Abstract
Staphylococcus aureus isolates from women with nasal, anal, or vaginal colonization were evaluated for population diversity by pulsed-field gel electrophoresis. Cluster analysis of restriction patterns revealed diversity indices of 0.89 and 0.99 for toxic shock syndrome toxin 1-positive and -negative isolates, respectively. Toxin-producing strains were isolated more frequently from the nares than from other sites.
Staphylococcus aureus is an endogenous microorganism colonizing the nasal cavities, skin, gastrointestinal tracts, anuses, and vaginal vaults of healthy women. Approximately 60% of women harbor this organism intermittently at one or more body sites (12). In this study, approximately 7% of women harbored toxin-producing S. aureus, while other studies have shown that as many as 25% harbor toxin producers (2). Thus, understanding the epidemiology of toxic shock syndrome (TSS) is clinically important because of the rare but potentially devastating symptoms caused by toxic shock syndrome toxin 1 (TSST-1), a potent exotoxin produced by S. aureus (1, 4, 5, 7, 10). Patients with TSS exhibit a number of clinical symptoms, including erythematous rash, fever, diarrhea, inability to maintain homeostasis, multiple-organ involvement, and desquamation of the skin, with the most severe cases being fatal (9). Of cases of TSS involving menstruation, almost all are associated with TSST-1 (1, 4, 5, 7, 10). Little information is available regarding the genetic diversity of TSST-1-producing S. aureus isolates and their favored human niche.
In this study, S. aureus samples were isolated from the anterior nares, anuses, and vaginas of overtly healthy women and evaluated for frequency of isolation, the presence of TSST-1 as determined by enzyme-linked immunosorbent assay (6), and the genetic diversity of TSST-1-positive (TPOS) and TSST-negative (TNEG) isolates as determined by genomic DNA restriction with SmaI and pulsed-field gel electrophoresis (PFGE) of the resulting fragments.
Samples were obtained by standard microbial techniques from 3,012 subjects during this study. Subjects were recruited to fit a demographic profile of four major ethnic groups, namely, whites (80%), blacks (12%), Hispanics (5%), and Asians (3%), that is based on the 1990 U.S. census. A total of 1,051 S. aureus isolates were recovered from 787 (26%) women; 7% of all subjects were colonized with TPOS S. aureus. The anterior nares were the most common anatomic site of carriage, accounting for 548 (52%) isolates, followed by the vagina (264 isolates [25%]) and anus (239 isolates [23%]). Of the TPOS isolates, 72% (177 of 245) were isolated from the anterior nares. This percentage represents a significant increase in the frequency of recovery relative to the recovery of TPOS strains from anal or vaginal samples (P < 0.001) (Table 1). Furthermore, the frequency of TPOS strain isolation from the anterior nares was significantly higher than the frequency of TNEG strain isolation from that site (72% versus 46%; P < 0.001). Among subjects from which S. aureus was isolated, 576 (73%) harbored S. aureus at one body site, 158 (20%) harbored the organism at two body sites, and 53 (7%) harbored the organism at all three body sites.
TABLE 1.
Sample population statistics
| Panela | Type of specimen | % of women colonized at:
|
% of all isolates
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| One site
|
Two sites
|
Three sites
|
|||||||||||
| TPOS | TNEG | Total | TPOS | TNEG | Total | TPOS | TNEG | Total | TPOS | TNEG | Total | ||
| A | Nasal | 91 | 56 | 72 | 39 | 21 | 25 | 53 | 28 | 33 | 72 | 46 | 52 |
| Anal | 5 | 13 | 11 | 33 | 40 | 38 | 24 | 36 | 33 | 15 | 25 | 23 | |
| Vaginal | 4 | 21 | 16 | 28 | 39 | 37 | 24 | 36 | 33 | 13 | 29 | 25 | |
| B | Nasal | 91 | 65 | 81 | 39 | 24 | 33 | 54 | 20 | 33 | 72 | 44 | 60 |
| Anal | 5 | 12 | 8 | 32 | 37 | 34 | 23 | 40 | 33 | 15 | 25 | 19 | |
| Vaginal | 4 | 22 | 11 | 28 | 39 | 33 | 23 | 40 | 33 | 13 | 31 | 21 | |
Panel A consisted of 3,012 women from five geographical locations producing 1,051 isolates; panel B consisted of 329 women from five geographical locations producing 434 isolates, which were evaluated by PFGE.
Boldface values indicate statistically significant differences between TPOS and TNEG isolate proportions (P < 0.01).
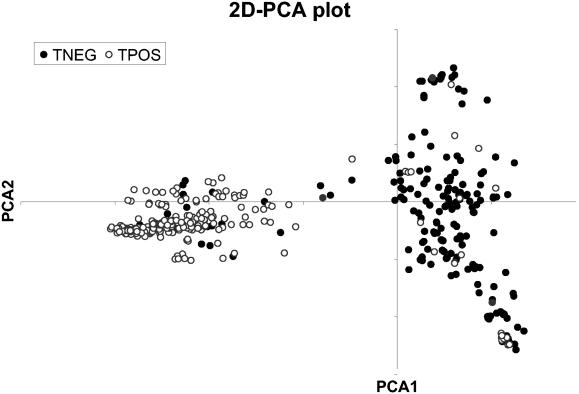
A total of 434 isolates were evaluated by PFGE, of which 245 were TPOS and 189 were TNEG. The smaller sample set was similar to the sample population by body site, and there was a slight trend toward a disproportionate increase in nasal carriage (Table 1) (chi-square test, P = 0.019) attributable to the increased representation of TPOS isolates. The proportions of woman harboring S. aureus at one, two, or three body sites were also comparable (chi-square test, P = 0.83). When these 434 isolates were evaluated by PFGE with a 75% similarity index, 125 clusters (subtypes) were partitioned from 213 unique PFGE patterns. The diversity index (DH) for this group of isolates was 0.96. When the respective toxigenic groups were evaluated, TPOS isolates formed a small subset (245 of 1,051 [23%]) of the entire S. aureus sample population and showed markedly less diversity (DH = 0.89) than the TNEG isolates (DH = 0.99) (Fig. 1). Furthermore, TPOS and TNEG isolates in this study were represented by 43 clusters and 100 clusters, respectively. For TPOS isolates, 30% were represented by a single cluster. No more than 4% of TNEG isolates were represented by any single cluster. Most TPOS isolates exhibited similar PFGE patterns (Fig. 2). For example, the four most prevalent TPOS isolates, which represented 53% (129 of 245) of all TPOS isolates, were closely related, with a mean band difference of only 2.18 and a maximal mean band difference of 3.39 (3, 8, 11, 13).
FIG. 1.
Two-dimensional principle component analysis (2D-PCA) of the PFGE pattern multivariate data with a standardized data set and covariance matrix. The TPOS isolates comprise a much smaller group than the TNEG isolates, and the two groups are quite well defined. The difference in group size is quite remarkable considering the fact that only 23% of the TNEG isolates are represented in the graph.
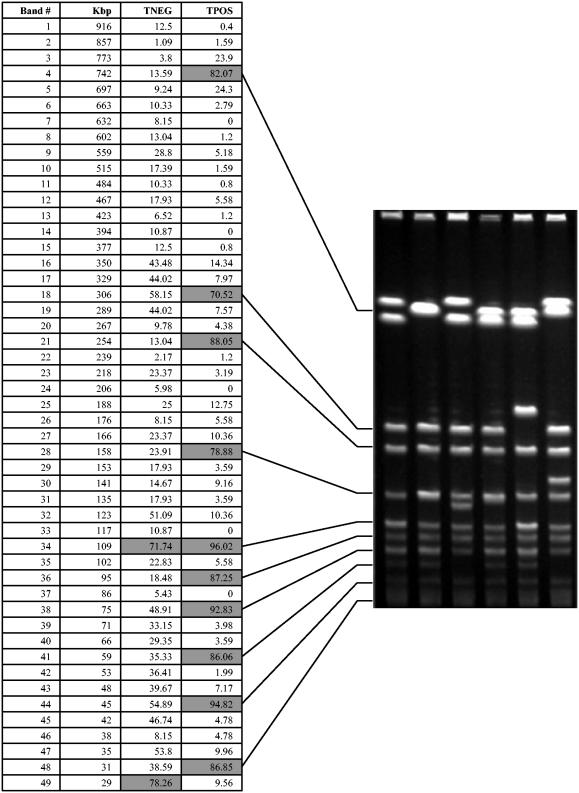
FIG. 2.
Estimated band type sizes in kilobase pairs and the frequencies of occurrence of the corresponding band types. Note the reoccurring DNA fragments at 742, 306, 254, 158, 109, 95, 75, 59, 45, and 31 kbp for the TPOS strains.
Evaluation of the data set for the most likely number of partitions by an analysis of the maximum successive differences between k and k + 1 partitions of the total sum-of-squares of all clusters for each possible number of groups, where k is the number of partitions (3, 11, 13), found that the most likely number of clusters was 2. Contingency table comparisons of these clusters for the production of TSST-1 found a sensitivity and specificity of 92% (225 of 245 isolates tested) and 86% (163 of 189 isolates tested), respectively, for TPOS S. aureus strains (Fig. 1).
Of women colonized by S. aureus at all three body sites, 100% were colonized with strains with identical PFGE types at two or more body sites; 36% (8 of 22) of these women had strains with identical PFGE types at all three body sites evaluated, 5% (1 of 22) had strains with identical PFGE types at the nasal and anal body sites, none had matched pairs at the nasal and vaginal body sites, and 59% (13 of 22) had strains with identical PFGE types at anal and vaginal body sites. Interestingly, 92% (12 of 13) of women with isolates that produced identical PFGE patterns from anal and vaginal sites were colonized with TNEG S. aureus at these sites. Of women whose isolates were evaluated by PFGE, 19% (61 of 329) yielded isolates from two body sites and 84% (51 of 61) of these woman harbored strains with identical PFGE types. Among the women colonized at two sites, the proportion of paired anal and vaginal isolates with identical PFGE patterns that were TNEG was significantly higher than the proportion of other PFGE pairs (chi-square test, P < 0.01) (Table 2).
TABLE 2.
Numbers of women colonized at two sites and the recovery locations of S. aureus strains producing identical PFGE patterns
| PFGE patterns | Type of isolate | No. of women with isolates froma:
|
Total | ||
|---|---|---|---|---|---|
| N, A | N, V | A, V | |||
| Identical | TPOS | 14 | 10 | 7 | 31 |
| TNEG | 4 | 3 | 11 | 18 | |
| Both | 0 | 1 | 1 | 2 | |
| Varied | TPOS | 1 | 0 | 0 | 1 |
| TNEG | 1 | 2 | 1 | 4 | |
| Both | 1 | 3 | 1 | 5 | |
| Total | 21 | 19 | 21 | 61 | |
N, A, nares and anus; N, V, nares and vagina; A, V, anus and vagina.
The homology of PFGE patterns among TPOS isolates evaluated during this study suggests a common lineage for the majority of TPOS S. aureus isolates, whereas the TNEG isolates appear to be a more diverse group (Table 3 and Fig. 1). Although TSST-1 expression has been associated with the entire breadth of the S. aureus population, it has been suggested that one lineage is highly associated with TSS involving the cervicovaginal niche (5). That conclusion is not refuted by the close relationship of most TPOS isolates in this study and their primary colonization of the anterior nares. This high association of the TPOS isolates with the human nasal cavity suggests that this site is a staging niche for subsequent colonization of an anal, vaginal, or wound site that could potentially lead to the onset of disease.
TABLE 3.
TNEG and TPOS groups and their frequencies
| Isolates | Group sizea (X) | Frequencyb (Y) | No. of isolatesc (X · Y) | %d |
|---|---|---|---|---|
| TNEG | 1 | 51 | 51 | 27.0 |
| 2 | 32 | 64 | 33.9 | |
| 3 | 8 | 24 | 12.7 | |
| 4 | 2 | 8 | 4.2 | |
| 5 | 3 | 15 | 7.9 | |
| 6 | 2 | 12 | 6.3 | |
| 7 | 1 | 7 | 3.7 | |
| 8 | 1 | 8 | 4.2 | |
| Total | 100 | 189 | 100 | |
| TPOS | 1 | 11 | 11 | 4.5 |
| 2 | 15 | 30 | 12.2 | |
| 3 | 3 | 9 | 3.7 | |
| 4 | 3 | 12 | 4.9 | |
| 5 | 2 | 10 | 4.1 | |
| 6 | 1 | 6 | 2.4 | |
| 8 | 1 | 8 | 3.3 | |
| 9 | 1 | 9 | 3.7 | |
| 10 | 1 | 10 | 4.1 | |
| 11 | 1 | 11 | 4.5 | |
| 12 | 1 | 12 | 4.9 | |
| 20 | 1 | 20 | 8.2 | |
| 23 | 1 | 23 | 9.4 | |
| 74 | 1 | 74 | 30.2 | |
| Total | 43 | 245 | 100 |
Number of isolates in a single PFGE group.
Frequency of group size occurrence.
Number of isolates in respective group size.
Percentage of the total number of isolates for the corresponding group size.
The increased frequency of identical PFGE patterns of paired anal-vaginal isolates over those of nasal and other body site pairs suggests cross-colonization between these sites as a more frequent event.
Daghistani et al. found that isolates from the anterior nares were more likely than those from wounds or vaginal sources to produce TSST-1 (2). The differences in recovery frequency between TPOS and TNEG isolates found in this study support this claim (Table 1). For women harboring TPOS S. aureus, 72% of the isolates were recovered from the anterior nares. For women colonized at all three body sites, 92% of paired anal and vaginal isolates with identical PFGE patterns did not produce toxin. For women colonized at two body sites, the percentage of paired TNEG anal and vaginal isolates with identical PFGE patterns was significantly greater than that of nasal-anal and anal-vaginal isolate pairs (Table 2) (P < 0.01). Of the TPOS isolates from women harboring S. aureus at a single body site, a staggering 91% were isolated from swabs of the anterior nares, while only 5 and 4% were recovered from anal and vaginal samples, respectively. These proportions are significantly different from those of TNEG isolates from women colonized at a single body site, of which 66% were from the anterior nares, 13% were from the anus, and 21% were from the vagina, respectively (Table 1) (chi-square test, P < 0.001). For all women harboring S. aureus in this study, and with a 95% confidence, the frequency of recovery of TPOS isolates from the anterior nares over the recovery from anal or vaginal body sites was between 20 and 33% higher than the recovery frequency of TNEG isolates (P < 0.001).
The data from this study indicate that TSST-1-producing strains of S. aureus isolated from the anterior nares, anuses, or vaginas of women are less diverse than non-toxin-producing strains from the same sources over a large geographic area. Moreover, TPOS isolates are more likely to be recovered from the anterior nares than from other anatomic locations, suggesting that these strains are perhaps less adaptable to demanding environments and therefore are concentrated in the nares—the primary niche for this species.
Acknowledgments
We thank Mary Delaney, Andrea DuBois, Matthew Lawlor, Wendy Osterling, Dave Aiello, and Paul Modern for S. aureus isolation and TSST-1 characterization and Hill Top Laboratories for their efforts in obtaining the samples.
This research was funded in part by Procter & Gamble Co., Cincinnati, Ohio.
REFERENCES
- 1.Crass, B. A., and M. S. Bergdoll. 1986. Toxin involvement in toxic shock syndrome. J. Infect. Dis. 153:918-926. [DOI] [PubMed] [Google Scholar]
- 2.Daghistani, H. I., A. A. Issa, and A. A. Shehabi. 2000. Frequency of nasal and wound isolates of Staphylococcus aureus associated with TSST-1 production in Jordanian population. FEMS Immunol. Med. Microbiol. 27:95-98. [DOI] [PubMed] [Google Scholar]
- 3.Krzanowski, W. J., and Y. T. Lai. 1988. A criterion for determining the number of groups in a data set using sum-of-squares clustering. Biometrics 44:23-34. [Google Scholar]
- 4.Lee, V. T., A. H. Chang, and A. W. Chow. 1992. Detection of staphylococcal enterotoxin B among toxic shock syndrome (TSS)- and non-TSS-associated Staphylococcus aureus isolates. J. Infect. Dis. 166:911-915. [DOI] [PubMed] [Google Scholar]
- 5.Musser, J. M., P. M. Schlievert, A. W. Chow, P. Ewan, B. N. Kreiswirth, V. T. Rosdahl, A. S. Naidu, W. Witte, and R. K. Selander. 1990. A single clone of Staphylococcus aureus causes the majority of cases of toxic shock syndrome. Proc. Natl. Acad. Sci. USA 87:225-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parsonnet, J., J. T. Mills, Z. A. Gillis, and G. B. Pier. 1985. Competitive, enzyme-linked immunosorbent assay for toxic shock syndrome toxin 1. J. Clin. Microbiol. 22:26-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ross, R. A., and A. B. Onderdonk. 2000. Production of toxic shock syndrome toxin 1 by Staphylococcus aureus requires both oxygen and carbon dioxide. Infect. Immun. 68:5205-5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Todd, J., M. Fishaut, F. Kapral, and T. Welch. 1978. Toxic-shock syndrome associated with phage-group-I Staphylococci. Lancet ii:1116-1118. [DOI] [PubMed] [Google Scholar]
- 10.Vergeront, J. M., S. J. Stolz, B. A. Crass, D. B. Nelson, J. P. Davis, and M. S. Bergdoll. 1983. Prevalence of serum antibody to staphylococcal enterotoxin F among Wisconsin residents: implications for toxic-shock syndrome. J. Infect. Dis. 148:692-698. [DOI] [PubMed] [Google Scholar]
- 11.Vogt, W., and D. Nagel. 1992. Cluster analysis in diagnosis. Clin. Chem. 38:182-198. [PubMed] [Google Scholar]
- 12.von Eiff, C., K. Becker, K. Machka, H. Stammer, G. Peters, et al. 2001. Nasal carriage as a source of Staphylococcus aureus bacteremia. N. Engl. J. Med. 344:11-16. [DOI] [PubMed] [Google Scholar]
- 13.Warner, J. E., and A. B. Onderdonk. 2003. Method for optimizing pulsed-field gel electrophoresis banding pattern data. J. Mol. Diagn. 5:21-27. [DOI] [PMC free article] [PubMed] [Google Scholar]