Abstract
Microsphaeropsis arundinis is an anamorphic fungal plant inhabitant belonging to the form class Coelomycetes. We describe two cases of M. arundinis soft tissue infections in immunosuppressed patients. This organism has not previously been described as causing disease in humans. It was identified on the basis of its typical ostiolate pycnidial conidiomata, ampulliform conidiogenous cells, and small, smooth-walled, brown, cylindrical conidia.
Members of the class Coelomycetes are emerging as an important group causing soft tissue infections in immunosuppressed patients (13, 16). Infections usually are localized, following traumatic implantation, but may progress to invasive subcutaneous disease (2, 4, 5, 7, 9, 11, 17). Airborne transmission of these fungi is unlikely since they typically produce their conidia within enclosed structures known as conidiomata (16). In this report we describe two subcutaneous infections caused by Microsphaeropsis arundinis in diabetic patients.
Species of the genus Microsphaeropsis Höhnel are coelomycetous anamorphic fungi that have probable affinities to the ascomycete genus Paraphaeosphaeria O. Eriksson (3). Microsphaeropsis species are typically found as saprobes and parasites of terrestrial plants. They inhabit branches and leaves of various plant hosts and are ubiquitous in soil and freshwater environments.
Case 1.
An 80-year-old non-insulin-dependent diabetic man presented with a 3-cm-diameter painless, deep granulomatous plaque on the dorsum of the right hand. The lesion had been slowly enlarging over a period of months. Past medical history included ischemic heart disease, chronic renal impairment, and a chronic inflammatory demyelinating polyneuropathy for which he was receiving long-term corticosteroid therapy. A punch biopsy specimen of the lesion was obtained and submitted for fungal culture and histology. Direct fluorescent microscopic examination using a Blankophor BA (Bayer) fluorescent stain revealed irregular, septate hyphae. The treating dermatologist, pending culture results, started treatment with oral terbinafine at standard doses. There was a greater than 50% reduction in plaque size by 12 weeks. Formal excision of the lesion was not required. Terbinafine therapy was ceased, and the lesion resolved over the next 8 weeks. Several months later, the patient developed a bloodstream infection with Candida albicans and received a prolonged course of oral fluconazole for this. There had been no recurrence of the lesion at the time of the patient's death, 2.5 years later.
Case 2.
A 56-year-old diabetic man was admitted to a hospital for investigation and management of recurring ulcers on both feet. He also had persistent osteomyelitis of the left fourth finger. Medical problems included chronic renal failure requiring hemodialysis and ankylosing spondyloarthropathy, for which he received corticosteroid therapy. His past history was notable for recurrent wound infections of his feet and amputation of two toes 4 years earlier. At the same time, osteomyelitis of the left fourth finger was diagnosed and culture from the finger yielded Phialophora verrucosa. Long-term itraconazole treatment was begun. The ulcers on the feet persisted, and cultures obtained 3 months prior to the present admission yielded bacterial skin flora and an unidentified fungal isolate. Repeat culturing from a different site again yielded the unidentified fungus. Since the significance of this isolate was unknown, it was not investigated any further.
At the present admission, tissue obtained from a necrotic lesion on the heel of the right foot was submitted for histology and culture. In addition, tissue samples for fungal and bacterial microscopy and culture were obtained from four different ulcer sites on the right foot and left calf. Direct Blankophor BA fluorescence microscope examination of three tissue samples obtained from different ulcer sites revealed the same irregular septate hyphae in all specimens. The fourth sample was insufficient for microscopy. Culture from these sites yielded the fungus now identified as M. arundinis. Despite antibiotic therapy and debridement, the infection worsened and the patient developed gangrenous right toes, requiring amputation of the right foot. Two months later, he developed gangrene in the left foot, requiring amputation of that foot as well. He was maintained on itraconazole treatment for the Phialophora osteomyelitis for 10 months following amputation. There was no subsequent relapse of infection in the legs.
Histologic examination.
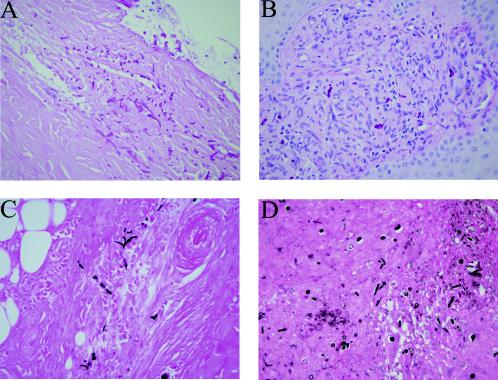
Histopathological examination of material from the first patient showed surface ulceration and an underlying mixed acute and chronic inflammatory response with a prominent granulomatous component. Special stains for fungi (methenamine silver and periodic acid-Schiff [PAS]) highlighted fungal elements as yeast forms and septate pseudohyphae. Many of these organisms were within multinucleate giant cells, and fungal elements were seen extending into the deeper layers of the dermis (Fig. 1A and C). Examination of material from the second patient showed similar features with widespread ulceration and extensive acute inflammation. Chronic inflammation was less prominent, and only a few multinucleate giant cells were discerned. Several of these giant cells contained fungal elements. Methenamine silver and PAS stains again highlighted the budding-yeast forms and septate pseudohyphae which extended into the underlying subcutaneous fat and connective tissue (Fig. 1B and D).
FIG. 1.
(A and B) Histological sections of tissue from case 1 (A) and case 2 (B) demonstrate invasive growth of fungal elements into connective tissue with an associated inflammatory response (PAS stain). (C and D) Methenamine silver stains from case 1 (C) and case 2 (D) further highlight the fungal elements as budding yeast forms and septate pseudohyphae. Magnifications, ×400.
Mycology.
Specimens collected from both patients were cultured onto BBL Sabouraud dextrose agar Emmons (SDA) containing chloramphenicol (0.16 g/liter) and gentamicin (0.16 g/liter). They were incubated at both 28 and 37°C. BBL Mycosel agar with cycloheximide (0.4 g/liter) (Becton, Dickinson & Co.) and BBL brain heart infusion agar (BHIA) with chloramphenicol (0.16 g/liter) and gentamicin (0.16 g/liter) were also set up at 28 and 37°C.
Growth was apparent within 3 days on SDA and BHIA at both 28 and 37°C, with optimal growth occurring at 28°C. The fungus failed to grow at temperatures above 42°C. Less abundant growth, which occurred more slowly, was detected on the Mycosel agar at 28°C. After 7 days of incubation, subcultures of the colonies measured 15 mm in diameter. They were initially pale grey, fluffy, folded, and spreading. The colonies filled the petri dish within 3 weeks, becoming dark brown to grey with a lighter-colored periphery. Microscopy of the initial cultures revealed septate, pigmented, and irregularly shaped hyphae, with swollen segments up to 4 μm in diameter. No conidia or asexual fruiting bodies (pycnidia) were evident after 6 weeks of subculture onto specialty agars including potato dextrose agar (PDA) (Oxoid, Basingstoke, England), Czapek solution agar (Difco Laboratories, Detroit, Mich.), cornmeal agar (Amyl Media Pty Ltd., Dandenong, Victoria, Australia), and 2% water agar.
Subcultures of the isolates were submitted to the Orange Agricultural Institute for specific identification. They were inoculated onto a variety of media to induce sporulation. Colonies on Difco PDA produced dense aerial mycelium, initially greenish-grey in the centre of the colony and whitish at the margin, later becoming dark brown to grey-brown. On BBL malt agar and BBL cornmeal agar, the aerial mycelium was brownish in the center, becoming pale grey-brown at the margins. Colonies were slow growing, reaching only 15 to 20 mm in diameter after 7 days at 25°C. Pycnidium production was slow on PDA, malt agar, and Difco oatmeal agar, occurring only after 8 to 10 weeks. Colonies on carnation leaf agar (tap water agar plus irradiated carnation leaves) produced pycnidia after 4 to 5 weeks under black light and a 12-h/12-h alternate dark-light regimen.
Identification of isolates.
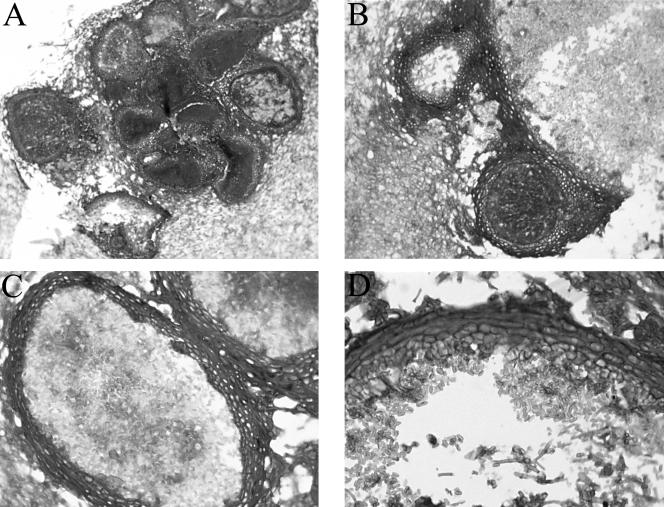
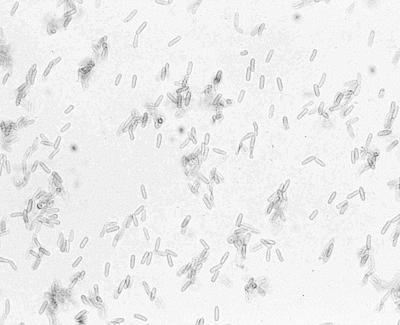
In vitro, all of our isolates produced subglobose pycnidial conidiomata with dark brown parenchymatous walls (textura angularis) and a single apical ostiole and measured 250 to 350 μm in diameter (Fig. 2A to C). Histological sections of the pycnidia of M. arundinis from case 2 were prepared by fixing portions of the colony with visible pycnidia (black dots) in 10% buffered formalin. Following overnight processing, 4-μm-thick paraffin sections were prepared and stained with PAS stain. The conidiogenous cells were ampulliform and up to 5 μm long and produced conidia enteroblastically without proliferation (Fig. 2D). The conidia were smooth walled, brown, and cylindrical and measured 3.5 to 4.5 by 1 to 1.5 μm (Fig. 3).
FIG. 2.
(A) Multiple M. arundinis pycnidia showing thick, darkened walls. Magnification, ×125. (B) Vertical section of two M. arundinis pycnidia showing thick, darkened walls. Magnification, ×312. (C) Vertical section of M. arundinis pycnidia. Magnification, ×500. (D) Part of the pycnidial wall of M. arundinis showing a thick, darkened outer wall becoming more hyaline in the innermost layer. Conidiophores, conidiogenous cells, and smooth-walled, ellipsoidal conidia are present. The wall consists of a layer of three cells. Magnification, ×788. All panels were stained with PAS stain.
FIG. 3.
Elongated, smooth-walled conidia of M. arundinis. Magnification, ×788.
The pycnidial nature of the conidiomata and the enteroblastic nature of the conidiogenesis clearly place them in the genus Microsphaeropsis as currently defined. Of the presently known species, the cylindrical shape and size of the conidia are consistent with an identification of M. arundinis (15). These isolates are also identical to a previous isolate from Australia (DAR 35822), which has been identified as M. arundinis at the International Mycological Institute.
M. arundinis was originally described as a species of Alveophoma Bausa Alcade by Ahmad (1). In the original description (in vivo), the conidiomata were described as being scattered or gregarious with one or two ostioles, and the walls were parenchymatous with thickened walls in the outer layers, becoming paler to hyaline in the innermost layer. The conidiomata measured 130 to 165 by 165 to 230 μm. The conidia were described as hyaline and oblong with rounded ends, measuring 6.5 to 7.8 by 1 to 2.5 μm. This fungus was transferred to the genus Microsphaeropsis by Sutton (15), who examined the type collection. In contrast to the original description, Sutton described the conidia as brown, cylindrical, and measuring only 4 to 4.5 by 1.5 μm.
M. arundinis is distinguished from the species M. olivacea and M. callista by its smooth, thin-walled, cylindrical, guttulate conidia measuring 4 to 4.5 by 1.5 μm. M. olivacea has oval to ellipsoidal, pale brown, smooth, thin-walled conidia measuring 4 to 8 by 2.3 to 5 μm. Conidia of M. callista measure 7 to 8 by 4.5 to 5.5 μm, are elliptical to fusiform and thick walled, have a central guttule, and are darker at each end (8). The isolates have been deposited in the culture collections of the Australian Medical Mycology Reference Laboratory (AMMRL); NSW Agriculture Plant Pathology Herbarium, Orange (DAR), and the University of Alberta Microfungus Collection and Herbarium (UAMH). The isolate from case 1 is deposited as AMMRL 159.00 and DAR 75043. Isolates from case 2 are deposited as AMMRL 159.01, AMMRL 159.02, and AMMRL 159.03, DAR 76499, DAR 76031, and DAR 76500, and UAMH 10394, UAMH 10393, and UAMH 10392.
Bacteriology.
Multiresistant Staphylococcus aureus was isolated from three separate tissue samples all collected from lesions on the right leg of the patient in case 2. The fourth tissue sample yielded mixed growth of multiresistant S. aureus and Stenotrophomonas maltophilia.
Antifungal susceptibility testing.
The M. arundinis isolates from these two patients were tested using the Sensititre Yeast One (Trek Diagnostic Systems Ltd.) colorimetric microbroth dilution method. The sample was incubated at 28°C for 96 h. This longer incubation time was required to allow adequate growth of the fungus, and 28°C rather than 35°C was selected as the better temperature for growth. The MIC of itraconazole was 0.25 mg/liter in both cases. The amphotericin B MIC was 0.25 mg/liter for the organism isolated from the patient in case 1, while two separate MIC tests were performed on isolates from the left calf and the right foot of the patient in case 2. The amphotericin B MICs obtained for these two isolates were 0.125 and 1 mg/liter, respectively.
Molecular analysis.
Subcultures of the isolates were submitted to the Department of Microbiology at Westmead Hospital for molecular analysis. DNA amplification of the D1 variable region of the 28S gene (large subunit) and the internal transcribed spacer region (ITS1 and ITS2 regions) of the gene coding for rRNA (rDNA) was performed on the three isolates from case 2 and one isolate from case 1. Sequence alignments of the two PCR products (LSU and ITS) using CLUSTAL W showed greater than 99% homology among the four isolates. The identity of the isolates could not be confirmed by DNA sequence analysis using both a BLAST search and a FASTA search, since Microsphaeropsis is not yet listed in the GenBank database. The closest match was to a Paraphaeosphaeria sp.
Discussion.
Microsphaeropsis arundinis is a plant inhabitant first described from the grass Arundo donax L. in West Pakistan (now Pakistan) (1). This grass has a worldwide distribution and is often used for musical instrument and handicraft production. In Australia, A. donax is a garden escape weed known as giant reed or elephant grass. It is typically found growing along watercourses, roadsides, and wetlands. It is a robust perennial that grows to a height of 6 m. Although the prevalence of M. arundinis on A. donax or any other plant species has not been determined in Australia, several other Microsphaeropsis species have been described from or reported to occur in Australia. Microsphaeropsis callista (H. Syd.) Sutton was isolated from Eucalyptus haemastoma leaves (14), while M. conielloides Sutton was isolated from leaves of Eucalyptus pauciflora and from air (14). M. olivacea (Bonord) Höhnel is a ubiquitous species, has been isolated from many plant genera, and has a worldwide distribution, including Australia (15).
M. arundinis is confirmed here as a cause of soft tissue infection in two patients, both of whom had diabetes. In the first case, the organism was isolated from a lesion on the dorsum of the hand. Fungal elements were observed by direct microscopy, and their presence was confirmed by histological examination. Treatment with terbinafine appeared to be curative.
The second case was more complicated. The lesions were long standing, and the ulcers became colonized with resistant nosocomial organisms following prolonged hospitalization of the patient. However, despite the mixed infections, we consider M. arundinis to be significant, since it was detected by histopathological examination, microscopy, and culture from four different lesions. Active inflammation including multinucleate giant cells engulfing fungal elements was present in deep tissue. In addition, a coelomycete was known to be present in the lesions before the patient became colonized with nosocomial organisms during a prolonged hospital admission. Both patients were diabetic and were receiving immunosuppressive medications for intercurrent illness. This would have predisposed them to opportunistic fungal infections. How these patients acquired their infections is not known. The lesion on the dorsum of the hand may well have been acquired by traumatic implantation. The patient never volunteered this information, but he may not have noticed a minor injury. The second patient had chronic nonhealing ulcers on his legs and would have been at risk of infection with multiple organisms. This patient lived on a rural property in Central New South Wales, Australia, an area in which A. donax grass is known to occur. However, he did not recall traumatic contact with plant material. He also had a persistent osteomyelitis of his finger as a result of infection with another fungus, Phialophora verrucosa, which is associated with traumatic implantation from plant material.
A third isolate of M. arundinis identified by this laboratory was obtained from the infected forelimb of a cat. The site of the lesion on the fourth digit was amputated, and the cat was treated with ketoconazole and topical terbinafine without subsequent relapse. This case has been reported elsewhere (8).
A fourth isolate was obtained in 1981 in Sydney, Australia, from an ankle nodule of a patient being treated for acute myeloid leukemia. Histopathology of the nodule showed a leukemic infiltrate and the presence of fungal elements. The fungus was grown on three separate occasions from samples of this site. Isolates have been deposited in the DAR (DAR 35822) and UAMH (UAMH 10391) culture collections (J. Walker, unpublished data). The significance of this isolate could not be established at the time.
Other Microsphaeropsis spp. have been implicated in soft tissue infections. M. olivacea was reported as causing a skin lesion in an otherwise healthy woman (6), and it was also isolated from the eye of a man with keratitis following traumatic injury (12). Coniothyrium-Microsphaeropsis complex was isolated from the ulcerated knee of a diabetic woman who had undergone a renal transplant (10).
Although the identity of our isolates could not be confirmed by molecular analysis, they were shown to have greater than 99% homology to each other, indicating that they are of the same species. Since the taxonomy of the coelomycetes is complex, detailed molecular analysis would be helpful in the definitive characterization of these isolates in particular, as well as in determining the relationships between the various species within the group in general.
Acknowledgments
We thank the Department of Anatomical Pathology (PaLMS) staff, in particular Dianne Reader, for the preparation and staining of the histological sections of the pycnidia. We also thank Catriona Halliday from the Department of Microbiology, ICPMR, Westmead Hospital, for performing the molecular analysis of the isolates.
REFERENCES
- 1.Ahmad, S. 1971. Contributions to the fungi of West Pakistan. X. Biologia (Lahore) 17:1-26. [Google Scholar]
- 2.Baker, J. G., I. F. Salkin, P. Forgacs, J. H. Haines, and M. E. Kemna. 1987. First report of subcutaneous phaeohyphomycosis of the foot caused by Phoma minutella. J. Clin. Microbiol. 25:2395-2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camara, M. P. S., M. E. Palm, P. van Berkum, and E. L. Stewart. 2001. Systematics of Paraphaeosphaeria: molecular and morphological approach. Mycol. Res. 105:41-56. [Google Scholar]
- 4.Castro, L. G. M., C. da Silva Lacaz, J. Guarro, J. Gené, E. M. Heins-Vaccari, R. S. de Freitas Leite, G. L. H. Arriagada, M. M. O. Reguera, E. M. Ito, N. Y. S. Valente, and R. S. Nunes. 2001. Phaeohyphomycotic cyst caused by Colleotrichum crassipes. J. Clin. Microbiol. 39:2321-2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chabasse, D., C. de Bievre, E. Legrand, J. P. Saint-Andre, L. de Gentile, B. Cimon, and J. P. Bouchara. 1995. Subcutaneous abscess caused by Pleurophomopsis lignicola Petr: first case. J. Med. Vet. Mycol. 33:415-417. [PubMed] [Google Scholar]
- 6.Guarro, J., E. Mayayo, J. Tapiol, C. Aguilar, and J. Cano. 1999. Microsphaeropsis olivacea as an etiological agent of human skin infection. Med. Mycol. 37:133-137. [PubMed] [Google Scholar]
- 7.Guarro, J., T. E. Svidzinski, L. Zaror, M. H. Forjaz, J. Gene, and O. Fischman. 1998. Subcutaneous hyalohyphomycosis caused by Colleotrichum gleosporioides. J. Clin. Microbiol. 36:3060-3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kluger, E. K., P. K. Della Torre, P. Martin, M. B. Krockenberger, and R. Malik. 2004. Concurrent Fusarium chlamydosporium and Microsphaeropsis arundinis infections in a cat. J. Feline Med. Surg. 6:271-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maslen, M. M., T. Collis, and R. Stuart. 1996. Lasiodiplodia theobromae isolated from a subcutaneous abscess in a Cambodian immigrant to Australia. J. Med. Vet. Mycol. 34:279-283. [DOI] [PubMed] [Google Scholar]
- 10.Miele, P. S., C. S. Levy, M. A. Smith, E. M. Dugan, R. H. Cooke, J. A. Light, and D. R. Lucey. 2002. Primary cutaneous fungal infections in solid organ transplantation: a case series. Am. J. Transplant. 2:678-683. [DOI] [PubMed] [Google Scholar]
- 11.Padhye, A. A., S. Karpati, A. Rosenthal, and E. Punithalinham. 2004. Subcutaneous phaeohyphomycotic abscess caused by Pleurophomosis lignicola. Med. Mycol. 42:129-134. [DOI] [PubMed] [Google Scholar]
- 12.Shah, C. V., D. B. Jones, and E. R. Holz. 2001. Microsphaeropsis olivacea keratitis and consecutive ophthalmitis. Am. J. Ophthalmol. 131:142-143. [DOI] [PubMed] [Google Scholar]
- 13.Sigler, L., R. C. Summerbell, L. Poole, M. Wieden, D. A. Sutton, M. G. Rinaldi, M. Aguirre, G. W. Estes, and J. N. Galgiani. 1997. Invasive Nattrassia mangiferae infections: case report, literature review, and therapeutic and taxonomic appraisal. J. Clin. Microbiol. 35:433-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sutton, B. C. 1971. Coelomycetes. IV. The genus Harknessia and similar fungi on Eucalyptus. Mycol. Papers 123:1-46. [Google Scholar]
- 15.Sutton, B. C. 1980. The coelomycetes: fungi imperfecti with pycnidia acervuli and stromata. Commonwealth Mycological Institute, Kew, England.
- 16.Sutton, D. A. 1999. Coelomycetous fungi in human disease: a review. Clinical entities, pathogenesis, identification and therapy. Rev. Iberoam. Micol. 16:171-179. [PubMed] [Google Scholar]
- 17.Sutton, D. A., W. D. Timm, G. Morgan-Jones, and M. Rinaldi. 1999. Human phaeohyphomycotic osteomyelitis caused by the coelomycete Phomopsis Saccardo 1905: criteria for identification, case history, and therapy. J. Clin. Microbiol. 37:807-811. [DOI] [PMC free article] [PubMed] [Google Scholar]