Abstract
In this retrospective study, the usefulness of a PCR performed on serum for primary diagnosis and monitoring of Mediterranean visceral leishmaniasis (MVL) was assessed. In the case of primary diagnosis of MVL, the serum PCR showed a sensitivity of 97% and a specificity of 95%, with positive and negative predictive values of 94 and 97%, respectively.
The biological diagnosis of suspected Mediterranean visceral leishmaniasis (MVL) due to Leishmania infantum should always be confirmed by the detection of the parasite, generally from bone marrow aspirates.
Recently, numerous PCR assays have been published which are routinely used for the diagnosis of Leishmania infection (3, 9, 11). Although the PCR method can be applied to various samples, only a few studies have reported the selection of serum sample for PCR diagnosis of canine leishmaniasis due to Leishmania chagasi (14) or visceral leishmaniasis (VL) due to Leishmania donovani (5). To date, no data are available concerning the value of serum PCR for the diagnosis of human MVL.
Serum samples from 33 patients with diagnosed MVL included 21 immunocompetent (ICT) children and adults and 12 immunocompromised (ICD) patients (10 with human immunodeficiency virus, 1 with bone marrow graft, and 1 with lymphoma). Samples at primary diagnosis and following chemotherapy were routinely stored at −20°C and retrospectively analyzed by PCR. In all cases but one (a subclinical paucisymptomatic MVL), the parasite has been detected by bone marrow microscopic examination and/or culture. In positive cultures (24 of 33), the isolated strains were typed as zymodeme MON-1 (Centre National de Référence des Leishmanioses, WHO collaborating Centre, Montpellier, France). For monitoring studies, a total of 79 samples were analyzed (an average of 2 to 4 samples per patient). Twenty-two presumably negative control sera were obtained from asymptomatic individuals living in areas of nonendemicity with no history of VL (Angers, Maine et Loire, France) or in areas of endemicity (Alpes-Maritimes, France). All these samples were negative by Western blotting (WB) and in particular did not react with typical 14- to 18-kDa antigens (8, 13). Finally, 17 sera from asymptomatic contacts living in areas of endemicity (Principality of Monaco and Alpes-Maritimes) exhibiting typical reactivity to 14- to 18-kDa L. infantum antigens by WB, indicating a previous exposure to L. infantum (8, 13), were also included in the study.
Total DNA was extracted from 0.5- to 2-ml serum samples previously adjusted to 5 ml with distilled water with the QIAGEN Maxi-kit (Courtaboeuf, France) according to the manufacturer's instructions.
PCRs were performed in duplicate as previously reported (7). The DNA target (139 bp) was a generic conserved sequence of the kinetoplast DNA minicircles (7). One parasite contains several thousand copies of the sequence.
No false positive was evidenced among the 22 presumably negative control samples. On the contrary, in 2 out of 17 asymptomatic contacts the DNA target sequence was amplified, confirming previous reports indicating that in such asymptomatic individuals, parasitemia episodes can occur (7). Among the 33 ICD or ICT patients with confirmed MVL, 32 (97%) were found positive by serum PCR at the time of primary diagnosis. The PCR-negative sample originated from the patient with subclinical paucisymptomatic confirmed MVL in which the parasite could be only detectable by PCR on whole blood and bone marrow. In addition, this patient showed a good response to specific therapy.
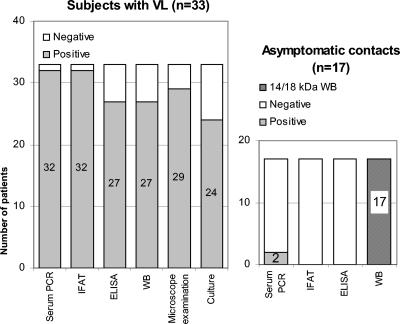
When compared to culture and direct examination, for the diagnosis of MVL serum PCR appeared more sensitive in detecting L. infantum presence than the two other parasite detection techniques taken individually (Fig. 1). In this series, serum PCR positivity was unexpectedly associated with positive serology by immunofluorescence antibody test (IFAT; cutoff value, 1/80). In 16 ICT MVL patients (60 samples; median range of 6 months), controls of serum PCR were negative as early as the 7th day and up to 3 years after primary diagnosis except for one child with a positive control on the 13th month after the clinical cure (without clinical relapse). In the opposite direction, in nine ICD MVL patients (19 samples, median range, 1 month) disappearance of PCR positivity occurred 3 months later than for ICT and one to three PCR-positive episodes were observed in each of four individuals. In two of these, PCR positivity was correlated with clinical relapse.
FIG. 1.
Sensitivity of serological tests and parasitological techniques in patients with confirmed VL and subjects without VL (asymptomatic contacts). Negative controls were all serum PCR negative. Asymptomatic contacts were selected on the basis of their reactivity toward 14- to 18-kDa antigens by WB. In this case, all serum samples tested were negative by IFAT and enzyme-linked immunosorbent assay (ELISA) but two exhibited PCR positivity.
For the MVL diagnosis, these results indicate that serum PCR, taken alone, shows a sensitivity of 97% and a specificity of 95% with positive and negative predictive values of 94 and 97%, respectively. Under our conditions, the technique appeared more sensitive than classical parasite detection techniques performed individually and was positive in all ICD patients (who occasionally exhibited negative serology). Moreover, it did not require invasive procedures and could be performed within 1 day. Therefore, serum PCR could be selected as a first approach to confirm VL diagnosis when whole blood is not available. Indeed, PCR testing of whole blood instead of serum improved PCR sensitivity (5) and infection parasite load is generally much higher in mononuclear cells than in plasma (10). Furthermore, our subclinical paucisymptomatic MVL could only be confirmed by PCR on blood. Thus, whole blood should be preferred whenever possible for PCR testing.
Concerning monitoring, in ICT patients we generally observed as previously reported by Cascio and al. (2) a disappearance of PCR positivity within an average of 1 week. On the contrary, in ICD subjects receiving anti-Leishmania therapy we found, in agreement with Lachaud et al. (6), that Leishmania DNA levels decreased more slowly than for ICT patients. In addition, as reported by Bossolasco et al. (1), Fisa et al. (4), and contrary to Pizzuto et al. (12), positivity of our PCR was not predictive of clinical relapse. Nevertheless, the relapses in our study were always preceded by positive PCR: the technique could be used for the survey of ICD patients with apparently cured MVL. Finally, as already described (7), the parasite was detectable in some asymptomatic contacts living in areas of endemicity and the serum PCR could be applied to retrospectively evaluate, from serum banks, the minimal frequency of asymptomatic carriage in these countries.
Because of its sensitivity and simplicity, serum PCR represents, therefore, a valuable tool for the retrospective diagnosis of MVL when whole blood is not available and for retrospective epidemiological studies on the asymptomatic carriage. Nevertheless, further studies including larger series and the use of a real-time quantitative PCR are expected particularly to improve its predictive value for clinical relapses.
Acknowledgments
This work was supported by the GACL (Groupe d'Action Contre la Leishmaniose).
We are grateful to Sophie Fornasero and Jean Villevieille for their excellent technical assistance and to Sophie Brun (Centre Hospitalier Universitaire Angers, France) for her collaboration. We wish to thank Emil Lou for revising the manuscript. We also thank the blood donors from the Blood Bank of Principality of Monaco for their altruistic participation.
REFERENCES
- 1.Bossolasco, S., G. Gaiera, D. Olchini, M. Gulletta, L. Martello, A. Bestetti, L. Bossi, L. Germagnoli, A. Lazzarin, C. Uberti-Foppa, and P. Cinque. 2003. Real-time PCR assay for clinical management of human immunodeficiency virus-infected patients with visceral leishmaniasis. J. Clin. Microbiol. 41:5080-5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cascio, A., S. Calattini, C. Colomba, C. Scalamogna, M. Galazzi, M. Pizzuto, R. Camilli, M. Gramiccia, L. Titone, M. Corbellino, and S. Antinori. 2002. Polymerase chain reaction in the diagnosis and prognosis of Mediterranean visceral leishmaniasis in immunocompetent children. Pediatrics 109:E27. [DOI] [PubMed] [Google Scholar]
- 3.Costa, J.-M., R. Durand, M. Deniau, D. Rivollet, M. Izri, R. Houin, M. Vidaud, and S. Bretagne. 1996. PCR enzyme-linked immunosorbent assay for diagnosis of leishmaniasis in human immunodeficiency virus-infected patients. J. Clin. Microbiol. 34:1831-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisa, R., C. Riera, E. Ribera, M. Gallego, and M. Portus. 2002. A nested polymerase chain reaction for diagnosis and follow-up of human visceral leishmaniasis patients using blood samples. Trans. R. Soc. Trop. Med. Hyg. 96:191-194. [DOI] [PubMed] [Google Scholar]
- 5.Hu, X. S., W. T. Yang, H. G. Lu, H. P. Yan, J. P. Cheng, Y. Ma, B. Q. Jin, and T. Zhang. 2000. Sequencing a specific kinetoplast DNA fragment of Leishmania donovani for polymerase chain reaction amplification in diagnosis of leishmaniasis in bone marrow and blood samples. J. Parasitol. 86:822-826. [DOI] [PubMed] [Google Scholar]
- 6.Lachaud, L., J. Dereure, E. Chabbert, J. Reynes, J. M. Mauboussin, E. Oziol, J. P. Dedet, and P. Bastien. 2000. Optimized PCR using patient blood samples for diagnosis and follow-up of visceral leishmaniasis, with special reference to AIDS patients. J. Clin. Microbiol. 38:236-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le Fichoux, Y., J.-F. Quaranta, J.-P. Aufeuvre, A. Lelievre, P. Marty, I. Suffia, D. Rousseau, and J. Kubar. 1999. Occurrence of Leishmania infantum parasitemia in asymptomatic blood donors living in area of endemicity in southern France. J. Clin. Microbiol. 37:1953-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marty, P., A. Lelievre, J. F. Quaranta, A. Rahal, M. Gari-Toussaint, and Y. Le Fichoux. 1994. Use of leishmanin skin test and western blot analysis for epidemiological studies in visceral leishmaniasis areas: experience in a highly endemic focus in Alpes-Maritimes (France). Trans. R. Soc. Trop. Med. Hyg. 88:658-659. [DOI] [PubMed] [Google Scholar]
- 9.Mathis, A., and P. Deplazes. 1995. PCR and in vitro cultivation for detection of Leishmania spp. in diagnostic samples from humans and dogs. J. Clin. Microbiol. 33:1145-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palatnik-de-Sousa, C. B., E. Paraguai-de-Souza, E. M. Gomes, F. C. Soares-Machado, K. G. Luz, and R. Borojevic. 1996. Transmission of visceral leishmaniasis by blood transfusion in hamsters. Braz. J. Med. Biol. Res. 29:1311-1315. [PubMed] [Google Scholar]
- 11.Piarroux, R., F. Gambarelli, B. Toga, H. Dumon, M. Fontes, S. Dunan, and M. Quilici. 1996. Interest and reliability of a polymerase chain reaction on bone marrow samples in the diagnosis of visceral leishmaniasis in immunocompromised patients. AIDS 10:452-453. [DOI] [PubMed] [Google Scholar]
- 12.Pizzuto, M., M. Piazza, D. Senese, C. Scalamogna, S. Calattin, L. Corsico, B. Persico, B. Adriani, C. Magni, G. Guaraldi, G. Gaiera, A. Ludovisi, M. Gramiccia, M. Galli, M. Moroni, M. Corbellino, and S. Antinori. 2001. Role of PCR in diagnosis and prognosis of visceral leishmaniasis in patients coinfected with human immunodeficiency virus type 1. J. Clin. Microbiol. 39:357-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suffia, I., J.-F. Quaranta, M. C. M. Eulalio, B. Ferrua, P. Marty, Y. Le Fichoux, and J. Kubar. 1995. Human T-cell activation by 14- and 18-kilodalton nuclear proteins of Leishmania infantum. Infect. Immun. 63:3765-3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Travi, B. L., C. J. Tabares, H. Cadena, C. Ferro, and Y. Osorio. 2001. Canine visceral leishmaniasis in Colombia: relationship between clinical and parasitological status and infectivity for sandflies. Am. J. Trop. Med. Hyg. 64:119-124. [DOI] [PubMed] [Google Scholar]