Abstract
To determine the genogroups and genotypes of bovine enteric caliciviruses (BECVs) circulating in calves, we determined the complete capsid gene sequences of 21 BECVs. The nucleotide and predicted amino acid sequences were compared phylogenetically with those of known human and animal enteric caliciviruses. Based on these analyses, 15 BECVs belonged to Norovirus genogroup III and genotype 2 (GIII/2) and were genetically distinct from human Norovirus GI and GII. Six BECVs had capsid gene sequences similar to that of the unclassified Nebraska (NB)-like BECV. The 15 bovine noroviruses (BoNVs) were more closely related to Bo/NLV/Newbury-2/76/UK (GIII/2) and other known genotype 2 BoNVs than to genotype 1 Bo/NLV/Jena/80/DE. The BoNV Bo/CV521-OH/02/US showed high nucleotide and amino acid identities (84 and 94%, respectively) with the capsid gene of Bo/NLV/Newbury-2/76/UK, whereas the nucleotide and amino acid sequences of the RNA polymerase gene were more closely related to those of Bo/NLV/Jena/80/DE (77 and 87% identities, respectively) than to those of Bo/NLV/Newbury-2/76/UK (69 and 69% identities, respectively), suggesting that Bo/CV521-OH/02/US is a genotype 1-2 recombinant. Gene conversion analysis by the recombinant identification program and SimPlot also predicted that Bo/CV521-OH/02/US was a recombinant. Six NB-like BECVs shared 88 to 92% nucleotide and 94 to 99.5% amino acid identities with the NB BECV in the capsid gene. The results of this study demonstrate genetic diversity in the capsid genes of BECVs circulating in Ohio veal calves, provide new data for coinfections with distinct BECV genotypes or genogroups, and describe the first natural BoNV genotype 1-2 recombinant, analogous to the previously reported human norovirus recombinants.
Caliciviruses are nonenveloped, single-stranded RNA viruses with positive-sense genomes of 7.4 to 8.3 kb in size (6). The family Caliciviridae is divided into four genera, i.e., Norovirus, Sapovirus, Vesivirus, and Lagovirus, based on genomic organization and genetic analysis (2, 10, 27). Genomes of noroviruses are composed of three open reading frames (ORFs), which encode a posttranslationally cleaved nonstructural polyprotein (ORF1), a single major structural capsid protein (ORF2), and a small basic protein (ORF3) (12, 31). Genomes of Sapoviruses consist of two to three ORFs, with the major capsid protein encoded in ORF1 contiguous with the nonstructural polyprotein and a small basic protein encoded in ORF2 (28). The capsid proteins of noroviruses are composed of two structural domains, the shell (S) and the protruding (P) domains. The P domain contains two subdomains, P1 and P2. The S domain is formed by the N-terminal 225 amino acids (aa), and the P domain is composed of aa 226 to the C-terminal end (32).
The enteric caliciviruses include the noroviruses and sapoviruses, which cause acute gastroenteritis in humans and animals (3, 7, 9, 11, 15, 38). Bovine enteric caliciviruses (BECVs) are classified as a third genogroup (GIII) of noroviruses, distinct from GI and GII human noroviruses (1, 5, 23, 29). They include Bo/NVL/Newbury-2/76/UK and Bo/NLV/Jena/80/DE (1). Recently, an unclassified BECV, Nebraska (NB) strain, which is most closely related to Sapovirus and Lagovirus, was recognized as a new NB-like genus or genogroup (34). The capsid genes of GIII noroviruses are composed of 1,560 to 1,569 bp, whereas the NB strain capsid gene is 1,650 bp, which is 81 to 90 bp larger than those of GIII noroviruses (23, 29, 35).
Determination of the antigenic relationship between genogroups and genotypes of enteric caliciviruses is difficult because most viruses, except for Cowden porcine sapovirus, do not grow in cell culture (8, 30). Based on genetic divergence in the RNA-dependent RNA polymerase (RdRp) and capsid genes, Norovirus and Sapovirus are each classified into at least three distinct genogroups, with multiple genotypes, which is indicative of the genetic diversity found within each genogroup (1, 10, 13, 36). However, the antigenic relationships among the genogroups of enteric caliciviruses have not been extensively analyzed. Calicivirus capsid proteins expressed in a baculovirus expression system self-assemble into virus-like particles that are antigenically and morphologically similar to native virions. The virus-like particles of human noroviruses have been used as antigens in enzyme immunoassays for serotyping of human noroviruses (14, 18).
Characterized bovine noroviruses (BoNVs) comprise two genotypes (1 and 2) in Norovirus GIII, of which Bo/Jena/80/DE and Bo/Newbury-2/76/UK are the prototype viruses of GIII/1 and GIII/2, respectively. With the exception of Bo/Jena/80/DE, most other recently reported BoNVs, such as Bo/Aberystwyth24/00/UK, Bo/CH126/98/NET, Bo/CH131/98/NET, Bo/CV95-OH/00/US, Bo/CV186-OH/00/US, Bo/Dumfries/94/UK, and Bo/Penrith55/00/UK, are classified as Norovirus GIII/2 (like Bo/Newbury-2/76/UK) based on the determined sequences of their RdRp and/or capsid genes (1, 29, 35). To further characterize the genetic diversity of genogroups and genotypes of BECVs circulating among calves and to determine their genetic relationships to human noroviruses and sapoviruses, we sequenced the complete capsid genes of 21 BECV strains detected in veal calves in Ohio.
MATERIALS AND METHODS
BECV strains.
BECV strains selected for capsid gene sequencing were previously reported (35). BECV strains in fecal samples collected from 2-week-old veal calves (1 week after farm placement) on farms A and B in 2002 in Ohio were detected by reverse transcription-PCR (RT-PCR). Calves introduced on each farm were obtained from auction barns sourcing dairy calves from four or five states (Ohio, West Virginia, Virginia, Pennsylvania, and New York). Of the 21 BECV strains characterized, 12 BECVs originated from feces of veal calves on farm A, with the balance detected in feces of veal calves from farm B. Calves in which BECVs were detected in fecal samples were either clinically normal or had diarrhea. The RdRp genes of 7 of the 21 BECVs were sequenced previously (35; J. R. Smiley and L. J. Saif, unpublished data).
RNA extraction and RT-PCR.
Viral RNA was extracted from fecal samples diluted 1:10 with phosphate-buffered saline (pH 7.4) by using the TRIzol reagent (Invitrogen Corporation, Carlsbad, Calif.) according to the manufacturer's instructions. RNA pellets were suspended in 50 μl of nuclease-free water and used to amplify the capsid gene by RT-PCR. The remainder of the RNA was stored at −70°C for future use.
Primers used to amplify the complete capsid gene were designed based on previously reported sequence data (5, 23, 34). The primer sets used to amplify the complete capsid gene of BECV were NAcap-F/R and Jenacap-F1/F2/R1/R2/R3 for GIII noroviruses and NBcap-F3/R for NB-like BECV (Table 1). Primers NAcap-F/R and Jenacap-F1/F2/R1/R2/R3 were designed based on published sequences of known GIII/2 BoNVs, including Bo/Newbury-2/76/UK (accession number AF097917) and Bo/Jena/80/DE (AJ011099), respectively. Primer set NBcap-F3/R was designed from the gene sequence of the NB strain (NC_004064). To amplify the partial RdRp and capsid genes of Bo/CV521-OH/02/US, primer RC35/1932 was designed based on its previously reported RdRp gene sequence (AY151257) and the capsid gene sequence determined in this study (AY549161). Internal primers were designed based on the conserved region of the capsid gene sequences of BECV strains characterized in this study and were used to sequence the internal regions of the capsid genes of noroviruses and NB-like BECVs.
TABLE 1.
Newly designed oligonucleotide primer sequences used for amplification of the capsid genes of BoNVs and NB-like BECVs
| Primer | Sense | Sequence (5′→3′) | Locationa | Annealing temp (°C) | Target virus | Sample no. of amplified BECVb |
|---|---|---|---|---|---|---|
| NAcap-F | + | TCC TTC CCG ATT TTG TAA | 767-784 | 50 | Norovirus GIII/2 | CV490, CV499, CV500, CV506, CV509, CV510, CV511, CV521, CV533, CV540, CV550, CV551, CV557 |
| NAcap-R | − | AAC CCC GCC GAG AAG AGA GGA GAA | 2,538-2,381 | 50 | Norovirus GIII/2 | |
| Jenacap-F1 | + | TGA TTT GTC GCT GTG GGA AGG T | 5,001-5,022 | 54 | Norovirus GIII/1 | |
| Jenacap-F2 | + | AGC GGC GGA ATG GAG AT | 4,939-4,955 | 50 | Norovirus GIII/1 | |
| Jenacap-R1 | − | AGT GGA AAT TGC CGC CGA TAC AGC | 6,636-6,659 | 50 | Norovirus GIII/1 | |
| Jenacap-R2 | − | GCA TCC TCT CAT CAT GTT GG | 6,725-6,744 | 50 | Norovirus GIII/1 | |
| Jenacap-R3 | − | AAT TGC CGC CGA TAC AG | 6,637-6,653 | 50 | Norovirus GIII/1 | |
| NBcap-F3 | + | GTG ATT TAA TTA GAG AAG GAA AC | 5,035-5,057 | 56 | NB-like BECV | CV504, CV519, CV526, CV531, CV548, CV562 |
| NBcap-R | − | CGT AGC AGC ACT AGC CAT A | 6,708-6,726 | 56 | NB-like BECV |
The primer sequences of NAcaq-F/R, Jenacap-F1/F2/R1/R2/R3, and NBcap-F3/R were located at genome sequences of Bo/Newbury-2/76/UK (accession number AF097917), Bo/Jena/80/DE (AJ011099), and Bo/NB/80/US (NC_004064), respectively.
Fecal samples, CV500, CV510, CV551, and CV557, which were RT-PCR positive with primer set NBU-F/NBU-R (35), were not positive with primer set NBcap-F3/NBcap-R, except for Bo/CV500- OH/02/US. The capsid gene of Bo/CV500-OH/02/US, which was weakly positive with primer set NBcap- F3/NBcap-R, was cloned and sequenced with a RT-PCR product which was amplified with primer set NAcap-F/NAcap-R, CV526 and CV531 were positive with CBECU-F/CBECU-R, which were specific primers for BoNV (35).
The BECV capsid genes were amplified by one-step RT-PCR. Three microliters of RNA was denatured at 70°C for 10 min and mixed with an RT-PCR mixture consisting of 5 μl of 10× PCR buffer (50 mM KCl, 10 mM Tris-HCl [pH 9.0], 0.1% Triton X-100), 5 μl of 25 mM MgCl2, 1 μl of 10 mM deoxynucleoside triphosphates, 0.5 μl of 10 mM (each) primers, 1 μl of Taq DNA polymerase (5 U/μl), 0.5 μl of RNasin RNase inhibitor (40 U/μl), and 0.5 μl of avian myeloblastosis virus reverse transcriptase (10 U/μl) in a total volume of 50 μl. All reagents were from Promega Corporation (Madison, Wis.). All one-step RT-PCRs were performed under the following conditions except for the annealing temperature. The annealing temperatures for each primer are described in Table 1. The conditions for one-step RT-PCR were one cycle of 45°C for 60 min and 94°C for 2 min; 35 cycles of 94°C for 30 s, 50 to 56°C for 30 s, and 72°C for 1 min 30 s; and 72°C for 10 min. At the completion of cycling, amplified RT-PCR products were kept at 4°C and were electrophoresed in a 1.2% agarose gel containing ethidium bromide.
Cloning and sequencing of the capsid gene.
The amplified RT-PCR products were purified with a gel extraction kit (Qiagen Inc., Valencia, Calif.) and cloned into PCR2.1 vectors by using the TOPO TA cloning kit (Invitrogen Corporation) according to the manufacturer's instructions. Plasmid DNA from transformed cells was extracted by using an alkaline lysis method (Qiagen Inc.), and the presence of BECV capsid gene inserts was confirmed by restriction enzyme mapping. Cloned capsid genes were sequenced by using an ABI Prism 377 DNA sequencer (Applied Biosystems, Foster, Calif.). Two to four clones for each BECV strain were sequenced to ascertain the consensus sequence.
Phylogenetic analysis.
Multiple alignments of nucleotide and amino acid sequences were performed with Clustal W as implemented in the Lasergene software package (DNASTAR Inc., Madison, Wis.), and nucleotide and amino acid identities were calculated by using the same package. Phylogenetic analyses of Clustal W nucleotide and amino acid alignments generated by the European Bioinformatics Institute (http://www.ebi.ac.uk) were performed by the UPGMA and neighbor-joining methods, and 1,000 bootstrap replicates generated by using the Molecular Evolutionary Genetics Analysis version 2.1 (MEGA2) software (21). Genetic distances among human, porcine, and bovine noroviruses and among Norovirus genogroups and genotypes were calculated by Kimura's two-parameter method (20).
Gene conversion analysis.
Genetic recombination was analyzed by using the Recombinant Identification Program (RIP) (33) and the sliding-window genetic diversity plot (SimPlot), version 3.2 beta, which was kindly provided by S. Ray, Johns Hopkins University School of Medicine, Baltimore, Md. (25). Bo/Newbury-2/76/UK and Bo/Jena/80/DE were used for background sequences. The analyzed ORF1 C-terminal region (partial RdRp gene) and ORF2 of Bo/CV521-OH/02/US, whose size was 1,898 bp, corresponded to nucleotides 1651 to 2232 of Bo/Newbury-2/76/UK and to nucleotides 4601 to 6489 of Bo/Jena/80/DE. The aligned sequences were analyzed by RIP with a window size of 100 bp and a threshold for statistical significance of 90% and by SimPlot with a window 200 bp wide and a step size of 20 bp.
Nucleotide sequence accession numbers.
The sequences of the complete capsid genes of BECV strains have been assigned GenBank accession numbers. The accession numbers for the BoNVs are as follows: Bo/CV490-OH/02/US, AY549153; Bo/CV499-OH/02/US, AY549154; Bo/CV500-OH/02/US, AY549155; Bo/CV506-OH/02/US, AY549156; Bo/CV509-OH/02/US, AY549157; Bo/CV510-OH/02/US, AY549158; Bo/CV511-OH/02/US, AY549159; Bo/CV511 M-OH/02/US, AY549160; Bo/CV521-OH/02/US, AY549161; Bo/CV533-OH/02/US,AY549162; Bo/CV540-OH/02/US, AY549163; Bo/CV550-OH/02/US, AY549164; Bo/CV551-OH/02/US, AY549165; Bo/CV551 M-OH/02/US, AY549166; and Bo/CV557-OH/02/US, AY549167. The accession numbers for NB-like BECVs are as follows: Bo/CV504-OH/02/US, AY549168; Bo/CV519-OH/02/US, AY549169; Bo/CV526-OH/02/US, AY549170; Bo/CV531-OH/02/US, AY549171; Bo/CV548-OH/02/US, AY549172; and Bo/CV562-OH/02/US, AY549173.
RESULTS
RT-PCR and cloning of the capsid gene.
The capsid genes of 21 BECV strains selected from samples identified in a previous report (35) were amplified from fecal samples by one-step RT-PCR (Table 1). The capsid genes of 15 BECV strains, which were RT-PCR positive with primer sets P290/P289, J11U/J11L, and/or CBECU-F/CBECU-R, were amplified from 13 fecal samples by RT-PCR with primer set NAcap-F/NAcap-R. The capsid genes of six NB-like BECVs were amplified with primer set NBcap-F3/NBcap-R. Fecal samples CV511 and CV551 were mixtures of two BoNVs based on the capsid gene sequences (Bo/CV511-OH/02/US and Bo/CV511 M-OH/02/US for CV511 and Bo/CV551-OH/02/US and Bo/CV551 M-OH/02/US for CV551. Although various reaction conditions that included different Mg2+ concentrations and annealing temperatures were applied to one-step RT-PCR, the capsid genes of Jena-like BECVs with primer sets Jenacap-F1/Jenacap-R1 and Jenacap-F2/Jenacap-R2 or combinations of these primer sets were not amplified from any of the fecal samples in which the 21 BECVs were detected.
Sequence identity and phylogenetic analysis of the capsid genes of BoNV and NB-like BECV.
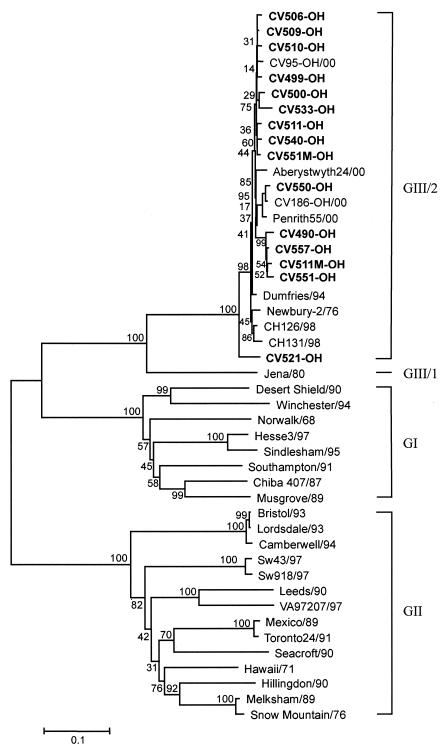
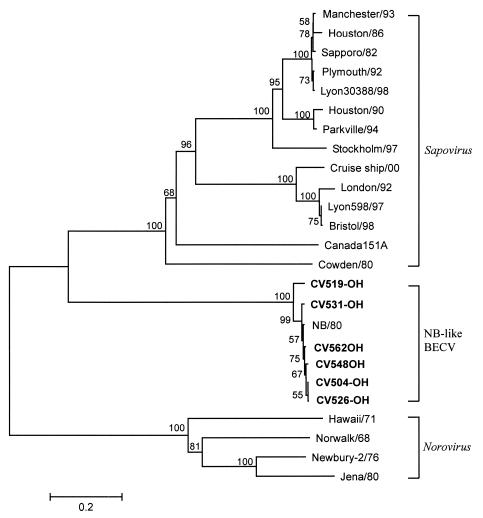
Multiple alignments and phylogenetic analysis of the capsid gene sequences of the BECVs sequenced in this study and of genetically characterized human, bovine, and porcine noroviruses as well as human, porcine, and mink sapoviruses were performed. Of the 21 BECV strains sequenced, 15, including Bo/CV511-OH/02/US, Bo/CV511 M-OH/02/US, Bo/CV551-OH/02/US and Bo/CV551 M-OH/02/US, belonged to Norovirus GIII/2, which includes most known BoNVs, except for Bo/Jena/80/DE (GIII/1) (Fig. 1 and Table 2). Six strains were classified as NB-like BECV (Fig. 2). The capsid genes of all 15 characterized BoNVs were comprised of 1,569 bp and were predicted to encode 522 aa. Each of the six NB-like BECV capsid genes characterized was 1,686 bp in size, which corresponds to the size determined for the BECV NB strain (34).
FIG. 1.
Phylogenetic tree, constructed by the neighbor-joining method, based on the amino acid sequence of the entire capsid gene of BoNV. The bootstrap value (percent) is given at each node. Viruses for which capsid gene sequences were used for phylogenetic analysis (GenBank accession numbers) included (i) GI human noroviruses Hu/NLV/Chiba407/87/JP (AB042808), Hu/NLV/Desert Shield395/90/SA (U04469), Hu/NLV/Hesse3/97/DE (AF093797), Hu/NLV/Musgrove/89/UK (AJ277614), Hu/NLV/Norwalk/68/US (M87661), Hu/NLV/Sindlesham/95/UK (AJ277615), Hu/NLV/Southampton/91/UK (L07418), and Hu/NLV/Winchester/94/UK (AJ277609); (ii) GII human noroviruses Hu/NLV/Bristol/93/UK (X76716), Hu/NLV/Camberwell1101922/94/AUS (AF145896), Hu/NLV/Hawaii/71/US (U07611), Hu/NLV/Hillingdon/90 (AJ277607), Hu/NLV/Leeds/90/UK (AJ277608), Hu/NLV/Lordsdale/93/UK (X86557), Hu/NLV/Melksham/89/UK (X81879), Hu/NLV/Mexico/89/MX (U22498), Hu/NLV/Seacroft/90/UK (AJ277620), Hu/NLV/Toronto24/91/CAN (U02030), Hu/NLV/Snow Mountain/76/US (U70059), and Hu/NLV/VA97207/97/US (AY038599); (iii) GII porcine noroviruses Sw/NLV/Sw43/97/JP (AB074892) and Sw/NLV/Sw918/97/JP (AB074893); and (iv) GIII bovine noroviruses: Bo/Aberystwyth24/00/UK (AY126475), Bo/CH126/98/NET (AF320625), Bo/CH131/98/NET (AF320113), Bo/CV95-OH/00/US (AF542083), Bo/CV186-OH/00/US (AF542084), Bo/Dumfries/94/UK (AY126474), Bo/Jena/80/DE (AJ011099), Bo/Newbury-2/76/UK (AF097917), and Bo/Penrith55/00/UK (AY126476).
TABLE 2.
Capsid gene nucleotide and amino acid identities between BoNV and representative human noroviruses
| Virus no., namea | % Capsid gene nucleotide and amino acid identityb with virus no.:
|
||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | |
| 1, CV490-OH | 96 | 95 | 96 | 96 | 96 | 96 | 98 | 92 | 94 | 95 | 95 | 97 | 96 | 98 | 95 | 95 | 94 | 96 | 95 | 95 | 96 | 95 | 69 | 47 | 48 | 47 | 44 | 44 | |
| 2, CV499-OH | 90 | 98 | 99 | 99 | 99 | 98 | 96 | 93 | 97 | 98 | 96 | 95 | 99 | 96 | 97 | 97 | 96 | 98 | 97 | 97 | 97 | 96 | 68 | 47 | 48 | 47 | 44 | 44 | |
| 3, CV500-OH | 89 | 96 | 98 | 98 | 98 | 98 | 95 | 93 | 97 | 98 | 96 | 95 | 98 | 96 | 96 | 97 | 96 | 98 | 97 | 97 | 97 | 96 | 68 | 47 | 48 | 47 | 44 | 44 | |
| 4, CV506-OH | 90 | 97 | 96 | 99 | 99 | 99 | 96 | 93 | 97 | 98 | 96 | 96 | 99 | 96 | 96 | 97 | 96 | 99 | 97 | 97 | 97 | 96 | 69 | 48 | 48 | 47 | 45 | 45 | |
| 5, CV509-OH | 90 | 97 | 96 | 98 | 99 | 99 | 96 | 93 | 97 | 99 | 97 | 96 | 99 | 97 | 97 | 97 | 97 | 99 | 98 | 98 | 98 | 97 | 68 | 48 | 49 | 47 | 45 | 45 | |
| 6, CV510-OH | 89 | 97 | 96 | 98 | 97 | 99 | 96 | 93 | 97 | 98 | 97 | 96 | 99 | 96 | 96 | 97 | 97 | 99 | 97 | 97 | 97 | 97 | 68 | 47 | 48 | 47 | 45 | 45 | |
| 7, CV511-OH | 90 | 95 | 93 | 95 | 95 | 95 | 96 | 94 | 97 | 99 | 96 | 95 | 99 | 97 | 97 | 98 | 97 | 98 | 97 | 98 | 97 | 97 | 69 | 48 | 49 | 48 | 45 | 45 | |
| 8, CV511M-OH | 96 | 90 | 89 | 89 | 90 | 89 | 90 | 92 | 94 | 95 | 96 | 98 | 96 | 99 | 95 | 95 | 94 | 96 | 96 | 95 | 96 | 95 | 69 | 48 | 48 | 47 | 43 | 44 | |
| 9, CV521-OH | 83 | 83 | 83 | 83 | 83 | 83 | 83 | 84 | 91 | 93 | 92 | 92 | 93 | 93 | 92 | 94 | 94 | 93 | 93 | 94 | 93 | 94 | 69 | 48 | 48 | 47 | 44 | 45 | |
| 10, CV533-OH | 89 | 97 | 96 | 97 | 97 | 98 | 94 | 89 | 82 | 96 | 95 | 94 | 97 | 95 | 95 | 95 | 94 | 97 | 96 | 95 | 95 | 95 | 67 | 48 | 48 | 47 | 45 | 44 | |
| 11, CV540-OH | 89 | 94 | 93 | 95 | 95 | 94 | 97 | 89 | 83 | 94 | 96 | 95 | 99 | 96 | 96 | 97 | 96 | 98 | 97 | 97 | 97 | 96 | 68 | 48 | 48 | 47 | 45 | 45 | |
| 12, CV550-OH | 90 | 92 | 91 | 92 | 92 | 92 | 92 | 90 | 84 | 92 | 92 | 96 | 96 | 96 | 96 | 96 | 95 | 96 | 99 | 95 | 98 | 96 | 69 | 47 | 48 | 47 | 45 | 45 | |
| 13, CV551-OH | 97 | 90 | 89 | 89 | 90 | 89 | 90 | 96 | 83 | 89 | 90 | 90 | 96 | 98 | 94 | 95 | 94 | 95 | 95 | 95 | 96 | 94 | 69 | 48 | 48 | 47 | 46 | 44 | |
| 14, CV551M-OH | 90 | 94 | 93 | 95 | 95 | 94 | 97 | 90 | 83 | 94 | 97 | 92 | 90 | 96 | 96 | 97 | 96 | 99 | 97 | 97 | 97 | 96 | 68 | 48 | 48 | 47 | 45 | 45 | |
| 15, CV557-OH | 97 | 90 | 90 | 90 | 90 | 90 | 90 | 97 | 83 | 89 | 90 | 91 | 97 | 90 | 95 | 96 | 95 | 96 | 96 | 95 | 96 | 95 | 69 | 48 | 48 | 48 | 44 | 44 | |
| 16, Aberystwyth24 | 86 | 87 | 87 | 87 | 88 | 87 | 87 | 86 | 84 | 86 | 87 | 87 | 85 | 87 | 86 | 96 | 96 | 96 | 96 | 96 | 96 | 96 | 68 | 47 | 48 | 47 | 45 | 45 | |
| 17, CH126 | 87 | 88 | 88 | 88 | 89 | 88 | 88 | 87 | 84 | 87 | 87 | 88 | 87 | 88 | 87 | 88 | 98 | 97 | 96 | 98 | 96 | 98 | 69 | 47 | 49 | 48 | 45 | 45 | |
| 18, CH131 | 87 | 89 | 88 | 89 | 89 | 88 | 89 | 88 | 84 | 88 | 88 | 87 | 87 | 88 | 88 | 88 | 95 | 97 | 96 | 97 | 95 | 97 | 69 | 48 | 49 | 48 | 45 | 45 | |
| 19, CV95-OH | 90 | 95 | 94 | 96 | 96 | 95 | 96 | 90 | 83 | 95 | 96 | 92 | 90 | 96 | 91 | 87 | 87 | 93 | 97 | 97 | 97 | 96 | 69 | 48 | 48 | 47 | 45 | 45 | |
| 20, CV186-OH | 90 | 92 | 92 | 92 | 92 | 92 | 92 | 91 | 84 | 92 | 93 | 96 | 90 | 93 | 91 | 88 | 87 | 87 | 93 | 96 | 98 | 96 | 69 | 48 | 48 | 47 | 46 | 45 | |
| 21, Dumfries | 87 | 89 | 87 | 88 | 88 | 88 | 88 | 88 | 84 | 87 | 88 | 87 | 86 | 88 | 87 | 88 | 88 | 89 | 88 | 87 | 96 | 98 | 69 | 48 | 49 | 47 | 45 | 45 | |
| 22, Penrith55 | 90 | 92 | 91 | 92 | 92 | 92 | 92 | 90 | 85 | 91 | 92 | 95 | 90 | 93 | 91 | 88 | 87 | 88 | 93 | 96 | 88 | 96 | 69 | 48 | 48 | 47 | 45 | 45 | |
| 23, Newbury-2 | 87 | 89 | 89 | 89 | 89 | 88 | 89 | 87 | 84 | 88 | 88 | 89 | 87 | 89 | 87 | 88 | 90 | 89 | 89 | 88 | 90 | 90 | 69 | 48 | 49 | 48 | 46 | 45 | |
| 24, Jena | 70 | 70 | 69 | 70 | 70 | 70 | 70 | 70 | 68 | 69 | 69 | 69 | 69 | 69 | 70 | 68 | 70 | 69 | 70 | 70 | 69 | 69 | 69 | 49 | 48 | 48 | 45 | 48 | |
| 25, Norwalk | 57 | 56 | 56 | 56 | 56 | 56 | 56 | 57 | 56 | 56 | 57 | 56 | 57 | 56 | 56 | 55 | 55 | 56 | 58 | 56 | 56 | 56 | 56 | 54 | 69 | 69 | 47 | 43 | |
| 26, Desert Shield | 54 | 56 | 55 | 55 | 55 | 55 | 55 | 54 | 55 | 55 | 55 | 55 | 54 | 54 | 55 | 55 | 54 | 55 | 55 | 55 | 56 | 54 | 55 | 30 | 68 | 67 | 46 | 42 | |
| 27, Sindlesham | 29 | 54 | 54 | 54 | 54 | 54 | 30 | 29 | 55 | 53 | 54 | 30 | 30 | 55 | 29 | 54 | 29 | 30 | 54 | 54 | 30 | 54 | 30 | 30 | 70 | 66 | 47 | 42 | |
| 28, Hawaii | 54 | 54 | 53 | 30 | 54 | 28 | 54 | 54 | 52 | 29 | 30 | 53 | 55 | 28 | 54 | 53 | 29 | 30 | 29 | 53 | 55 | 53 | 54 | 29 | 32 | 58 | 33 | 65 | |
| 29, Lordsdale | 30 | 28 | 23 | 23 | 23 | 28 | 23 | 30 | 24 | 23 | 23 | 25 | 30 | 25 | 30 | 27 | 28 | 24 | 23 | 25 | 31 | 25 | 30 | 26 | 32 | 33 | 32 | 67 | |
Genogroups/genotypes are as follow: no. 1 to 23, GIII/2; no. 24, GIII/1; no. 25, GI/1; no. 26, GI/3; no. 27, GI/6; no. 28, GII/1; no. 29, GII/4.
Percent amino acid and nucleotide identities are in the upper and lower triangles, respectively.
FIG. 2.
Phylogenetic tree, constructed by the neighbor-joining method, based on the amino acid sequence of the entire capsid gene of NB-like BECV. The bootstrap value (percent) is given at each node. Viruses for which capsid gene sequences were used for phylogenetic analysis (GenBank accession numbers) included (i) GI human sapoviruses Hu/SLV/Houston/86/US (U95643), Hu/SLV/Houston/27/90/US (U95644), Hu/SLV/Manchester/93/UK (X86560), Lyon30388/98 (AJ251991), Hu/SLV/Parkville/94/US (U73124), Hu/SLV/Plymouth/92/UK (X86559), Hu/SLV/Sapporo/82/JP (U65427), and Hu/SLV/Stockholm/318/97/SE (AF194182); (ii) GII human sapoviruses Hu/SLV/Bristol/98/UK (AJ249939), Hu/SLV/cruise ship/00/US (AY289804), Hu/London/29845/92/UK (U95645), and Hu/SLV/Lyon/598/97/F (AJ271056); (iii) porcine sapovirus Sw/Cowden//UA (AF182760); (iv) mink sapovirus Canada 151A (AY144337); and (v) unclassified NB-like BECV Bo/NB/80/US (NC_004064).
The nucleotide and amino acid identities of the complete capsid genes of the 15 BoNVs are summarized in Table 2. Nucleotide and amino acid sequence identities among the 15 BoNVs ranged from 82 to 98% and 91 to 99%, respectively. Bo/CV521-OH/02/US had the lowest amino acid identity (92 to 93%) with previously characterized BoNVs. The 15 BoNVs had 67 to 99% amino acid identity (68 to 96% nucleotide identity) to other known BoNVs, including Bo/Jena/80/DE. The BoNVs were more closely related to Bo/Newbury-2/76/UK than to Bo/Jena/80/DE, sharing 94 to 97% amino acid identity (84 to 89% nucleotide identity) with Bo/Newbury-2/76/UK and 67 to 69% amino acid identity (68 to 70% nucleotide identity) to Bo/Jena/80/DE. The 15 BoNVs had similar but lower amino acid identities to GI and GII human noroviruses, which ranged from 45 to 50% amino acid identity (29 to 57% nucleotide identity) to GI human noroviruses and from 43 to 46% amino acid identity (21 to 54% nucleotide identity) to GII human noroviruses. The 15 characterized BoNVs shared 44 to 46% amino acid identity (27 to 56% nucleotide identity) to GII porcine noroviruses. The amino acid identity of the 15 BoNVs to other GIII/2 BoNVs ranged from 92 to 99%, which was similar to the range calculated between the different BoNVs sequenced in this study.
The genetic distances based on the amino acid sequences among the 15 BoNVs were 0.008 to 0.082 (0.032 ± 0.019). The 15 BoNVs had genetic distances of 0.015 to 0.073 (0.035 ± 0.012) from GIII/2 bovine noroviruses and of 0.344 to 0.367 (0.355 ± 0.006) from the GIII/1 bovine norovirus, Bo/Jena/80/DE, compared with 0.659 ± 0.014 and 0.761 ± 0.023 from GI and GII noroviruses, respectively. The genetic distances between Norovirus GI and GII, GI and GIII, and GII and GIII were 0.733 ± 0.025, 0.655 ± 0.013, and 0.755 ± 0.022, respectively. Based on sequence identities and genetic distances, BoNVs were more closely related to GI noroviruses than to GII noroviruses.
The motifs IDPWI, LAGNA, and LYTP, located in the S domain, and QNGR, located in P1A subdomain, were conserved in all noroviruses, including the 15 BoNVs. The motif LAGNA, located at the three- and fivefold axes of symmetry of Hu/NLV/Norwalk/68/US, has been suggested to be of structural importance (29). The stop codon of the 15 BoNVs was TGA, like that of other BoNVs and human GI and GII noroviruses.
Phylogenetic analysis of NB-like BECV.
The amino acid sequences of six NB-like BECVs shared 94 to 99.5% identity (87 to 99.8% nucleotide identity) with each other and 93 to 99% identity (88 to 92% nucleotide identity) with the NB strain. The amino acid identity of the six NB-like BECVs to BoNVs, human noroviruses, and sapoviruses, including porcine and mink sapoviruses, was less than 23%. The genetic distances of NB-like BECVs based on the amino acid sequence were 0.002 to 0.058 (0.027 ± 0.024) from each other and 0.08 to 0.013 (0.01 ± 0.002) from the NB strain. However, the genetic distances of NB-like BECVs were higher from human noroviruses and human, mink, and porcine sapoviruses (1.343 to 1.765). Bo/CV519-OH/02/US showed the lowest amino acid (93%) and nucleotide (87%) identities and also the highest genetic distance (0.56 to 0.06) within the six NB-like BECVs.
Comparative phylogenetic analysis of the whole capsid protein and the S and P domains of BoNV.
The nucleotide and amino acid identities of the capsid genes of the 15 BoNVs, other characterized BoNVs, and Hu/NLV/Norwalk/68/US are summarized in Table 3. Analyses were conducted on the S (176 aa), P1A (53 aa), P2 (127 aa), and P1B (117 aa) domains, corresponding to those described for Hu/NLV/Norwalk/68/US (32). The S domain was the most conserved region between noroviruses. The 15 BoNVs had the lowest amino acid identity between both genogroups and genotypes in the P2 domain, which includes the hypervariable region of the capsid gene. The amino acid identity of the S domain of 15 BoNVs was highest with Bo/Newbury-2/76/UK. The amino acid identity for the S domain with Bo/Jena/80/DE (Table 3) was higher at 85 to 90% (76 to 78% nucleotide identity), compared to 67 to 69% (68 to 70% nucleotide) identity for the whole capsid gene (Table 3).
TABLE 3.
Comparison of amino acid and nucleotide sequence identities of the S and P domains of the capsid region in bovine and human noroviruses
| Norovirus (genogroup/genotype)a | % Amino acid (nucleotide) sequence identitya
|
||||
|---|---|---|---|---|---|
| Capsid gene | S domaind (aa 50-225) | P domaind
|
|||
| P1A (aa 226-278) | P2 (aa 279-405) | P1B (aa 406-522) | |||
| BoNV (GIII/2)b | 91-99 (82-98) | 97-100 (82-99) | 83-100 (76-99) | 87-100 (79-98) | 88-99 (82-98) |
| BoNV (GIII/2)c | 92-99 (83-96) | 97-100 (84-96) | 87-100 (78-98) | 87-95 (81-96) | 90-100 (83-96) |
| Bo/Newbury-2/76/UK (GIII/2) | 94-97 (84-89) | 96-100 (85-92) | 87-96 (81-87) | 87-93 (82-95) | 92-98 (83-91) |
| Bo/Jena/80/DE (GIII/1) | 67-69 (68-70) | 85-90 (76-78) | 55-59 (50-59) | 41-43 (58-61) | 66-70 (67-70) |
| Hu/Norwalk/68/US (GI/1) | 47-48 (56-57) | 67-69 (65-67) | 47-51 (15-56) | 17-19 (5-8) | 44-48 (58-61) |
The amino acid and nucleotide sequences of each norovirus were compared with those of 15 BoNVs sequenced in this study.
The amino acid and nucleotide sequences were compared among the 15 BoNVs.
Bo/Aberystwyth24/00/UK (AY126475), Bo/CH126/98/NET (AF320625), Bo/CH131/98/NET (AF320113), Bo/CV95-OH/00/US (AF542083), Bo/CV186-OH/00/US (AF542084), Bo/Dumfries/94/UK (AY126474), and Bo/Penrith55/00/UK (AY126476) were included.
The S domain is formed by the N-terminal 225 aa, and the P domain consists of aa 226 to the C- terminal end (32).
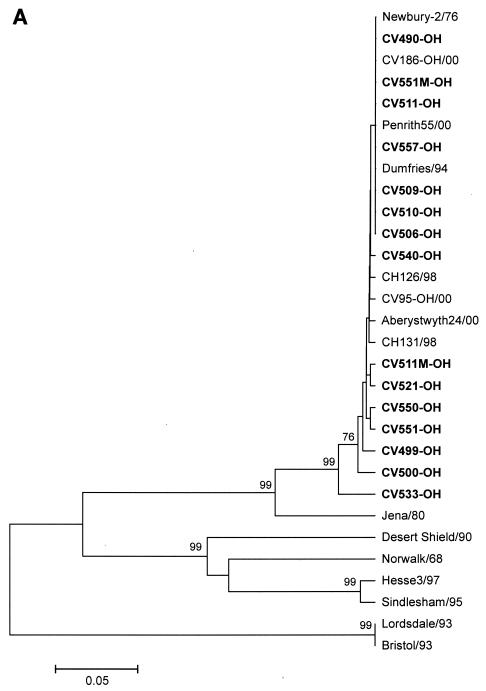
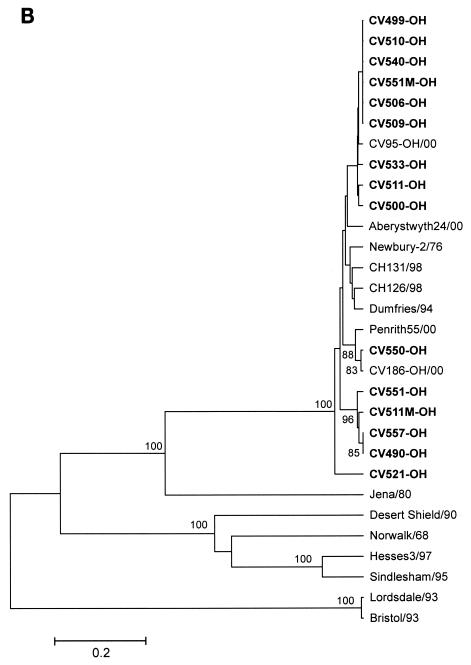
Phylogenetic trees of the complete capsid and isolated S and P domains classified the 15 BoNVs into Norovirus GIII/2 although the amino acid sequences of the S domains of the 15 BoNVs had higher sequence identity to Bo/Jena/80/DE than did those of the P2 domains (Fig. 3).
FIG. 3.
Comparative phylogenetic trees, constructed by the unweighted-pair-group-method-with-arithmetic method, based on amino acid sequence of bovine noroviruses and representative human noroviruses. (A) S domain; (B) P2 domain. Bootstrap values of greater than 70% are given at each node.
Sequence and gene conversion analyses of RdRp and capsid genes of Bo/CV521-OH/02/US.
Based on the partial RdRp gene sequence, Bo/CV521-OH/02/US was most closely related to Bo/Jena/80/DE, indicative of Norovirus GIII/1 (35). However, based on the capsid gene sequence determined in this study, Bo/CV521-OH/02/US belonged to Norovirus GIII/2 (Fig. 1). An RT-PCR product of 1,898 bp was amplified with primer set RC35/RC1932, which was designed to amplify both the partial C terminus of the RdRp gene (464 bp) and the capsid gene (1,448 bp). The nucleotide sequence of the overlapping RdRp segment of the RC35/RC1932 RT-PCR product was the same as that the RdRp gene sequence previously reported for Bo/CV521-0H/02/US (accession number AY151257). The RdRp gene (ORF1) of Bo/CV521-OH/02/US overlaps ORF2 by 14 nucleotides, similar to the case for other characterized BoNVs. The amino acid (nucleotide) identities of the RdRp of Bo/CV521-OH/02/US were 95% (83%) and 88% (77%) to Bo/Jena/80/DE and Bo/Newbury-2/76/UK, respectively. Bo/CV521-OH/02/US shared 69% amino acid (68% nucleotide) and 94% amino acid (84% nucleotide) identities of the entire capsid gene to Bo/Jena/80/DE and Bo/Newbury-2/76/UK, respectively.
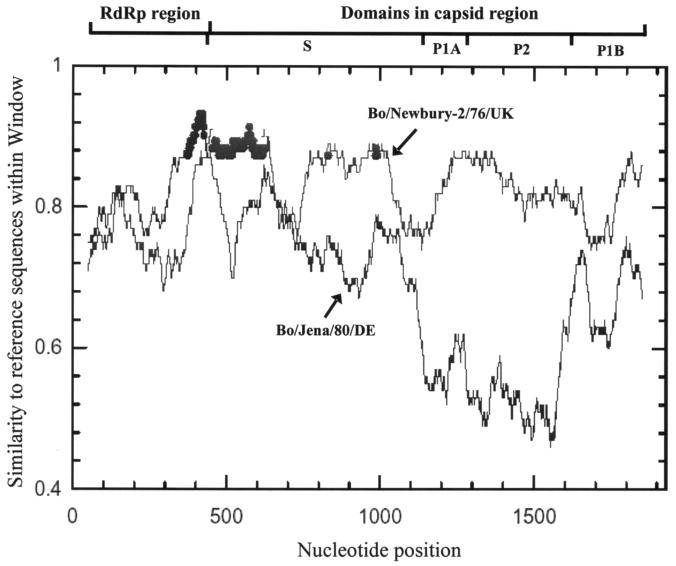
From the results of RIP analysis, Bo/CV521-OH/02/US showed higher similarity with Bo/Jena/80/DE in the RdRp region and, in contrast to RdRp, with Bo/Newbury-2/76/UK in the capsid region (Fig. 4). The results of SimPlot were equivalent to those of RIP; graphics from the RIP analysis are presented in this report. Collectively, these results suggested that Bo/CV521-OH/02/US was a recombinant generated by genetic recombination between Jena-like and Newbury-2-like BECVs.
FIG. 4.
Gene conversion analysis of Bo/CV521-OH/02/US compared with Bo/Jena/80/DE (black) and Bo/Newbury-2/76/UK (gray), using RIP (http://hivweb.lanl.gov/RIP/RIPsubmit.html). Program options were a window size of 100 and a threshold for statistical significance of 90%. The RdRp and capsid regions are indicated.
DISCUSSION
Fecal samples used in this study were selected on the basis of previously published RT-PCR results (35) and were categorized as (i) positive with primer sets P290/P289 and CBECU-F/CBECU-R, (ii) positive with primer NBU-F/NBU-R, (iii) positive with CBECU-F/CBECU-R and negative with P290/P289 and NBU-F/NBU-R, (iv) positive with CBECU-F/CBECU-R and NBU-F/NBU-R, and (v) positive with all primer sets. The capsid genes of BoNVs were amplified and cloned from positive samples in categories i, iii, and v. The capsid genes of NB-like BECVs were amplified from positive fecal samples categorized in groups ii and iv. The RdRp genes of both BoNVs and NB-like BECVs were amplified from samples CV510, CV526, CV531, CV551, and CV557, using the primers CBECU-F/CBECU-R and NBU-F/NBU-R, respectively, which is indicative of coinfections and shedding of genetically different BECV by the same calf. These findings suggest the need to clone BECVs prior to their use in sequencing or pathogenesis studies. However, at this time the biological cloning of BECVs is not feasible due to the lack of a cell culture system. Therefore, fecal samples intended for use as inocula in animal studies should be tested for the presence of genetically different BECVs.
Based on comparison of the amino acid and nucleotide sequences of the complete capsid gene, the 21 BECV strains detected in fecal samples from two veal calf farms in Ohio were classified as Norovirus GIII/2 (Newbury-2-like, 15 strains) and NB-like BECV (6 strains). These viruses were phylogenetically distinct from human and porcine noroviruses and from human, porcine, and mink sapoviruses. Analyses of the complete capsid genes of the 15 BoNVs showed they shared lower, in comparison to GIII bovine noroviruses, amino acid and nucleotide identities with GI and GII human noroviruses, being more closely related to GI human noroviruses, which is consistent with a previous report (23). This finding also suggests that most bovine and human noroviruses may be genetically distinct. However, as a caveat to this finding, there are few sequence analyses of BoNVs and counterpart human noroviruses from developing countries, where closer contact between cattle and humans is expected. Bovine rotaviruses were thought to be distinctive from human rotaviruses until strains from cattle and children in India were compared and found to share common serotypes (16).
We analyzed the capsid genes of BECVs detected from two veal calf farms in 2002. The calves introduced into each farm were obtained from regional sale barns auctioning calves from four or five states, which suggests that these BECV strains have different geographic origins. Thus, the capsid gene diversity of BECVs detected on these farms is most likely a reflection of this diverse geographic sourcing. It was unclear whether calves were infected with genetically different BECVs before or after they were introduced to the veal farms. Two genetically different BoNV strains, such as (i) Bo/CV511-OH/02/US and Bo/CV511 M-OH/02/US and (ii) Bo/CV551-OH/02/US and Bo/CV551 M-OH/02/US were detected from the same sample, suggesting that calves were coinfected with the same genotype but with genetically different BECVs. Similarly, the RT-PCR results also showed some calves to be coinfected with different genogroups, GIII BoNV and the NB-like BECV. These coinfections with genetically different BECVs in a calf may result in natural genetic recombination between BoNVs, as suggested by our findings (Bo/CV521-OH/02/US), and/or possibly with NB-like BECVs. Genetic recombination may be a common mechanism for the generation of a diversity of genotypes or genogroups, although there is no evidence of recombination between the latter. Several natural recombinants between distinct human norovirus genotypes have been reported, as discussed below (17, 24, 37).
Phylogenetic trees generated from the complete capsid gene sequences of seven of the 15 BoNVs were compared to ones based on the RdRp gene sequences (Smiley and Saif, unpublished data). Except for Bo/CV521-OH/02/US, six BoNVs were classified as Norovirus GIII/2 irrespective of their RdRp or capsid gene sequences. Bo/CV521-OH/02/US was classified as Norovirus GIII/2 based on the whole capsid gene, but Smiley et al. (35) reported Bo/CV521-OH/02/US as Norovirus GIII/I, which was a Jena-like BoNV based on the RdRp gene sequence. This divergent result may be from coinfection by genetically different BECVs in a calf or from genetic recombination of BECVs. To exclude the possibility of amplification of different BECV due to contamination of genetically different BECVs in the same fecal sample, a contiguous portion of the RdRp and capsid genes of Bo/CV521-OH/02/US was amplified with primer set RC35/RC1932. Following analyses of the gene sequence and gene conversion, Bo/CV521-OH/02/US was found to likely be a recombinant, with Norovirus GIII/1-like origin for the RdRp gene and Norovirus GIII/2-like origin for the capsid gene. Natural genetic recombination between various genotypes or clusters of GI and GII human noroviruses has been detected for the WUG1 strain (19), the Arg320 strain (17), Hu/Snow Mountain/76/US (24), and Hu/Wortley/90/UK (37). However, to our knowledge, naturally occurring recombinants for BoNVs have not been previously reported or documented.
The putative region of recombination in human norovirus recombinants has been suggested to be the ORF1-ORF2 junction region (17, 19). For Bo/CV521-OH/02/US, we also suspect that recombination occurred in the ORF1-ORF2 junction (Fig. 4). This finding suggests that such recombination makes it difficult to classify noroviruses based on the C-terminal region of the RdRp or on complete and partial capsid gene sequences, but our subsequent analysis (Table 3) validated this approach. The capsid genes of noroviruses are composed of two domains, S and P (32). The S domain, which is located in the N terminus of the capsid gene, is the most conserved region. The P2 subdomain (aa 279 to 405 of the capsid protein) includes the hypervariable region and is the least conserved region. These patterns of sequence conservation were also consistent for the 15 BoNVs. The S domains of 15 BoNVs shared the highest amino acid identity with Hu/NLV/Norwalk/68/US, in contrast to the P1A, P2, and P1B subdomains (Table 3). The P1B subdomain of GIII/2 BoNVs was more conserved within a genotype than the P1A subdomain. Comparison of phylogenetic trees determined for the complete capsid gene or each domain of BoNVs and human noroviruses showed no significant differences in their placement, since only bootstrap values of greater than 70% were considered significant (Fig. 3). These results suggest that both the C-terminal region of the RdRp gene (ORF1) and the S domain of the capsid gene (ORF2) junction are reliable regions for genotyping noroviruses and discovering a recombinant.
The conserved motifs GXXPGIGKT in the 2C nucleoside triphosphatase, GDGC in the 3C protease, and GLPSG and YGDD in the RdRp regions of caliciviruses were known (4, 26). We identified the one previously described motif, LAGNA (S domain) (33), and the three new motifs, IDPWI (S domain), LYTP (S domain), and QNGR (P1A domain), in the capsid genes of noroviruses, including the 15 GIII BoNVs sequenced in this study. The motifs LAGNA and LYTP of all noroviruses were included in the globular domain as predicted with SMART/Pfam domain and defined by Linding et al. in GlobPlot version 2.1 (http://globplot.embl.de/) (22). However, the motifs IDPWI and QNGR of GII noroviruses were a potential globular domain, but these motifs of GIII BoNVs were not included in the globular domain. The IDPWI and QNGR motifs of some GI noroviruses, such as Hu/NLV/Norwalk/68/US and Hu/NLV/Desert Shield395/90/SA, were located in a globular domain. The role of each motif needs to be clarified for the noroviruses.
In summary, the results of this study demonstrate genetic diversity in the capsid genes of BECVs circulating in Ohio veal calves, provide new data for coinfections with distinct BECV genotypes or genogroups, and describe what is to our knowledge the first natural BoNV recombinant, analogous to the previously reported human norovirus recombinants.
Acknowledgments
We thank S. Sreevatsan, J. LeJeune, and D. Jackwood for critical review of the manuscript. We also thank the Molecular and Cellular Imaging Center of the Ohio Agricultural Research and Development Center for DNA sequencing.
This work was supported by grant R01 AI49716 from the National Institute of Allergy and Infectious Diseases, National Institutes of Health. M. G. Han was partially supported by the Postdoctoral Fellowship Program of the Korea Science and Engineering Foundation.
REFERENCES
- 1.Ando, T., J. S. Noel, and R. L. Fankhauser. 2000. Genetic classification of “Norwalk-like viruses.” J. Infect. Dis. 181(Suppl. 2):S336-S348. [DOI] [PubMed] [Google Scholar]
- 2.Berke, T., B. Golding, X. Jiang, D. W. Cubitt, M. Wolfaardt, A. W. Smith, and D. O. Matson. 1997. Phylogenetic analysis of the caliciviruses. J. Med. Virol. 52:419-424. [DOI] [PubMed] [Google Scholar]
- 3.Bridger, J. C., G. A. Hall, and J. F. Brown. 1984. Characterization of a calici-like virus (Newbury agent) found in association with astrovirus in bovine diarrhea. Infect. Immun. 43:133-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clarke, I. N., and P. R. Lambden. 1997. The molecular biology of caliciviruses. J. Gen. Virol. 78:291-301. [DOI] [PubMed] [Google Scholar]
- 5.Dastjerdi, A. M., J. Green, C. I. Gallimore, D. W. Brown, and J. C. Bridger. 1999. The bovine Newbury agent-2 is genetically more closely related to human SRSVs than to animal caliciviruses. Virology 254:1-5. [DOI] [PubMed] [Google Scholar]
- 6.Estes, M. K., R. Atmar, and M. Hardy. 1997. Norwalk and related diarrhea viruses, p. 1073-1095. In D. Richman, D. Whitley, and F. Hayden (ed.), Clinical virology, vol. 1. Churchill Livingstone, New York, N.Y. [Google Scholar]
- 7.Fankhauser, R. L., J. S. Noel, S. S. Monroe, T. Ando, and R. I. Glass. 1998. Molecular epidemiology of “Norwalk-like viruses” in outbreaks of gastroenteritis in the United States. J. Infect. Dis. 178:1571-1578. [DOI] [PubMed] [Google Scholar]
- 8.Flynn, W. T., and L. J. Saif. 1988. Serial propagation of porcine enteric calicivirus-like virus in primary porcine kidney cell cultures. J. Clin. Microbiol. 26:206-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flynn, W. T., L. J. Saif, and P. D. Moorhead. 1988. Pathogenesis of porcine enteric calicivirus-like virus in four-day-old gnotobiotic pigs. Am. J. Vet. Res. 49:819-825. [PubMed] [Google Scholar]
- 10.Green, J., J. Vinje, C. I. Gallimore, M. Koopmans, A. Hale, D. W. Brown, J. C. Clegg, and J. Chamberlain. 2000. Capsid protein diversity among Norwalk-like viruses. Virus Genes 20:227-236. [DOI] [PubMed] [Google Scholar]
- 11.Green, K. Y. 1997. The role of human caliciviruses in epidemic gastroenteritis. Arch. Virol. Suppl. 13:153-165. [DOI] [PubMed] [Google Scholar]
- 12.Green, K. Y., R. M. Chanock, and A. Z. Kapikian. 2001. Human caliciviruses, p. 841-874. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 13.Green, S. M., K. E. Dingle, P. R. Lambden, E. O. Caul, C. R. Ashley, and I. N. Clarke. 1994. Human enteric Caliciviridae: a new prevalent small round-structured virus group defined by RNA-dependent RNA polymerase and capsid diversity. J. Gen. Virol. 75:1883-1888. [DOI] [PubMed] [Google Scholar]
- 14.Hale, A. D., S. E. Crawford, M. Ciarlet, J. Green, C. Gallimore, D. W. Brown, X. Jiang, and M. K. Estes. 1999. Expression and self-assembly of Grimsby virus: antigenic distinction from Norwalk and Mexico viruses. Clin. Diagn. Lab. Immunol. 6:142-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall, G. A., J. C. Bridger, B. E. Brooker, K. R. Parsons, and E. Ormerod. 1984. Lesions of gnotobiotic calves experimentally infected with a calicivirus-like (Newbury) agent. Vet. Pathol. 21:208-215. [DOI] [PubMed] [Google Scholar]
- 16.Jagannath, M. R., R. R. Vethanayagam, B. S. Reddy, S. Raman, and C. D. Rao. 2000. Characterization of human symptomatic rotavirus isolates MP409 and MP480 having ′long' RNA electropherotype and subgroup I specificity, highly related to the P6[1],G8 type bovine rotavirus A5, from Mysore, India. Arch. Virol. 145:1339-1357. [DOI] [PubMed] [Google Scholar]
- 17.Jiang, X., C. Espul, W. M. Zhong, H. Cuello, and D. O. Matson. 1999. Characterization of a novel human calicivirus that may be a naturally occurring recombinant. Arch. Virol. 144:2377-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang, X., D. O. Matson, G. M. Ruiz-Palacios, J. Hu, J. Treanor, and L. K. Pickering. 1995. Expression, self-assembly, and antigenicity of a snow mountain agent-like calicivirus capsid protein. J. Clin. Microbiol. 33:1452-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katayama, K., H. Shirato-Horikoshi, S. Kojima, T. Kageyama, T. Oka, F. Hoshino, S. Fukushi, M. Shinohara, K. Uchida, Y. Suzuki, T. Gojobori, and N. Takeda. 2002. Phylogenetic analysis of the complete genome of 18 Norwalk-like viruses. Virology 299:225-239. [DOI] [PubMed] [Google Scholar]
- 20.Kimura, M. 1980. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 16:111-120. [DOI] [PubMed] [Google Scholar]
- 21.Kumar, S., K. Tamura, I. B. Jakobsen, and M. Nei. 2001. MEGA2: molecular evolutionary genetics analysis software. Bioinformatics 17:1244-1245. [DOI] [PubMed] [Google Scholar]
- 22.Linding, R., R. B. Russell, V. Neduva, and T. J. Gibson. 2003. GlobPlot: exploring protein sequences for globularity and disorder. Nucleic Acids Res. 31:3701-3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu, B. L., P. R. Lambden, H. Gunther, P. Otto, M. Elschner, and I. N. Clarke. 1999. Molecular characterization of a bovine enteric calicivirus: relationship to the Norwalk-like viruses. J. Virol. 73:819-825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lochridge, V. P., and M. E. Hardy. 2003. Snow Mountain virus genome sequence and virus-like particle assembly. Virus Genes 26:71-82. [DOI] [PubMed] [Google Scholar]
- 25.Lole, K. S., R. C. Bollinger, R. S. Paranjape, D. Gadkari, S. S. Kulkarni, N. G. Novak, R. Ingersoll, H. W. Sheppard, and S. C. Ray. 1999. Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J. Virol. 73:152-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matson, D. O., T. Berke, M. B. Dinulos, E. Poet, W. M. Zhong, X. M. Dai, X. Jiang, B. Golding, and A. W. Smith. 1996. Partial characterization of the genome of nine animal caliciviruses. Arch. Virol. 141:2443-2456. [DOI] [PubMed] [Google Scholar]
- 27.Mayo, M. A. 2002. A summary of taxonomic changes recently approved by ICTV. Arch. Virol. 147:1655-1656. [DOI] [PubMed] [Google Scholar]
- 28.Numata, K., M. E. Hardy, S. Nakata, S. Chiba, and M. K. Estes. 1997. Molecular characterization of morphologically typical human calicivirus Sapporo. Arch. Virol. 142:1537-1552. [DOI] [PubMed] [Google Scholar]
- 29.Oliver, S. L., A. M. Dastjerdi, S. Wong, L. El-Attar, C. Gallimore, D. W. Brown, J. Green, and J. C. Bridger. 2003. Molecular characterization of bovine enteric caliciviruses: a distinct third genogroup of noroviruses (Norwalk-like viruses) unlikely to be of risk to humans. J. Virol. 77:2789-2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parwani, A. V., W. T. Flynn, K. L. Gadfield, and L. J. Saif. 1991. Serial propagation of porcine enteric calicivirus in a continuous cell line. Effect of medium supplementation with intestinal contents or enzymes. Arch. Virol. 120:115-122. [DOI] [PubMed] [Google Scholar]
- 31.Pfister, T., and E. Wimmer. 2001. Polypeptide p41 of a Norwalk-like virus is a nucleic acid-independent nucleoside triphosphatase. J. Virol. 75:1611-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prasad, B. V., M. E. Hardy, T. Dokland, J. Bella, M. G. Rossmann, and M. K. Estes. 1999. X-ray crystallographic structure of the Norwalk virus capsid. Science 286:287-290. [DOI] [PubMed] [Google Scholar]
- 33.Siepel, A. C., A. L. Halpern, C. Macken, and B. T. Korber. 1995. A computer program designed to screen rapidly for HIV type 1 intersubtype recombinant sequences. AIDS Res. Hum. Retroviruses 11:1413-1416. [DOI] [PubMed] [Google Scholar]
- 34.Smiley, J. R., K. O. Chang, J. Hayes, J. Vinje, and L. J. Saif. 2002. Characterization of an enteropathogenic bovine calicivirus representing a potentially new calicivirus genus. J. Virol. 76:10089-10098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smiley, J. R., A. E. Hoet, M. Traven, H. Tsunemitsu, and L. J. Saif. 2003. Reverse transcription-PCR assays for detection of bovine enteric caliciviruses (BEC) and analysis of the genetic relationships among BEC and human caliciviruses. J. Clin. Microbiol. 41:3089-3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vinje, J., H. Deijl, R. van der Heide, D. Lewis, K. O. Hedlund, L. Svensson, and M. P. Koopmans. 2000. Molecular detection and epidemiology of Sapporo-like viruses. J. Clin. Microbiol. 38:530-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vinje, J., J. Green, D. C. Lewis, C. I. Gallimore, D. W. Brown, and M. P. Koopmans. 2000. Genetic polymorphism across regions of the three open reading frames of “Norwalk-like viruses.” Arch. Virol. 145:223-241. [DOI] [PubMed] [Google Scholar]
- 38.Woode, G. N., and J. C. Bridger. 1978. Isolation of small viruses resembling astroviruses and caliciviruses from acute enteritis of calves. J. Med. Microbiol. 11:441-452. [DOI] [PubMed] [Google Scholar]