Abstract
This work examines the effects of potassium tellurite (K2TeO3) on the cell viability of the facultative phototroph Rhodobacter capsulatus. There was a growth mode-dependent response in which cultures anaerobically grown in the light tolerate the presence of up to 250 to 300 μg of tellurite (TeO32−) per ml, while dark-grown aerobic cells were inhibited at tellurite levels as low as 2 μg/ml. The tellurite sensitivity of aerobic cultures was evident only for growth on minimal salt medium, whereas it was not seen during growth on complex medium. Notably, through the use of flow cytometry, we show that the cell membrane integrity was strongly affected by tellurite during the early growth phase (≤50% viable cells); however, at the end of the growth period and in parallel with massive tellurite intracellular accumulation as elemental Te0 crystallites, recovery of cytoplasmic membrane integrity was apparent (≥90% viable cells), which was supported by the development of a significant membrane potential (Δψ = 120 mV). These data are taken as evidence that in anaerobic aquatic habitats, the facultative phototroph R. capsulatus might act as a natural scavenger of the highly soluble and toxic oxyanion tellurite.
Potassium tellurite (K2TeO3) has long been recognized as toxic to eukaryotic and prokaryotic cells; furthermore, because of its antimicrobial properties, it has been used in selective media for the isolation of a number of naturally tellurite-resistant bacterial species (19). Over the past few decades, tellurium has also found wide use in areas such metallurgy and the electronics industry. As a consequence, the toxic effects associated with the accumulation of tellurium compounds, particularly the water-soluble oxyanion tellurite (TeO32−), has become a concern (19).
Some gram-positive bacteria show an intrinsic low-level resistance to TeO32− (50 to 120 μg/ml) (18), while high-level resistance (500 to 2500 μg/ml) has been determined in certain gram-negative obligate aerobic photosynthetic species (14, 28). As a reference, Escherichia coli growth is inhibited at a tellurite concentration as low as 1 μg/ml. Growth in the presence of tellurite is often associated with the reduction of the oxyanion to elemental tellurium (Te0), which leads to blackening of the cells due to either internal or periplasmic accumulation of Te (19, 22). In some instances the capacity to grow at higher tellurite concentrations has been shown to depend on the presence of genetic determinants carried on IncHI, IncHII, and IncP plasmids (25). In addition, chromosomal genes important for growth in the presence of K2TeO3 have been identified in a few species, but their role has not been clearly determined (7, 20, 21).
The purple nonsulfur bacteria include several species that show intrinsic high-level resistance to potassium tellurite ranging from 50 to 1,000 μg/ml (9). Rhodobacter sphaeroides is characterized by growth mode dependence on the tellurite resistance level: complex media confer much lower resistance (10 to 80 μg/ml) than minimal media (100 to 1,000 μg/ml), and aerobic cultures are more resistant (800 to 1,000 μg/ml) than photosynthetic cultures (400 to 700 μg/ml). In R. sphaeroides, two loci involved in determining high-level resistance to tellurite oxyanions have been identified (11). The close relative Rhodobacter capsulatus is also able to withstand high concentrations of tellurite and to internally accumulate a considerable amount of elemental tellurium, as demonstrated by transmission electron microscopy and X-ray microanalysis (2). It has recently been shown that the uptake of tellurite by R. capsulatus depends on the ΔpH component of the transmembrane proton motive force (3). In this facultative phototroph, it was also reported that, during photosynthetic growth, the presence of potassium tellurite caused changes in the structure of the branched respiratory chain leading to a decrease in the content of c-type cytochromes, paralleled by a low cytochrome c oxidase activity (2). An analogous effect has been seen in Pseudomonas pseudoalcaligenes KF707 (4).
In this work we analyzed the effects of potassium tellurite on the viability of R. capsulatus cells. We report that photosynthetic anaerobic cultures are able to grow in the presence of high concentrations of tellurite (250 to 300 μg of TeO32− per ml); conversely, dark-grown aerobic cultures are highly sensitive to low tellurite concentrations (2 μg/ml) only for growth in minimal salt medium. We also show by flow cytometric analysis that the high proportion of damaged and dead cells seen at the early growth phase is drastically reduced at the end of the growth curve in parallel with a consistent intracellular accumulation of elemental tellurium. These results suggest that facultative photosynthetic bacteria might play a significant environmental role in intracellularly precipitating the toxic oxyanion tellurite (TeO32−) into a form, Te0, that is less toxic and less available to other organisms.
MATERIALS AND METHODS
Bacterial strain and growth conditions.
R. capsulatus B100 was grown under chemotrophic conditions at 30°C in Erlenmeyer flasks shaken at 150 rpm and under phototrophic conditions in completely filled screw-cap tubes and bottles at 30°C with an incident light intensity of 200 W m−2. The cells were cultivated in both rich (YPS) and minimal (RCV) media (26). YPS medium contained yeast extract, 0.3%; peptone, 0.3%; CaCl2, 2 mM; and MgSO4, 2 mM, in deionized water, pH 6.8. RCV medium contained dl-malic acid, 0.1%; (NH4)2SO4, 0.4%; EDTA, 0.002%; MgSO4 · 7H2O, 0.02%; trace elements, 1 ml/liter; CaCl2 · 2H2O, 0.0075%; FeSO4 · 7H2O, 0,0012%; thiamine hydrocloride, 10−4%; and KPO4 buffer, 10 mM, in deionized water, pH adjusted to 6.8 before autoclaving. Trace elements solution contained, per 250 ml of deionized water, MnSO4 · H2O, 397.5 mg; H3BO3, 700 mg; Cu(NO3)2 · 3H2O, · 10 mg; ZnSO4 · 7H2O, 60 mg; and NaMoO4 · 2H2O, 187.5 mg. Anaerobic growth in the dark was achieved by adding fructose (20 mM) and dimethyl sulfoxide (60 mM) to filled screw-cap tubes containing either YPS or RCV medium and incubating at 30°C in the dark. Potassium tellurite (K2TeO3) was added to the growing cultures at the concentrations specified for each experiment in the Results section.
MIC.
The MIC of potassium tellurite was determined on agar plates with either rich or minimal medium prepared with increasing concentrations of K2TeO3; 50-μl drops of one- to fivefold dilutions of fully grown cultures were laid on the surface of the plates, which were then incubated at 30°C under either respiratory, photosynthetic, or anaerobic-dark conditions for 3 to 14 days, depending on the growth mode. Anaerobic incubations were performed in closed anaerobic jars in the presence of the Anaerocult A anaerobic system (Merck). The blackening of the area corresponding to the culture drop, as a result of tellurite reduction to elemental tellurium by the cells, was scored as positive for growth.
Biochemical methods.
The protein content of whole cells was determined by the method of Lowry et al. (8) after a 1-min incubation with 0.1 N NaOH in boiling water. Crystalline bovine serum albumin (Sigma) was used as the protein standard. The quantitative determination of potassium tellurite in liquid media was done with the reagent diethyldithiocarbamate (Sigma) as described by Turner et al. (23). The dissolved oxygen concentration in the growth media and respiratory activities were determined through the use of a Clark-type oxygen electrode (YSI 53; Yellow Springs Instruments Inc., Yellow Springs, Ohio).
Transmission electron microscopy.
Bacterial cells grown in the presence of tellurite (25 to 50 μg/ml) were harvested after the cultures became black and processed for electron microscopic analysis. Cell pellets were first washed in 0.05 M cacodylate buffer (pH 7.2) and then fixed for 2 h in 0.05 M cacodylate-1.5% (wt/vol) glutaraldehyde (pH 7.2). The same buffer was then used for overnight washing of the sample, followed by 2 h of fixation with 2% (wt/vol) osmium tetroxide and dehydration with ethanol. Finally, the samples were embedded in Durcopan, and thin sections prepared with an LKB Ultratome Nova were double stained with uranyl acetate and lead citrate (13). Specimens were examined with a Philips CM-100 transmission electron microscope.
Flow cytometry.
Flow cytometry experiments were done according to Ziglio et al. (29). Briefly, cell pellets were diluted with 0.22-μm-pore-size-filtered Tricine buffer (0.1 M Tricine, 10 mM MgCl2 at pH 7.4) and stained with SYBR Green I (SYBR I) and propidium iodide (Molecular Probes Inc., Eugene, Oreg.) by adding 10 μl of both fluorochromes per ml of sample containing about 106 to 107 cells. The double-staining equilibrium was assumed to be reached within 15 min of incubation at room temperature in the dark. Samples were analyzed with a Bryte-HS flow cytometer (Bio-Rad, Hercules, Calif.) with a high light-scattering sensitivity (16) and equipped with a short-arc xenon lamp (Hamamatsu). Samples were excited at 470 to 490 nm, and fluorescence emission was detected at 515 to 565 nm for SYBR I (λex = 488 nm, λem = 525 nm) and at 590 to 720 nm for propidium iodide (λex = 530 nm, λem = 620 nm). Samples were analyzed for 3 to 4 min at a flow rate of 1.5 μl min−1 for cell enumeration. Data acquisition was triggered to reduce interference by nonfluorescent particles (debris), while photomultiplier gains were set in the logarithmic mode, and data were recorded as list mode files by WinBryte software (Bio-Rad).
TPP+ uptake measurements.
A polyvinyl chloride membrane selectively permeable to tetraphenylphosphonium (TPP+) was constructed as described (5), and TPP+ uptake measurements were performed as reported by Rugolo and Zannoni (14). An internal cell volume of 102 μl per μmol of bacteriochlorophyll was assumed, according to Kell et al. (6).
RESULTS
MIC determination under different growth conditions.
Rhodobacter capsulatus, a member of the family Rhodobacteraceae, is metabolically highly versatile and, as a consequence, able to grow under a wide variety of conditions. The MICs of TeO32− were determined for cells growing by aerobic respiration, photosynthesis, and anaerobic respiration (with dimethyl sulfoxide as the exogenous oxidant) on different media. It is immediately apparent from Table 1 that aerobic conditions greatly influenced the response of the cells grown on minimal RCV medium to the presence of tellurite. Under these conditions R. capsulatus was highly resistant to tellurite under photosynthetic-anaerobic conditions (250 μg/ml) but very sensitive (2 μg/ml) in the presence of oxygen. Anaerobic nonphotosynthetic conditions gave a medium level of resistance (20 μg/ml).
TABLE 1.
MICs of K2TeO3 for R. capsulatus grown with different media and growth conditions
| Mediuma | MIC (μg/ml)
|
||
|---|---|---|---|
| Aerobic | Photosynthetic | Anaerobic-dark | |
| YPS | 400 | 500 | |
| + malate | 50 | 400 | |
| RCV | 2 | 250 | |
| + 0.06% Y + 0.06% P | 10 | 300 | |
| + 0.3% Y + 0.3% P | 50 | 400 | |
| + 0.3% Y | 50 | 300 | |
| + 0.3% P | 50 | 300 | |
| YPS + fructose + DMSO | 150 | ||
| RCV + fructose + DMSO | 20 | ||
Y, yeast extract; P, peptone; DMSO, dimethyl sulfoxide.
The resistance profile was different for cells grown on complex YPS medium. Under all growth conditions, there was a high level of resistance. Aerobiosis did not appear to have any evident role in influencing the MIC of tellurite on this medium. Under aerobic conditions, the addition to minimal RCV medium of the components of complex YPS medium (yeast extract and peptone) did not restore the high MIC typical of these cultural conditions, even when added together and at the same concentrations used in YPS medium (Table 1). When these components were added at a low concentration (0.06%), the MIC increased from 2 to 10 μg/ml, but if they were added at the concentration typical of complex medium (0.3%), the MIC increased to 50 μg/ml. The same effect on the MIC was seen if the two components were added separately. The opposite effect was seen if YPS medium plates were amended with malate under aerobic conditions. Under these conditions the cells became more sensitive to tellurite relative to growth on normal rich medium, suggesting that malate is a key element in determining the level of tolerance of R. capsulatus cells to potassium tellurite.
Growth in the presence of tellurite.
In accord with the MICs determined on agar plates, liquid cultures of R. capsulatus with 50 μg of potassium tellurite per ml developed only in the absence of oxygen or on complex medium, with no growth occurring on minimal medium under aerobic conditions. An unexpected observation was that even photosynthetic cultures inoculated in completely filled screw-cap tubes containing RCV minimal medium started to grow and to reduce tellurite only after a lag period of approximately 120 h. This finding is in sharp contrast to the observation that photosynthetic growth and blackening of the cells on agar plates amended with tellurite were clearly visible within 48 h of inoculation.
To reconcile these contrasting findings, it was considered that true anaerobiosis was normally reached only for agar plates anaerobically incubated in jars where all the gaseous O2 was rapidly (30 min) consumed by the anaerobiosis-generating system, while in freshly inoculated screw-cap tubes there was enough dissolved O2 (about 190 μM, compared to a concentration of 250 μM for O2-saturated water, as measured by a Clark-type oxygen electrode) to prevent growth in the presence of tellurite as seen under aerobic conditions. Accordingly, in newly inoculated tubes kept in the dark at 30°C for 20 h to allow the metabolic activity of the cells to consume the dissolved oxygen, no measurable (≤20 μM by polarography) dissolved oxygen in the growth medium was present. As a consequence of this treatment, the lag period seen before the onset of growth decreased to about 5 h (Table 2). The same dark incubation was performed in the presence of 2 μg of the redox indicator resazurin per ml, which turned colorless, confirming that reducing-anaerobic conditions had been reached (not shown).
TABLE 2.
Lag periods before the onset of growth and dissolved oxygen concentrations of R. capsulatus photosynthetic cultures grown on minimal medium in the presence of potassium telluritea
| Time of addition of TeO32− | Lag period (h) | Dissolved oxygen (μM)a |
|---|---|---|
| Immediate | 120 ± 20 | 188 ± 14 |
| After 20 h of incubation in the dark | 5 ± 1 | 20 ± 2.5 |
| With H2O2 after 20 h of incubation in the dark | 120 ± 25 | 237 ± 17 |
| At the beginning of the dark incubation period | 140 ± 23 | 205 ± 15 |
Potassium tellurite was added at 50 μg/ml. Dissolved oxygen was measured with a Clark-type oxygen electrode.
As a control for the oxygen effect, hydrogen peroxide (H2O2) was added after the dark incubation period at a final concentration of 0.73 mM in the presence of 50 μg/ml tellurite, and the cultures were put in the light. Under these conditions, there was a catalase-dependent production of about 240 μM oxygen and the initial 120-h lag period was restored (Table 2). Control cultures with no tellurite added showed that the same concentration of H2O2 had no measurable effect on photosynthetic growth. In order to better define the effect of the oxyanion on cellular metabolism, the dark incubation was performed in the presence of tellurite. At the end of the incubation period, no oxygen had been consumed by the cells, and the cultures still showed the long lag period before the onset of photosynthetic growth (Table 2). These findings point to tellurite inhibition of oxidative metabolism.
Tellurium uptake by cells.
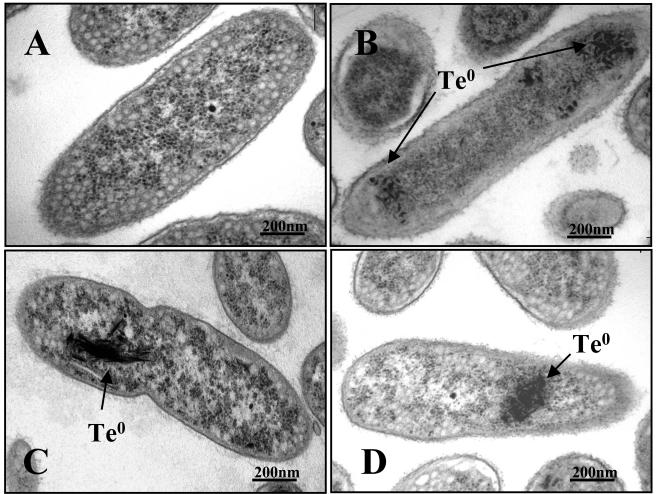
R. capsulatus cultures growing in the presence of tellurite take up the oxyanion from the medium and accumulate it inside the cells as black deposits of elemental tellurium (Te0) (2). This activity requires an energized cytoplasmic membrane and is a ΔpH-dependent process (3). Transmission electron microscopy photographs (Fig. 1) clearly showed that Te0 accumulated in the cytoplasm in shapes and sizes that were dependent on the growth conditions. Aerobically grown cells showed rod-like and barrel-like particles that were 40 to 50 nm in size (Fig. 1B), whereas cells grown under photosynthetic reducing conditions showed black deposits of splinterlike shape, varying in length from 75 to more than 350 nm (Fig. 1C and D).
FIG. 1.
Electron micrographs of R. capsulatus B100 cells grown in the absence (A) or in the presence (B, C, D) of 50 μg of K2TeO3 per ml. (A) Photosynthetic-anaerobic growth. (B) Aerobic growth. (C) Photosynthetic-anaerobic growth on minimal medium. (D) Photosynthetic-anaerobic growth on rich medium.
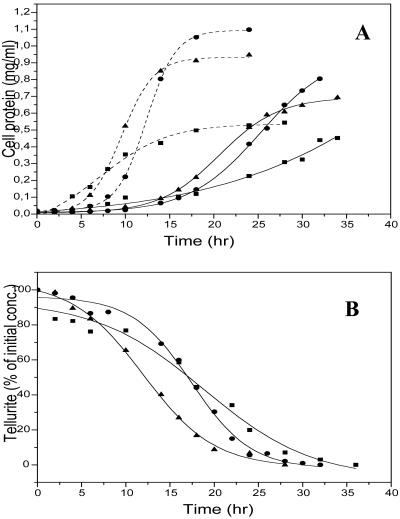
The uptake of potassium tellurite by the cells was measured by monitoring the disappearance of the oxyanion from the growth medium in both aerobic and photosynthetic cultures. At an initial tellurite concentration of 25 μg/ml, the oxyanion completely disappeared from the medium in approximately 30 h under aerobic conditions on rich medium and after 22 to 24 h under photosynthetic-anaerobic conditions (Fig. 2B). Similar results were obtained at an initial concentration of 50 μg of tellurite per ml, with the oxyanion disappearing from the medium in approximately 34 h (not shown). The presence of tellurite in the medium negatively affected the growth rate of the culture to a degree that was influenced by the growth mode (Fig. 2A). At 25 μg of K2TeO3 per ml, photosynthetic cultures showed a more than twofold increase in doubling time, 2.7 for cells grown on rich medium and 2.3 for cells grown on minimal medium, versus a 3.8-fold increase for aerobic cultures. Growing the cultures at 50 μg of tellurite per ml further increased the measured doubling times, as expected (data not shown).
FIG. 2.
(A) Growth curves of cells grown in the presence of 25 μg of K2TeO3 per ml. Solid lines, K2TeO3-grown cultures; dashed lines, control cultures. (B) Variation of tellurite concentration in the growth medium. The same symbols represent the same growth conditions in the two panels. Symbols: ▪, aerobic conditions on rich medium; •, photosynthetic conditions on minimal medium; ▴, photosynthetic conditions on rich medium.
Cell membrane integrity as determined by flow cytometry.
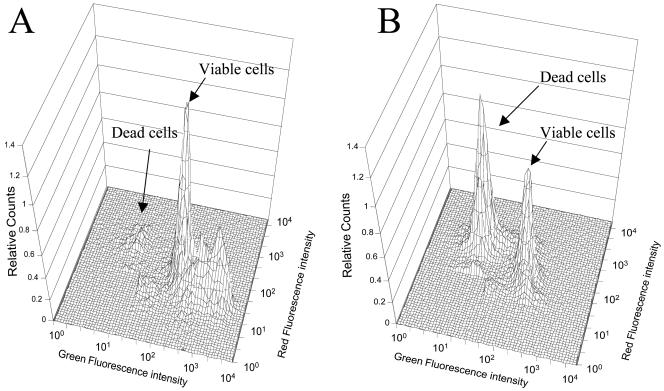
In this study, the determination of viable and dead R. capsulatus cells grown in the presence and absence of potassium tellurite was done on the basis of their membrane integrity as determined by staining with SYBR I and propidium iodide, two high-affinity nucleic acid dyes that give bright staining of cells (1, 27). Although no specific information is available on the structure and chemical properties of SYBR I or on its mode of binding to DNA (10), it has widely been shown to be capable of staining all cells, living or dead. Conversely, the polarity of propidium iodide allows it to penetrate only inactive cell membranes, which are characteristic of dead or damaged cells. In dead cells, the simultaneous presence of SYBR I and propidium iodide activates energy transfer between SYBR I and propidium iodide so that the fluorescence emission of SYBR I is no longer visible. In this way it is possible to distinguish viable cells (green fluorescent) from dead cells (red fluorescent).
In the cytograms shown in Fig. 3 two regions are defined, in the three-dimensional representation, by peaks of fluorescence signals which are indicative of different bacterial physiological states, dead and damaged cells and viable cells. R. capsulatus cells harvested from a control culture in the exponential growth phase (Fig. 3A) showed multiple peaks in a region of high-intensity green fluorescence and low-intensity red fluorescence. A minute peak corresponding to dead cells was visible in the low-green-fluorescence area. Cells grown in the presence of potassium tellurite (Fig. 3B) showed, on the contrary, a tall peak in the corresponding dead cells area. The data from flow cytometry experiments are collected in Table 3. Here it is shown that at the beginning of the growth phase (4 h), approximately 94% of the cells in the control cultures and 51% of the cells in tellurite-grown cultures were viable, suggesting significant damage of the cytoplasmic membrane by the toxic oxyanion at 50 μg/ml, corresponding to a tellurite-cell ratio of 1 ng/5,000. The percentage of damaged and dead cells decreased drastically after 24 h of growth, parallel to a complete disappearance of tellurite from the growth medium over the same period (Table 3; Fig. 2). At the end of the growth period (48 h), with no tellurite present in the medium, a strong recovery of the cytoplasmic membrane integrity in tellurite-grown cells was apparent, with more than 90% of cells viable, similar to the control cultures (Table 3).
FIG. 3.
Three-dimensional fluorescence cytograms of R. capsulatus cells grown on minimal medium and stained with SYBR I and propidium iodide. (A) Cells grown in the absence of potassium tellurite; total count, 2.8 × 108 cells/ml. (B) Cells grown in the presence of 50 μg of potassium tellurite per ml; total count, 3.3 × 108 cells/ml.
TABLE 3.
Cell viability in photosynthetic cultures grown on minimal medium in the presence of 50 μg of potassium tellurite per ml
| Culture | Growth period (h) | % Damaged cells | No. of cells/ml |
|---|---|---|---|
| Control | |||
| 4 | 6.6 ± 0.5 | 2.84 × 108 ± 8.52 × 107 | |
| 8 | 6.1 ± 0.4 | 1.74 × 109 ± 5.22 × 108 | |
| 24 | 6.5 ± 0.5 | 3.65 × 109 ± 1.15 × 109 | |
| + TeO32− | |||
| 4 | 49.2 ± 3.8 | 2.52 × 108 ± 7.56 × 107 | |
| 10 | 58.1 ± 4.5 | 3.32 × 108 ± 9.96 × 107 | |
| 14 | 56.1 ± 4.3 | 4.52 × 108 ± 1.30 × 108 | |
| 24 | 16.1 ± 1.2 | 2.34 × 109 ± 7.02 × 108 | |
| 48 | 9.4 ± 0.7 | 3.06 × 109 ± 9.20 × 108 |
Cell membrane permeability as determined by TPP+ cation uptake.
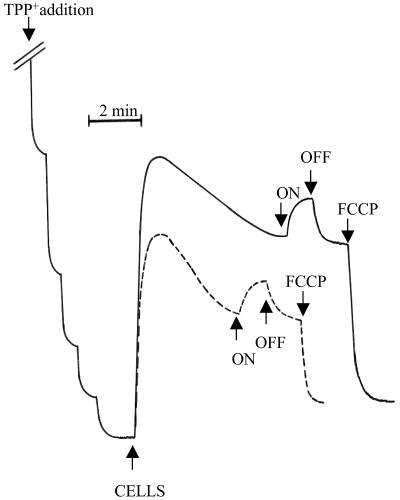
To test the capacity of the cell membrane to generate and maintain a consistent electric potential (Δψ) produced by proton extrusion coupled to either respiratory or photosynthetic electron transport, the distribution of the lipophilic cation TPP+ was analyzed. Figure 4 (continuous trace) shows that after the addition of R. capsulatus cells grown for 24 h in the absence of tellurite (control), a rapid upward deflection of the trace, indicative of TPP+ uptake (development of a negative potential inside the cells), was seen. Notably, the initial level of membrane potential (Δψ) cannot be maintained by respiration, possibly due to rate-limiting oxygen diffusion through the external membrane or wall structure of R. capsulatus. Under steady-state respiratory conditions (reached after approximately 5 min), the estimated Δψ was approximately 114 ± 3 mV, while under both respiration and continuous illumination (light on), the Δψ went up to 136 ± 3 mV. Δψ = 0 was defined by the TPP+ level seen in the dark after addition of a 1.5 μM concentration of the protonophore FCCP (carbonyl cyanide p-trifluoromethoxyphenylhydrazone).
FIG. 4.
Light-induced and oxygen-dependent uptake of TPP+ ions by R. capsulatus cells grown in the absence (continuous trace) or in the presence (interrupted trace) of potassium tellurite. Following the calibration addition of TPP+ (1 μM final concentration), cells (2.8 and 3.3 mg of protein for control and tellurite-grown cells, respectively) were added to 2 ml of air-saturated medium in 50 mM TES buffer, pH 7.5-10 mM KCl at 28°C. TPP+, tetraphenylphosphonium cation; FCCP, carbonyl cyanide p-trifluoromethoxyphenylhydrazone; ON, light on; OFF, light off.
The interrupted trace in Fig. 4 shows TPP+ uptake by R. capsulatus cells grown (24 h) in the presence of tellurite. Apparently, while the general TPP+ uptake pattern is qualitatively similar to that seen in cells grown in the absence of tellurite (continuous line), the Δψ values reached by respiration (104 ± 3 mV) and respiration plus photosynthesis (120 ± 3 mV) were lower than those seen with control cells. It is noteworthy that the Δψ values calculated for tellurite-grown cells were already corrected for a decreased total internal volume (Vi) due to an 11% increase in damaged cells and a 30% increase in bacteriochlorophyll content (3). Respiratory measurements performed in parallel with TPP+ determinations indicated that the endogenous respiration by cells of R. capsulatus grown in the presence of tellurite was inhibited 40% by light, whereas it was severely inhibited (80%) in control cells (not shown). This suggests, in line with early reports (14), that the light-generated Δψ exhibits strong control over respiration under normal growth conditions, whereas this phenomenon is much less evident in tellurite-grown cells. The reduced capacity of tellurite-grown cells to inhibit respiration during continuous illumination might be due to a restricted light-dependent electron flow caused by a lower cytochrome c content (2) (see Discussion).
DISCUSSION
The facultative photosynthetic bacterium Rhodobacter capsulatus shows a high level of resistance to potassium tellurite, like other phototrophic species (9), and accumulates it in the cytoplasm, upon reduction, as elemental tellurium (Fig. 1) (2). We determined the MICs of tellurite under a variety of growth conditions and showed that the increasing level of resistance depends primarily on the absence of oxygen (Table 1). Aerobic conditions on minimal medium allowed very low resistance (2 μg/ml), while under photosynthetic-anaerobic conditions on the same medium, the cells were able to grow in the presence of up to 250 μg of potassium tellurite per ml. Even growth under anaerobic conditions in the dark, made possible by the addition of dimethyl sulfoxide as an electron sink, which represents an energetically less favorable situation than growth by aerobic respiration (15), showed a resistance level of 20 μg/ml.
A second factor influencing the level of resistance to TeO32− was the composition of the medium. YPS rich medium determined an increase of resistance under all growth conditions, mostly in aerobiosis (Table 1). This finding was taken as an indication that the components of the rich medium may exert a protective, antioxidative action on the cells in the presence of potassium tellurite, which is believed to have strong oxidant properties (17). Interestingly, when MIC determinations were made on media of mixed composition, there was a significant effect under aerobic conditions: the addition of malate to rich medium was sufficient to drastically decrease the level of resistance to tellurite, while the addition of yeast extract and peptone, either together or separately, to minimal medium had a protection effect that only marginally increased the measured MIC (Table 1). Under photosynthetic conditions there was no such effect. These data suggest that malate might have an important role in the mechanism determining the susceptibility of R. capsulatus cells to potassium tellurite.
The effects of growth conditions and medium composition on the resistance pattern to tellurite, as shown here, appear in sharp contrast to previous observations made for the closely related species R. sphaeroides (9). In this species, the effect of medium composition on TeO32− resistance, even under aerobic conditions, was indeed the opposite of that seen in R. capsulatus. These contrasting findings may possibly be reconciled by considering that species belonging to the genus Rhodobacter show a relatively high degree of metabolic differences and that the method used for determining resistance to TeO32−, i.e., MICs measured on liquid media (9), probably masked the selection of hyperresistant variants, resulting in an increase in the apparent MICs (11).
The effect of oxic conditions was also evident in liquid cultures. Aerobic incubation on minimal medium in the presence of tellurite prevented the growth of R. capsulatus cells. Even completely filled screw-cap tubes, used for phototrophic growth, contained enough dissolved oxygen (188 μM) to inhibit growth in the presence of the oxyanion. The protective action of the rich medium components was seen in the liquid cultures as for growth on agar plates. The effect of O2 on growth on minimal medium in the presence of tellurite was confirmed in experiments in which the level of dissolved oxygen was changed, taking advantage of the metabolic activity of the cells, or by addition of H2O2 to the culture tubes (Table 2).
Two hypothetical explanations can be considered to rationalize these observations. First, oxygen above a certain threshold level induces the production of molecules involved in aerobic metabolism, which are preferred targets for the oxidative action of the TeO32− anion and whose inactivation prevents aerobic growth. In this scenario, components of the rich medium could exert a protective, antioxidative action, thereby making aerobic growth possible. As a second hypothesis, tellurite inhibits the cellular response to oxidative stresses, as would happen during the subculturing of photosynthetic cultures, in which cells adapted to photosynthetic-anaerobic conditions must confront a sudden increase of oxygen due to the high level (75 to 80% of saturation) of O2 normally present in fresh medium. Under normal conditions, R. capsulatus cells can easily overcome this type of oxidative stress, while the presence of tellurite would prevent the adaptive response. The decreased tellurite resistance under oxic conditions might be due to tellurite damage of proteins and free thiols as well as displacement of other metal ions from biomolecules (25). Indeed, oxidation of free thiol groups on proteins and small redox buffers such as glutathione, cytochromes, and dihydroascorbate can be a source of oxidative stress (24). Thus, proteins acting in defense against the oxidative stress could be the immediate targets of tellurite oxyanions.
The ability of R. capsulatus cells to reduce tellurite to elemental tellurium and internalize it, as part of their tellurite response mechanism, is evident in transmission electron microscopic images in which deposits of Te0 are clearly visible in the cytoplasm (Fig. 1) (2). Apparently differences in the size and internal distribution of the tellurium deposits are related to the growth mode of the cultures (Fig. 1). Whether these various forms of elemental tellurium depend on different reduction mechanisms remains to be determined.
Growth in the presence of potassium tellurite increased the doubling time of the culture (Fig. 2A) to a degree that depended on the initial concentration of the oxyanion (not shown). At all initial concentrations and under all growth conditions tested, R. capsulatus cultures were able to completely take up the tellurite oxyanion from the medium and accumulate it in the cytoplasm. Interestingly, as soon as the cells overcame the initial tellurite-induced stress (tellurite/cell ratio of 1 ng/5,000), presumably upon activation of a consistent tellurite uptake that leads to complete disappearance of the toxic oxyanion from the medium (Fig. 2B), the membrane integrity, which was severely impaired in the early growth phase, was substantially restored, as indicated by flow cytometric analysis (Table 3; Fig. 3) and by membrane potential measurements (Fig. 4). In this respect, various parameters such as (i) reproductive growth, (ii) metabolic activity, and (iii) membrane integrity have recently been discussed in the light of the most suitable markers defining the concept of cell viability (10). Apparently, reproductive growth represents the most stringent proof of viability because it requires both conditions ii and iii. However, cells with an intact membrane are supposed to be capable of metabolic activity and show reproductive growth under appropriate conditions. Conversely, dead bacterial cells contain a permeabilized cytoplasmic membrane, freely exposed to the environment.
Here we have shown by flow cytometry that potassium tellurite affects the membrane integrity of R. capsulatus cells in their early growth phase (Table 3), while the membrane permeability of stationary-phase cells is similar to that of control cells. Furthermore, the data in Table 3 show that the photosynthetic growth capacity of control cells and cells grown in the presence of tellurite is similar. These results, along with those recently obtained with membrane vesicles (chromatophores) isolated from light-grown cells in the presence of tellurite and harvested at their stationary phase of growth (2) can be taken as evidence of the presence of an efficient photosynthetic metabolism and cell viability. Herein, we have also shown that tellurite toxicity is strongly enhanced in R. capsulatus by aerobic conditions, particularly in minimal salt medium. Finally, the capacity of R. capsulatus to massively accumulate the highly soluble tellurite in the form of insoluble elemental tellurium crystallites suggests that facultative phototrophs might play a significant environmental role in subtracting the toxic oxyanion from polluted aquatic sites.
Acknowledgments
We thank MIUR for supporting this work (PRIN2003).
We are indebted to R. Randi and F. Pennetta for their skillful assistance with transmission electron microscopy and tellurium uptake determinations, respectively.
REFERENCES
- 1.Barbesti, S., S. Citterio, M. Labra, M. D. Baroni, M. G. Neri, and S. Sgorbati. 2000. Two and three-color fluorescence flow cytometric analysis of immunoidentified viable bacteria. Cytometry 10:214-218. [PubMed] [Google Scholar]
- 2.Borsetti, F., R. Borghese, F. Francia, M. R. Randi, S. Fedi, and D. Zannoni. 2003. Reduction of potassium tellurite to elemental tellurium and its effect on the plasma membrane redox components of the facultative phototroph Rhodobacter capsulatus. Protoplasma 221:153-161. [DOI] [PubMed] [Google Scholar]
- 3.Borsetti, F., A. Toninello, and D. Zannoni. 2003. Tellurite uptake by cells of the facultative phototroph Rhodobacter capsulatus is a ΔpH dependent process. FEBS Lett. 554:315-318. [DOI] [PubMed] [Google Scholar]
- 4.Di Tomaso, G., S. Fedi, M. Carnevali, M. Manegatti, C. Taddei, and D. Zannoni. 2002. The membrane-bound respiratory chain of Pseudomonas pseudoalcaligenes KF707 cells grown in the presence or absence of potassium tellurite. Microbiology 148:1699-1708. [DOI] [PubMed] [Google Scholar]
- 5.Kamo, N., M. Muratsuga, R. Hongoh, and Y. Kobatake. 1979. Membrane potential of mitochondria measured with an electrode sensitive to tetraphenyl phosphonium and relationship between proton electrochemical potential and phosphorylation potential. J. Biol. Membr. 49:105-121. [DOI] [PubMed] [Google Scholar]
- 6.Kell, D. B., S. J. Ferguson, and P. John. 1978. Measurement by a flow dialysis technique of the steady-state proton-motive force in chromatophores from Rhodospirillum rubrum. Comparison with phosphorylation potential. Biochim. Biophys. Acta 502:111-126. [DOI] [PubMed] [Google Scholar]
- 7.Liu, M., and D. E. Taylor. 1999. Characterization of Gram-positive tellurite resistance encoded by the Streptococcus pneumoniae tehB gene. FEMS Microbiol. Lett. 174:385-392. [DOI] [PubMed] [Google Scholar]
- 8.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 9.Moore, M. D., and S. Kaplan. 1992. Identification of intrinsic high-level resistance to rare-earth oxides and oxyanions in members of the class Proteobacteria: characterization of tellurite, selenite, and rhodium sesquioxide reduction in Rhodobacter sphaeroides. J. Bacteriol. 174:1505-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nebe-von-Caron, G., P. J. Stephens, C. J. Hewitt, J. R. Powell, and R. A. Badley. 2000. Analysis of bacterial function by multi-colour fluorescence flow cytometry and single sorting. J. Microbiol. Methods 42:97-114. [DOI] [PubMed] [Google Scholar]
- 11.O'Gara, J. P., M. Gomelsky, and S. Kaplan. 1997. Identification and molecular genetic analysis of multiple loci contributing to high-level tellurite resistance in Rhodobacter sphaeroides 2.4.1. Appl. Environ. Microbiol. 63:4713-4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rathgeber, C., N. Yurkova, E. Stackebrandt, J. T. Beatty, and V. Yurkov. 2002. Isolation of tellurite- and selenite-resistant bacteria from hydrothermal vents of the Juan de Fuca ridge in the Pacific ocean. Appl. Environ. Microbiol. 68:4613-4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reynolds, E. S. 1963. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 17:208-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rugolo, M., and D. Zannoni. 1983. Oxygen-induced inhibition of light-dependent uptake of tetraphenyl phosphonium ions as a probe of a direct interaction between photosynthetic and respiratory components in cells of Rhodopseudomonas capsulata. Biochem. Biophys. Res. Commun. 113:155-162. [DOI] [PubMed] [Google Scholar]
- 15.Schultz, J. E., and P. F. Weaver. 1982. Fermentation and anaerobic respiration by Rhodospirillum rubrum and Rhodopseudomonas capsulata. J. Bacteriol. 149:181-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steen, H. B., and T. Lindmo. 1979. Flow cytometry: a high resolution instrument for everyone. Science 204:403-404. [DOI] [PubMed] [Google Scholar]
- 17.Summers, A. O., and G. A. Jacoby. 1977. Plasmid-determined resistance to tellurium compounds. J. Bacteriol. 129:276-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Summers, A. O., and S. Silver. 1978. Microbial transformation of metals. Annu. Rev. Microbiol. 32:637-672. [DOI] [PubMed] [Google Scholar]
- 19.Taylor, D. E. 1999. Bacterial tellurite resistance. Trends Microbiol. 7:111-115. [DOI] [PubMed] [Google Scholar]
- 20.Taylor, D. E., Y. Hou, R. J. Turner, and J. H. Weiner. 1994. Location of a potassium tellurite resistance operon (tehAtehB) within the terminus of Escherichia coli K-12. J. Bacteriol. 176:2740-2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toptchieva, A., G. Sisson, L. J. Bryden, D. E. Taylor, and P. S. Hoffman. 2003. An inducible tellurite-resistance operon in Proteus mirabilis. Microbiology 149:1285-1295. [DOI] [PubMed] [Google Scholar]
- 22.Trutko, A. M., V. K. Akimenko, N. E. Suzina, L. A. Anisimova, M. G. Shlyapnikov, B. P. Baskunov, V. I. Duda, and A. M. Boronin. 2000. Involvement of the respiratory chain of Gram-negative bacteria in the reduction of tellurite. Arch. Microbiol. 173:178-186. [DOI] [PubMed] [Google Scholar]
- 23.Turner, R. J., J. H. Weiner, and D. E. Taylor. 1992. Use of diethyldithiocarbamate for quantitative determination of tellurite uptake by bacteria. Anal. Biochem. 204:292-295. [DOI] [PubMed] [Google Scholar]
- 24.Turner, R. J., J. H. Weiner, and D. E. Taylor. 1999. Tellurite-mediated thiol oxidation in Escherichia coli. Microbiology 145:2549-2557. [DOI] [PubMed] [Google Scholar]
- 25.Walter, E. G., and D. E. Taylor. 1992. Plasmid-mediated resistance to tellurite: expressed and cryptic. Plasmid 27:52-64. [DOI] [PubMed] [Google Scholar]
- 26.Weaver, P. F., J. D. Wall, and H. Gest. 1975. Characterization of Rhodopseudomonas capsulata. Arch. Microbiol. 105:207-216. [DOI] [PubMed] [Google Scholar]
- 27.Weinbauer, M. G., C. Beckmann, and M. G. Höfle. 1998. Utility of green fluorescent nucleic acid dyes and aluminum oxide membrane filters for rapid epifluorescence enumeration of soil and sediment bacteria. Appl. Environ. Microbiol. 64:5000-5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yurkov, V., J. Iappè, and A. Vermeglio. 1996. Tellurite resistance and reduction by obligately aerobic photosynthetic bacteria. Appl. Environ. Microbiol. 62:4195-4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ziglio, G., G. Andreottola, S. Barbesti, G. Boschetti, L. Bruni, P. Foladori, and R. Villa. 2002. Assessment of activated sludge viability with flow cytometry. Water Res. 36:460-468. [DOI] [PubMed] [Google Scholar]