Abstract
Human herpesvirus 6 (HHV-6) has occasionally been associated with cases of interstitial pneumonitis, mainly in individuals with impaired cellular immunity. Here we report for the first time severe interstitial pneumonitis with simultaneous HHV-6 and Pneumocystis carinii infections in the lung tissue of a young patient with hypogammaglobulinemia.
CASE REPORT
A previously healthy 31-year-old man sought medical attention because of fever and respiratory symptoms in April 2001. A chest X ray revealed infiltrates in the lower lobes of both lungs, and cefadroxil therapy was commenced. After the antimicrobial treatment course, the patient continued to be symptomatic, with cough, pronounced sweating, and malaise. He was admitted to the hospital in the middle of May 2001 because of persistent respiratory symptoms.
On admission, physical examination revealed prominent nonproductive cough and mild dyspnea. The C-reactive protein level was only minimally elevated, at 17 mg/liter, and the erythrocyte sedimentation rate was normal. The white blood cell count was 1.8 × 109/liter, with 29% neutrophils, 41% lymphocytes, and 24% monocytes. The number of platelets was reduced to 95 × 109/liter. A chest X ray showed large infiltrates bilaterally, and abdominal ultrasonography revealed an enlarged spleen, the largest diameter being 20 cm. High-resolution computed tomography (HRCT) of the lungs revealed nonspecific findings of multiple parenchymal infiltrates bilaterally and a series of paratracheal and para-aortic lymph nodes that were not sufficiently prominent to be considered pathological. Treatment with ceftriaxone was initiated and continued for 2 weeks.
Bone marrow aspiration showed signs of peripheral consumption of leukocytes and platelets, and since the maturation of cell lines was normal, hypersplenism was considered to explain the cytopenia. Treatment with granulocyte-macrophage colony-stimulating factor was initiated and resulted in a prompt, but not marked, influence on the levels of peripheral blood leukocytes. Laboratory examinations showed markedly reduced levels of all immunoglobulins: immunoglobulin A (IgA), <0.2 g/liter; IgM, <0.2 g/liter; and IgG, 3.4 g/liter. Therefore, substitution therapy with intravenous immunoglobulins every 2 weeks was commenced. The number of CD4+ cells was normal, and the numbers of CD8+ and NK cells were reduced to 136 × 106/liter and 82 × 106/liter, respectively. The lymphocyte proliferation responses to phytohemagglutinin, concanavalin A, pokeweed mitogen, and purified protein derivative were normal.
Bronchoscopy was performed 1 week after admission. Microbiological examination of bronchoalveolar lavage (BAL) fluid revealed positive PCR results for Pneumocystis carinii (20); microscopic examination revealed negative results for staining with Grocott's methenamine-silver stain. Other microbiological test results for the BAL fluid specimen were negative. These tests included bacterial, fungal, and mycobacterial cultures; Legionella sp. cultures on buffered charcoal-yeast extract (Oxoid Ltd., Basingstoke, United Kingdom); and PCR assays for Aspergillus fumigatus (16), Mycobacterium tuberculosis (Abbott Laboratories, Abbott Park, Ill.), and Legionella spp. (15). The results of cultivation of BAL fluid for viruses and a cytomegalovirus (CMV) PCR assay (12) were negative. A human herpesvirus 6 (HHV-6) PCR assay (11) for the detection of both HHV-6 variant A and HHV-6 variant B yielded positive results for the peripheral blood sample, but no HHV-6 IgM or IgG antibodies (Panbio, Brisbane, Queensland, Australia) were detected in the serum. The results of human immunodeficiency virus (HIV) (Nuclisens; bioMérieux, Boxtel, The Netherlands) and CMV (12) PCR assays of blood samples as well as HIV antibody tests (Dade Behring, Marburg, Germany) were negative.
The diagnosis of definite P. carinii pneumonia could not be made based merely on the positive P. carinii PCR results for the BAL fluid. However, because the patient was immunocompromised and the involvement of P. carinii in the disease was considered possible, he was treated with trimethoprim-sulfamethoxazole for 3 weeks. This therapy was subsequently discontinued due to an allergic reaction. During these therapies, the general condition of the patient was relatively good, and he was afebrile. Another chest X ray, however, revealed no improvement in the infiltrates. Therefore, an open lung biopsy procedure was carried out in July 2001.
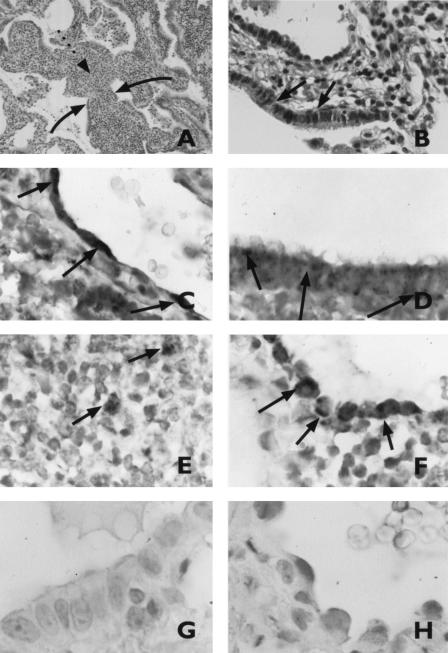
Microscopic examination of the lung biopsy specimens showed moderately severe changes of lymphoid interstitial pneumonitis (LIP), with features reminiscent of follicular bronchiolitis (Fig. 1A and B). A PCR assay yielded positive results for HHV-6 in the biopsy specimens. Since a tissue sample may be positive in a PCR assay due to viremia or circulating blood cells harboring the virus, in situ hybridization for HHV-6 was performed and revealed the localization of HHV-6 in inflammatory cells as well as in endothelial cells of large vessels, the respiratory epithelium of bronchi, and in alveolar pneumocytes (Fig. 1C to F). In contrast, the results of in situ hybridization for HHV-6 in lung biopsy specimens from a patient with bronchiolitis obliterans with organized pneumonia (Fig. 1G and H) and three patients with normal tissue morphology were negative.
FIG. 1.
Routine histopathological analysis of lung tissue from the case report patient with LIP. (A) Widened alveolar walls are packed with a polymorphous lymphoid cellular infiltrate (broken arrow). Alveolar epithelium is intact (solid arrows). (B) Ciliated cells (arrows) line a small airway with mild infiltration of the stroma by lymphoid cells. (C to H) In situ hybridization for HHV-6 DNA in lung biopsy specimens from the case report patient (C to F) and from a patient with bronchiolitis obliterans with organized pneumonia (G and H). (C) Endothelial cells of a large vessel (vein). (D) Metaplastic bronchiolar epithelium lining the alveolar wall. (E) Inflammatory cells (monocytes/macrophages). (F and H) Alveolar pneumocytes. (G) Respiratory epithelium. The arrows indicate the positive signal from in situ hybridization.
Treatment with valaciclovir at 1 g three times daily was instituted for 10 days. In August 2001, pulmonary HRCT revealed no significant new findings other than one new infiltrate on the left side. At the beginning of September 2001, oral methylprednisolone at 60 mg daily was initiated and then was reduced gradually to 30 mg daily after 2 months. Both clinical and radiological responses to treatment with corticosteroids were good: after 1 month, a chest X ray showed only minimal findings, and after 2 months, pulmonary HRCT findings were nearly normal, with only mild parenchymal infiltrates and local peribronchial consolidation.
A new bronchoscopy with BAL was carried out in November 2001. Negative findings for P. carinii were obtained by microscopy with silver staining and immunofluorescence and by PCR, and negative findings for HHV-6 were obtained by PCR. After January 2002, the interval between immunoglobulin infusions was increased to 4 weeks; the average concentration of serum IgG before infusion was ca. 7.0 g/liter. The amounts of peripheral blood leukocytes and platelets were normal. The number of CD4+ cells was normal (458 × 106/liter), but the numbers of CD8+ and NK cells were still low, at 84 × 106/liter and 37 × 106/liter, respectively. In July 2002, pulmonary HRCT revealed slight progression in the findings for both parenchymal infiltrates and mediastinal lymph node enlargement. Valaciclovir treatment for 10 days was restarted. The results of repeated examinations of diffusing capacity normalized in August 2002. After February 2003, the dose of methylprednisolone was reduced to 5 mg every other day. Clinically, the patient has been free of symptoms since then.
HHV-6 is a member of the subfamily of beta-herpesviruses. Two subgroup variants, HHV-6A and HHV-6B, have been identified (1). Primary infection occurs mainly by the age of 2 years (14). The most common manifestations of primary infection by HHV-6B are exanthema subitum, a generalized rash occurring most commonly in early childhood, and febrile illness (22). So far, no clear disease entities have been associated with HHV-6A. During primary infection, HHV-6 causes a persistent infection which may be reactivated later. Symptomatic reactivations, e.g., febrile illness, encephalitis, hepatitis, and pneumonia, have been shown to occur mainly during immunosuppression, such as after bone marrow or solid organ transplantation or in HIV-infected patients (8, 13). Here we report for the first time severe interstitial pneumonitis with simultaneous HHV-6 and P. carinii infections in the lung tissue of a patient with hypogammaglobulinemia.
LIP is characterized as a pulmonary inflammatory reaction to an unknown stimulus. Histopathologically, polymorph lymphoid cell infiltrates are seen in the pulmonary alveolar interstitium (19). LIP has been shown to occur in patients with autoimmune diseases, but viruses have also been proposed as potential triggering factors. Some case reports and retrospective studies have suggested the potential role of HHV-6 infection in LIP, but the pathogenic role of HHV-6 in that disease is largely unknown. Most of the cases have occurred in immunosuppressed patients with bone marrow transplantation or HIV infection (3, 4, 6). Interstitial pneumonitis was reported once in an immunocompetent adult during simultaneous infection with HHV-6 and Legionella pneumophila (17).
In the literature, the diagnosis of HHV-6-associated pneumonia has been based on isolation of the virus from blood, bone marrow, and BAL fluid samples or on immunohistochemical detection of HHV-6-positive cells in lung tissue (4, 5). Immunohistochemical staining of lung biopsy specimens has revealed HHV-6-positive macrophages, but infected lymphocytes also have been found (4). The finding that lung tissue of bone marrow transplantation patients as well as—surprisingly—that of healthy individuals commonly harbors HHV-6 DNA when analyzed by PCR (5, 7) makes it difficult to prove the causality between pneumonitis and HHV-6 infection. In our case report patient, not only was HHV-6 DNA detected in lung biopsy specimens and peripheral blood mononuclear cells by PCR, but also in situ hybridization revealed the localization of HHV-6 in inflammatory cells as well as in endothelial cells of large vessels, the respiratory epithelium of bronchi, and alveolar pneumocytes. This widespread invasion by HHV-6 and the finding that HHV-6 DNA was not detected in lung tissue obtained from other patients and examined in our laboratory by this method strongly indicate active virus replication in the lung tissue of our case report patient.
Cell-mediated immunity is crucial for the clearance of herpesviruses. Patients with defects in NK cell function are especially prone to severe herpesvirus infections (2, 9). In general, virus infections in which cell-mediated immunity is critical for virus clearance are rare in patients with hypogammaglobulinemia (21). For the herpesviruses, susceptibility to severe herpes simplex recurrence and severe CMV infection has been reported in hypogammaglobulinemia patients with concomitant impairment in cellular immunity (10, 18). In our case report patient, cell proliferation responses of lymphocytes to phytohemagglutinin, concanavalin A, pokeweed mitogen, and purified protein derivative were normal, indicating normal cell-mediated immunity. However, the amounts of CD8+ and NK cells were slightly reduced at the onset of the disease and remained so during the follow-up period of 1 year. That situation may have contributed to the low level of clearance of the virus and to the severity of the infection. The simultaneous infection with P. carinii also may have exacerbated the infection in the lung tissue.
In conclusion, our case report patient developed interstitial pneumonitis as the presenting manifestation and first sign of hypogammaglobulinemia. In situ hybridization revealed widespread invasion of lung tissue by HHV-6, indicating active virus replication. This finding suggests that HHV-6 may have played a pathogenic role in the development of interstitial pneumonitis in this patient, thus supporting earlier suggestions regarding the potential association between HHV-6 and LIP. Due to hypogammaglobulinemia, viral serological analysis was not helpful in defining whether the patient had a primary infection with HHV-6 or a reactivation. Our case report patient differs from previously described patients with HHV-6 reactivation in that he had a deficiency in humoral immunity.
Acknowledgments
We thank Stephen Venn for revising the language of the manuscript.
REFERENCES
- 1.Aubin, J. T., H. Collandre, D. Candotti, D. Ingrand, C. Rouzioux, M. Burgard, S. Richard, J. M. Huraux, and H. Agut. 1991. Several groups among human herpesvirus 6 strains can be distinguished by Southern blotting and polymerase chain reaction. J. Clin. Microbiol. 29:367-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biron, C. A., K. S. Byron, and J. L. Sullivan. 1989. Severe herpesvirus infection in an adolescent without natural killer cells. N. Engl. J. Med. 320:1731-1735. [DOI] [PubMed] [Google Scholar]
- 3.Buchbinder, S., A. H. Elmaagacli, U. W. Schaefer, and M. Roggendorf. 2000. Human herpesvirus 6 is an important pathogen in infectious lung disease after allogeneic bone marrow transplantation. Bone Marrow Transpl. 26:639-644. [DOI] [PubMed] [Google Scholar]
- 4.Carrigan, D. R., W. R. Drobyski, S. K. Russler, M. A. Tapper, K. K. Knox, and R. C. Ash. 1991. Interstitial pneumonitis associated with human herpesvirus-6 infection after marrow transplantation. Lancet 338:147-149. [DOI] [PubMed] [Google Scholar]
- 5.Cone, R. W. 1995. Human herpesvirus 6 as a possible cause of pneumonia. Semin. Respir. Infect. 10:254-258. [PubMed] [Google Scholar]
- 6.Cone, R. W., R. C. Hackman, M. L. W. Huang, R. A. Bowden, J. D. Meyers, M. Metcalf, J. Zeh, R. Ashley, and L. Corey. 1993. Human herpesvirus-6 in lung tissue from patients with pneumonitis after bone-marrow transplantation. N. Engl. J. Med. 329:156-161. [DOI] [PubMed] [Google Scholar]
- 7.Cone, R. W., M. L. W. Huang, R. C. Hackman, and L. Corey. 1996. Coinfection with human herpesvirus 6 variants A and B in lung tissue. J. Clin. Microbiol. 34:877-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dockrell, D. H., and C. V. Paya. 2001. Human herpesvirus-6 and -7 in transplantation. Rev. Med. Virol. 11:23-36. [DOI] [PubMed] [Google Scholar]
- 9.Fleishner, G., S. Starr, N. Koven, H. Kamiya, S. D. Douglas, and W. Henle. 1982. A non-x-linked syndrome with susceptibility to severe Epstein-Barr virus infection. J. Pediatr. 100:727-730. [DOI] [PubMed] [Google Scholar]
- 10.Freeman, H. J., T. K. Shnitka, J. R. A. Piercey, and W. M. Weinstein. 1977. Cytomegalovirus infection of gastrointestinal tract in a patient with late onset immunodeficiency syndrome. Gastroenterology 73:1397-1403. [PubMed] [Google Scholar]
- 11.Hemling, N., M. Röyttä, J. Rinne, P. Pöllänen, E. Broberg, V. Tapio, T. Vahlberg, and V. Hukkanen. 2003. Herpesviruses in brains in Alzheimer's and Parkinson's diseases. Ann. Neurol. 54:267-271. [DOI] [PubMed] [Google Scholar]
- 12.Hukkanen, V., and T. Vuorinen. 2002. Herpesviruses and enteroviruses in infections of the central nervous system: a study using time-resolved fluorometry. J. Clin. Virol. 25:S87-S94. [DOI] [PubMed] [Google Scholar]
- 13.Knox, K. K., and D. R. Carrigan. 1994. Disseminated active HHV-6 infections in patients with AIDS. Lancet 343:577-578. [DOI] [PubMed] [Google Scholar]
- 14.Okuno, T., K. Takahashi, K. Balachandra, K. Shiraki, K. Yamanishi, M. Takahashi, and K. Baba. 1989. Seroepidemiology of human herpesvirus 6 infection in normal children and adults. J. Clin. Microbiol. 27:651-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rantakokko-Jalava, K., and J. Jalava. 2001. Development of conventional and real-time PCR assays for detection of Legionella DNA in respiratory specimens. J. Clin. Microbiol. 39:2904-2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rantakokko-Jalava, K., S. Laaksonen, J. Issakainen, J. Vauras, J. Nikoskelainen, M. K. Viljanen, and J. Salonen. 2003. Semiquantitative detection by real-time PCR of Aspergillus fumigatus in bronchoalveolar lavage fluids and tissue biopsy specimens from patients with invasive aspergillosis. J. Clin. Microbiol. 41:4304-4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Russler, S. K., M. A. Tapper, K. K. Knox, A. Liepins, and D. R. Carrigan. 1991. Pneumonitis associated with coinfection by human herpesvirus 6 and Legionella in an immunocompetent adult. Am. J. Pathol. 138:1405-1411. [PMC free article] [PubMed] [Google Scholar]
- 18.Straus, S. E., M. Seidlin, H. Takiff, D. Jacobs, D. Bowen, and H. A. Smith. 1984. Oral acyclovir to suppress recurring herpes simplex virus infections in immunodeficient patients. Ann. Intern. Med. 100:522-524. [DOI] [PubMed] [Google Scholar]
- 19.Swigris, J. J., G. J. Berry, T. A. Raffin, and W. G. Kuschner. 2002. Lymphoid interstitial pneumonia—a narrative review. Chest 122:2150-2164. [DOI] [PubMed] [Google Scholar]
- 20.Wakefield, A. E., F. J. Pixley, S. Banerji, K. Sinclair, R. F. Miller, E. R. Moxon, and J. M. Hopkin. 1990. Detection of Pneumocystis carinii with DNA amplification. Lancet 336:451-453. [DOI] [PubMed] [Google Scholar]
- 21.Webster, A. D. 1994. Virus infections in primary immunodeficiency. J. Clin. Pathol. 47:965-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamanishi, K., K. Shiraki, T. Kondo, T. Okuno, M. Takahashi, Y. Asano, and T. Kurata. 1988. Identification of human herpesvirus-6 as a causal agent for exanthem subitum. Lancet 1:1065-1067. [DOI] [PubMed] [Google Scholar]