 | 12 | 48 | 5.4, 5.9 | |
| Transconjugant | >256 | 1 | 3 | 0.19 | 6 | 1.5 | 64 | 6 | 12 | 24 | TEM-15 | 5.4, 5.9 |
| SS012833 | >256 | 6 | 16 | 0.19 | >32 | 32 | >256 | 12 | 6 | 32 | 5.4, 5.9 | |
| Transconjugant | >256 | 2 | 8 | 0.19 | 32 | 32 | >256 | 12 | 8 | 16 | TEM-15 | 5.4, 5.9 |
Electrophoresis of the plasmid DNA from each wild-type S. sonnei strain and the transconjugants showed that the transconjugants acquired plasmids ranging in size from 5.2 to 135 kb. Transfer of ESBL-encoding genes was confirmed by Southern hybridization (data not shown).
IEF.
In an attempt to classify the β-lactamases produced by each isolate, the isoelectric points (pI) of the β-lactamases were determined by IEF. Among the 20 strains of S. sonnei, whose susceptibilities to oxyimino-β-lactams were enhanced by clavulanic acid, 17 strains and their transconjugants produced β-lactamases with pIs of 5.4 and 5.9. Strain SS990292 and its transconjugant expressed other enzymes with pIs of 5.4 and 8.0. Strain SS002028 expressed enzymes with pIs of 5.4, 5.9, and 8.0, but its transconjugant expressed enzymes with pIs of 5.9 and 8.0 only. SS001989 and its transconjugant expressed an enzyme with a pI of 8.0. Detection of β-lactamase with a pI of 5.4 corresponded to the presence of TEM-1, TEM-19, or TEM-20. A pI of 5.9 corresponded to the presence of TEM-15 or TEM-52 (14, 25). The enzyme with a pI of 8.0 was the CTX-M-14 β-lactamase. In some TEM-19 single-enzyme-producing strains, the band at pI 5.9 may have indicated the presence of another β-lactamase, but it was not identified in this study.
PCR amplification and sequencing of β-lactamase genes.
In order to classify the enzymes presumed to be ESBL, ESBL type-specific PCRs were performed for the isolated strains and their transconjugants. For the 20 S. sonnei isolates, ESBL production was confirmed by phenotype; blaTEM genes were amplified from 19 isolates and their transconjugants, and blaCTX-M genes were amplified from three isolates and their transconjugants. Two isolates (SS990292 and SS002028) and their transconjugants carried both blaTEM and blaCTX-M. No amplification products were obtained with blaSHV-specific primers from any of the 20 tested isolates and their transconjugants (data not shown).
Using amplified PCR products from the transconjugants, we sequenced the β-lactamase-encoding genes. In amplified blaTEM gene products, DNA sequencing and deduced amino acid sequence analysis revealed TEM-52-specific mutations in five strains expressing pI 5.9 β-lactamases, i.e., mutations occurred at lysine 104, threonine 182, and serine 238, numbered according to the scheme of Ambler et al. (3, 23). TEM-15-specific mutations, i.e., affecting lysine 104 and serine 238, were detected in five strains expressing pI 5.9 β-lactamases. A TEM-19-specific mutation (serine 238) was detected in eight strains expressing pI 5.4 β-lactamases. SS000207 harbored both a TEM-17 mutation (lysine 104) and TEM-20 mutations (threonine 182 and serine 238). Regardless of the type, the blaTEM genes of 14 isolates contained an additional silent point mutation at amino acid position 27 (ACG to ACA; threonine), compared to known TEM-1 gene sequences. However, the blaTEM genes of strains SS990292, SS991505, SS993093, SS000207, and SS002582 did not display this point mutation. The blaTEM-52 genes of SS990511 showed an additional point mutation at position 396 (GCT to GCG; alanine), and the blaTEM-19 genes of SS990292 also showed a point mutation at position 396.
In contrast, all amplified blaCTX-M gene products were identified as CTX-M-14 by sequence analysis. The amino acid sequence of CTX-M-14 differed by one amino acid from that of CTX-M-9 (Ala231Val) and was identical to those of β-lactamases recently found in a Korean S. sonnei isolate (22).
PFGE.
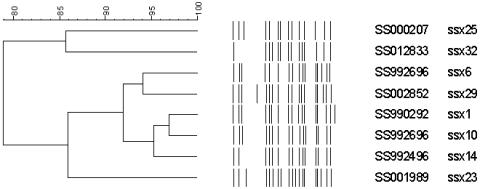
Because the clinical isolates used in this study expressed a limited number of ESBLs, i.e., TEM-15, TEM-19, TEM-52, and CTX-M-14, clonal spreading of the organisms was suspected. In order to examine this possibility, PFGE was performed. As shown in Fig. 1 and 2, XbaI-digested PFGE patterns of ESBL-producing S. sonnei isolates were classified into eight types. In our laboratory, a PFGE type database was constructed for S. sonnei strains isolated in Korea post-1991. We compared the PFGE patterns obtained in this study with established PFGE types, and all the PFGE patterns obtained in this study grouped with the existing types. Among the 20 tested strains, the most frequent PFGE type was ssx1. Eleven ESBL-producing S. sonnei isolates belonged to type ssx1. Two isolates belonged to type ssx6, which was the next most common type. Because only one to three bands of difference existed, and because computer-calculated relatedness varied from 78.95 to 96.97% among these eight PFGE types, it was assumed that the PFGE types were genetically related to each other. However, each PFGE type was related to an isolated region and time rather than an ESBL, as was found with non-ESBL-producing S. sonnei isolates.
FIG. 2.
Clustering of XbaI-digested PFGE patterns for ESBL-producing S. sonnei strains.
DISCUSSION
The major previously reported types of ESBLs produced in Korea were TEM-52, SHV-1, and SHV-2a, and most of these were produced by E. coli or K. pneumoniae recovered from hospitalized patients (11, 22, 23). Isolation of the first strain of S. sonnei producing ESBLs was reported in 1999 (22), and there was public concern about its potential spread. We therefore screened the ESBL-producing Shigella strains isolated in Korea after 1991. We found 20 such isolates producing six kinds of ESBLs, and among these, TEM-17, TEM-19, and TEM-20 are the first of their kind reported in Korea. In addition, these types of ESBLs have not been detected before in Shigella.
All the S. sonnei strains producing ESBLs isolated in 1999 were from Gyeongnam and Busan prefectures in southeastern Korea (Fig. 1). In 1999, 1,781 cases of shigellosis were reported in Korea, and among these, 976 (54%) were reported from these prefectures (20). Thus, local preponderance of strains of S. sonnei producing ESBLs in 1999 is clear, and this could suggest a clonal spread of this form. However, as shown in Fig. 1 and 2, even the PFGE types of ESBL-producing S. sonnei isolates in 1999 showed >91% relatedness; moreover, the PFGE types of ESBL-producing strains were not related to their produced ESBL types. Therefore, the S. sonnei isolates producing ESBLs could not have originated from a limited number of ESBL-producing strains by clonal spread. In 2000, there were 302 cases of shigellosis among the 2,462 cases reported from Gyeongnam and Busan prefectures (20), but no Shigella strain producing ESBLs was isolated. Four strains of S. sonnei isolates producing ESBLs were isolated from regions distant from Gyeongnam and Busan prefectures in 2000. The PFGE types of these isolates differed from each other and, except for SS002028, were different from the PFGE type of ESBL-producing S. sonnei strains isolated in 1999. This supports our conclusion that clonal spread was not involved in the transfer of ESBL productive capacity.
The existence of ESBL phenotype-related conjugative plasmids was discovered from the results of conjugation and plasmid profile experiments. Therefore, the dissemination of extended-spectrum cephalosporin resistance is partly due to the horizontal transfer of endemic resistance plasmids. Moreover, because the production of TEM-15 by K. pneumoniae, of TEM-52 by E. coli and K. pneumoniae, and of CTX-M-14 by S. sonnei, E. coli, and K. pneumoniae has been reported recently in Korea (22, 23, 24), it can be assumed that the TEM-15-, TEM-52-, and CTX-M-14-type ESBLs in S. sonnei strains identified in this study were transmitted through interspecies spread between medical facilities and the community in Korea. It can also be assumed that TEM-17-, TEM-19-, and TEM-20-type ESBLs mutated from these transmitted TEM types or that mutated types were transmitted.
The bla genes encoding various types of ESBLs that can exist in a single strain have been reported elsewhere (6, 17). In this study, we confirmed that strain SS002028 contains blaCTX-M-14 and blaTEM-52 and that strain SS00207 contains blaTEM-17, blaTEM-20, and blaTEM-52. Because the pI values of most TEM-type ESBLs detected by IEF were 5.4 or 5.9, and because the primer set used for sequencing blaTEM can amplify all types of these genes, all PCR-amplified blaTEM genes were cloned into vectors and then sequenced. However, as shown in Table 1, the MIC data and ESBL type were not matched in some strains. This may have been caused by hyperproduction of narrow-spectrum β-lactamases, such as TEM-1, or it may suggest the existence of other ESBL types that were not identified in this study. Strain SS991503, especially, showed decreased susceptibility to cefoxitin and imipenem. This suggests that there may be another type of β-lactamase rather than another ESBL.
After the isolation of an ESBL-producing S. sonnei strain was first reported, we screened ESBL production in almost all Shigella strains isolated after 1991 in Korea. Although ESBL-producing strains comprise <0.5% of all Shigella isolates after 1991 in Korea, S. sonnei has been identified as the leading cause of shigellosis in industrialized countries (2, 8), and the disease is endemic in Korea. The emergence of ESBL production in S. sonnei could thus become a serious threat to public health. More active surveillance and effective controls for shigellosis are clearly needed to minimize the spread of ESBL-producing S. sonnei isolates.
Acknowledgments
We are very grateful to H. Pai for providing the four strains producing TEM-52, SHV-1, SHV-12, and CMY-1 β-lactamases and for her helpful advice.
This study was supported by a grant from the Ministry of Health and Welfare, Republic of Korea (01-PJ6-PG5-01P21-0003).
REFERENCES
- 1.Ahamed, J., and M. Kundu. 1999. Molecular characterization of the SHV-11 β-lactamase of Shigella dysenteriae. Antimicrob. Agents Chemother. 43:2081-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alamonas, Y., V. Maipa, S. Levidiotou, and E. Gessouli. 2000. A community waterborne outbreak of gastroenteritis attributed to Shigella sonnei. Epidemiol. Infect. 125:499-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ambler, R. P., A. F. W. Coulson, J. M. Frere, J. M. Ghuysen, B. Joris, and M. Forsman. 1991. A standard numbering scheme for the class A β-lactamases. Biochem. J. 276:269-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradford, P. A. 2001. Extended spectrum β-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin. Microbiol. Rev. 14:933-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bush, K., and G. Jacoby. 1997. Nomenclature of TEM β-lactamases. J. Antimicrob. Chemother. 39:1-3. [DOI] [PubMed] [Google Scholar]
- 6.Colom, K., J. Perez, R. Alonso, A. Fernandez-Aranguiz, E. Larino, and R. Cisterna. 2003. Simple and reliable multiplex PCR assay for detection of blaTEM, bla(SHV) and blaOXA-1 genes in Enterobacteriaceae. FEMS Microbiol. Lett. 223:147-151. [DOI] [PubMed] [Google Scholar]
- 7.Gautom, R. 1997. Rapid PFGE protocol for typing of E. coli O157:H7 and other gram-negative organisms in 1 day. J. Clin. Microbiol. 35:2977-2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isenbarger, D. W., C. W. Hoge, A. Srijan, C. Pitarangsi, and N. Vithayasai. 2002. Comparative antibiotic resistance of diarrheal pathogens from Vietnam and Thailand, 1996-1999. Emerg. Infect. Dis. 8:175-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacoby, G. A., and A. A. Medeiros. 1991. More extended-spectrum β-lactamases. Antimicrob. Agents Chemother. 35:1697-1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacoby, G. A., and P. Han. 1996. Detection of extended-spectrum β-lactamases in clinical isolates of Klebsiella pneumoniae and Escherichia coli. J. Clin. Microbiol. 34:908-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim, J., J. Kwon, H. Pai, J. W. Kim, and D. T. Cho. 1998. Survey of Klebsiella pneumoniae strains producing extended-spectrum β-lactamases: prevalence of SHV-12 and SHV-2a in Korea. J. Clin. Microbiol. 34:1446-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim, S., J. Kim, H. Jang, Y. Kang, and B. Lee. 2003. Characterization of multidrug resistant Shigella sonnei in Korea 1998 to 2002, abstr. 247. Abstr. 1st FEMS Congr. Eur. Microbiologists. Federation of European Microbiological Societies, Delft, The Netherlands.
- 13.Kim, S., J. Kim, H. Jang, Y. Kang, and B. Lee. 2003. Review of antimicrobial resistance of Shigella flexneri isolated in Korea during 1998 to 2002, abstr. 247. Abstr. 1st FEMS Congr. Eur. Microbiologists. Federation of European Microbiological Societies, Delft, The Netherlands.
- 14.Mabilat, C., and P. Courvalin. 1990. Development of oligotyping for characterization and molecular epidemiology of TEM β-lactamases in members of the family Enterobacteriaceae. Antimicrob. Agents Chemother. 34:2210-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mabilat, C. L., and S. Goussard. 1993. PCR detection and identification of genes for extended-spectrum β-lactamases, p. 553-563. In D. H. Persing, T. F. Smith, F. C. Tenover, and T. J. White (ed.), Diagnostic molecular microbiology: principles and applications. American Society for Microbiology, Washington, D.C.
- 16.Matthew, M., M. Harris, M. J. Marshall, and G. W. Rose. 1975. The use of analytical isoelectric focusing for detection and identification of β-lactamases. J. Gen. Microbiol. 88:169-178. [DOI] [PubMed] [Google Scholar]
- 17.Melano, R., A. Corso, A. Petroni, D. Centron, B. Orman, A. Pereyra, N. Moreno, and M. Galas. 2003. Multiple antibiotic-resistance mechanisms including a novel combination of extended-spectrum beta-lactamases in a Klebsiella pneumoniae clinical strain isolated in Argentina. J. Antimicrob. Chemother. 52:36-42. [DOI] [PubMed] [Google Scholar]
- 18.National Committee for Clinical Laboratory Standards. 2000. Performance standards for antimicrobial disk susceptibility tests, 7th ed. Approved standard M2-A7. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 19.National Committee for Clinical Laboratory Standards. 2000. Disk diffusion supplemental tables. M100-S10 (M2). National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 20.National Institute of Health in Korea. 2003. Communicable diseases statistical yearbook 2002, p. 59. National Institute of Health in Korea, Seoul, Korea.
- 21.Oh, J. Y., H. S. Yu, S. K. Kim, S. Y. Seol, and D. T. Cho. 2003. Changes in patterns of antimicrobial susceptibility and integron carriage among Shigella sonnei isolates from southwestern Korea during epidemic periods. J. Clin. Microbiol. 41:421-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pai, H., E. Choi, H. Lee, J. Hong, and G. A. Jacoby. 2001. Identification of CTX-M-14 extended spectrum β-lactamase in clinical isolates of Shigella sonnei, Escherichia coli, and Klebsiella pneumoniae in Korea. J. Clin. Microbiol. 39:3747-3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pai, H., S. Lyu, J. H. Lee, J. Kim, Y. Kwon, J. Kim, and K. Choe. 1999. Survey of extended-spectrum β-lactamases in clinical isolates of Escherichia coli and Klebsiella pneumoniae: prevalence of TEM-52 in Korea. J. Clin. Microbiol. 37:1758-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pai, H., H. J. Lee, E. Choi, J. Kim, and G. Jacoby. 2001. Evolution of TEM-related extended-spectrum β-lactamases in Korea. Antimicrob. Agents Chemother. 45:3651-3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poyart, C., P. Mugnier, G. Quesne, P. Berche, and P. T. Cuot. 1998. A novel extended spectrum TEM-type β-lactamase (TEM-52) associated with decreased susceptibility to moxalactam in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 42:108-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shannon, K. P., A. King, I. Phillips, M. H. Nicolas, and A. Philippon. 1990. Importance of organisms producing broad-spectrum SHV-group β-lactamases in the United Kingdom. J. Antimicrob. Chemother. 25:343-351. [DOI] [PubMed] [Google Scholar]
- 27.Siu, L. K., J. Y. C. Lo, K. Y. Yuen, P. Y. Chau, M. H. Ng, and P. L. Ho. 2000. β-Lactamase in Shigella flexneri isolates from Hong Kong and Shanghai and a novel OXA-1 like β-lactamase, OXA-30. Antimicrob. Agents Chemother. 32:397-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Southern, E. M. 1975. Detection of specific sequences among DNA fragments separated by agarose gel electrophoresis. J. Mol. Biol. 98:503-517. [DOI] [PubMed] [Google Scholar]
- 29.Winokur, P. L., A. Brueggemann, D. L. Desalvo, L. Hoffmann, M. D. Apley, E. K. Uhlenhopp, M. A. Pfaller, and G. V. Doern. 2000. Animal and human multidrug-resistant, cephalosporin-resistant Salmonella isolates expressing a plasmid-mediated CMY-2 AmpC β-lactamase. Antimicrob. Agents Chemother. 44:2777-2783. [DOI] [PMC free article] [PubMed] [Google Scholar]