Abstract
Diagnosis of acute hepatitis A virus (HAV) infection is based on the detection of HAV immunoglobulin M (IgM). However, IgM could be detected due to nonspecific polyclonal activation of the immune system. An avidity test for anti-HAV IgG was developed to distinguish acute infection, where low-avidity antibodies are detected, from immune reactivation. The assay was tested on 104 samples, including 11 sera from patients with past infection, 15 sera from patients with acute infection and 4 collected after recovery, 10 sera from vaccinated subjects, 4 sera from patients with suspected immune reactivation, and 60 unselected HAV-IgM positive sera, collected over 1 year in a routine laboratory. The avidity index (AI) was expressed as percentage. The results were provided as the mean ± one standard deviation. Patients with a history of prior infection had AIs of >70% (mean, 86% ± 10), whereas the mean AI was 36% ± 16 during acute HAV infection (P < 0.001). Within the first month after the onset of hepatitis, avidity was either noncalculable due to a very low IgG titer or <50%. In patients with immune reactivation, avidity was >70% (88% ± 10%), a finding consistent with a prior infection. Among the 60 unselected sera, 35 (58%) had a noncalculable or <50% avidity, and most of them had a detectable HAV RNA, confirming HAV infection. In contrast, 16 (27%) had an avidity of >70%, and none was reverse transcription-PCR positive, suggesting immune reactivation. These 16 patients were significantly older than the others (50 ± 16 years versus 26 ± 14 years). The new anti-HAV IgG avidity assay we developed could improve HAV infection diagnosis, particularly in elderly patients.
Hepatitis A virus (HAV) is the most common cause of acute viral hepatitis in the world (9). As a result of the improvement in public sanitation and hygiene conditions, there has been a striking reduction in HAV endemicity in western countries over the past few decades (10, 17). The shift from high to intermediate or low endemicity leads to a change in the age of individuals susceptible to hepatitis A, from children to adolescents or adults. In general, infection during childhood is asymptomatic or anicteric, whereas infection in adults is often more severe. Diagnosis of acute hepatitis A is based on the detection of the immunoglobulin M (IgM) antibody to HAV (HAV-IgM) in patients who present with clinical features of hepatitis. Nevertheless, since many cases of hepatitis A are asymptomatic, HAV-IgM can be found without clinical symptoms or biological abnormalities (18). IgM antibodies directed against specific viral antigens can be detected due to nonspecific polyclonal activation of memory cells from a previous infection with an unrelated agent. Immune cells may became activated during viral infections or immune diseases (1, 5, 12, 14). Thus, Anti-HAV IgM detection could also correspond to immune reactivation in some cases.
To confirm HAV infection in HAV-IgM-positive patients, HAV RNA can be tested for in blood and stools, but its detection can be transitory (15), and this test is not performed in regular laboratories. Therefore, complementary tests may be needed for the positive diagnosis of this infection. The measurement of the specific IgG avidity index (AI) has proven to be useful in a number of viral infections in immunocompetent patients, including rubella virus (8), cytomegalovirus (3), varicella-zoster virus (11), Epstein-Barr virus (2), and parvovirus B19 (16). Low-avidity antibodies are detected in cases of acute infection, whereas high-avidity antibodies are present in subjects with a history of prior infection. The normal immune response includes maturation from low- to high-avidity antibodies that are maintained for life. In the present study, we developed and analyzed the performance of an avidity test for HAV IgG antibodies.
MATERIALS AND METHODS
Serum samples.
The study was performed on 104 serum samples stored at −20°C until use. Sera were as follows. Group 1 consisted of 11 samples from patients known to be immune (they had had a prior HAV infection), i.e., they were total HAV antibody (HAV-IgG+IgM) positive and HAV-IgM negative. Group 2 consisted of 15 samples from patients with acute hepatitis A diagnosed on the basis of detectable HAV-IgM and clinical symptoms. Acute HAV infection was further confirmed by the detection of HAV RNA in serum in 10 of 12 tested cases. Group 3 was composed of four specimens from 3 of the previous 15 patients collected after recovery from acute HAV infection. The sera were sampled more than 2 months after the onset of symptoms and tested for avidity in the same assay as the early sample. Group 4 included 10 sera from HAV-vaccinated patients; these samples were obtained 3 to 84 months after vaccination. Group 4 was made up of 4 samples from patients with probable immune reactivation: HAV-IgM positive and negative for HAV viremia and hepatic cytolysis attributable to other causes than HAV. The clinical data for these patients were as follows. One male patient, 81 years old, also had detectable IgM to cytomegalovirus and Epstein-Barr virus, in the context of a chronic active hepatitis of indeterminate cause. Two of the patients, an 87-year-old man 87 and a 78-year-old woman, had mild hepatic cytolysis related to drug toxicity during antituberculosis treatment. HAV IgM also tested positive in sera sampled more than 1 year previously, and HAV viremia was negative. Finally, one 57-year-old woman presented with mild hepatic cytolysis, attributed to drug toxicity during the treatment of a hematological malignancy. The last group, group 6, included 60 sera collected between November 2002 and November 2003 that were positive for HAV-IgM supplied by a nonacademic routine laboratory; no clinical data were available for these sera.
Procedures. (i) HAV routine serology.
The sera of groups 1 to 4 were tested for HAV-IgG+IgM with the ETI-AB-HAVK-3 kit (DiaSorin, Sallugia, Italy) and for HAV-IgM with the Vidas IgM assay (bioMérieux, Marcy l'Etoile, France). The sera of group 5 were tested for HAV-IgM with the HAVAB-M version 2 AxSYM (Abbott Laboratories, Abbott Park, Ill.).
(ii) HAV IgG detection.
The current diagnostic assays for HAV antibodies are competition-based enzyme-linked immunosorbent assays (ELISAs). These assays detect both IgG and IgM and do not permit the measurement of avidity. We thus developed an in-house assay for anti-HAV IgG detection. Samples were tested by using ELISA plates coated with HAV antigens (VAI ELISA plates; Viral Antigen, Inc., Memphis, Tenn.). The procedure recommended by the manufacturer was followed. Briefly, samples and controls were diluted 1:21 with sample diluent provided by the manufacturer. Dilutions were dispensed in each well and incubated 30 min at room temperature. Plates were washed three times with wash buffer (1 M phosphate-buffered saline, 1% bovine serum albumin, 0.05% Tween 20), and then IgG conjugate was added (alkaline phosphatase-conjugated rabbit anti-human IgG diluted 1:500 in wash buffer) (Dakocytomation, Trappes, France). After 30 min of incubation at room temperature, the plates were washed three times with wash buffer and substrate was added (p-NPP [Sigma] diluted in 5 ml of 0.1 M diethanolamide [Sigma-Aldrich, Saint Quentin Fallavier, France]). After 15 min incubation at room temperature, stop solution (1 M NaOH) was added. The absorbance was read at 405 nm.
The cutoff value of the assay was set at three standard deviations (SDs) above the mean optical density values obtained with three sera, negative for both HAV-IgM and HAV-(IgG+IgM), included in each plate.
(iii) HAV-IgG avidity test.
To measure HAV-IgG avidity, samples were tested with the HAV-IgG test carried out as described above and, in parallel, by using a 6 M urea wash step after the first incubation as previously described (7). Each serum sample was incubated in duplicate, and then the wells were washed three times (5 min each) with wash buffer containing or not containing 6 M urea. A final fourth wash was done in all wells. The subsequent steps were performed according to the manufacturer's instructions.
The avidity index (AI) was expressed as a percentage as follows: AI = (the absorbance reading with urea wash/the absorbance reading without urea wash) × 100.
(iv) RT-PCR for HAV genome detection.
Patients with available stored sera were tested for HAV RNA. Viral RNA was extracted from 140 μl of serum by using a QIAmp Viral RNA kit (Qiagen Courtaboeuf, France). Ten microliters of extracted RNA was subjected to reverse transcription (RT) and PCR amplification by using a One-Step RT-PCR kit (Qiagen) with previously described primers (4). A 512-bp fragment encompassing the VP1/2A junction of the HAV genome was amplified. The sensitivity of the RT-PCR assays was 43 IU/ml as assessed on serial dilutions of the World Health Organization HAV RNA standard purchased from the National Institute for Biological Standards and Control (Hertfordshire, United Kingdom).
Statistical analysis.
The results are presented as means ± SDs. Pearson χ2 and Student t test were used for statistical analysis.
RESULTS
Characterized samples.
In 4 of 15 samples from acutely infected patients, the AIs could not be calculated due to very low IgG titers (below the cutoff). Three of these four patients could be tested for HAV RNA and were positive.
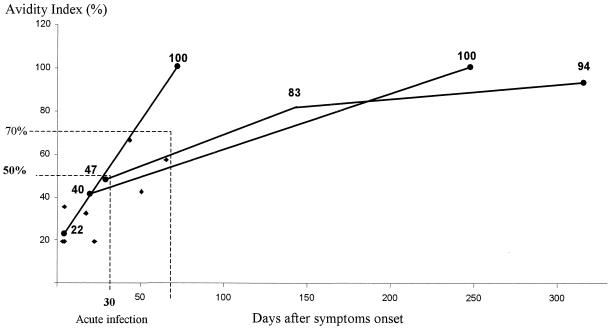
The AIs of the remaining 11 acutely infected patients are shown in Fig. 1. AIs were <50% (mean, 29% ± 12%) in 9 of 11 patients, 8 of 9 being tested within the 30 days after the onset of symptoms. Two patients sampled 60 and 45 days, respectively, after symptoms onset had AIs of 57 and 66% with a positive HAV RNA. HAV RNA could not be tested in 2 of the 11 cases. The AIs of these two cases were 22 and 19%, respectively. One of these two patients had a late specimen 2 months later, with an increase in AI from 22 to 100%. The increase of AI over time was evident for two other patients. An index of >70% was detected 2 to 6 months after disease onset.
FIG. 1.
AI values for 11 patients with acute infections according to the date of symptom onset. The evolution over time is indicated for three patients.
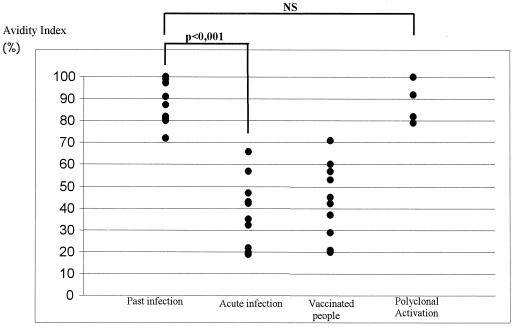
Avidity results according to clinical status are shown in Fig. 2. Samples from the 11 patients with a prior HAV infection showed AIs of >70% (mean, 86% ± 10%), whereas sera from the 11 patients with acute hepatitis had AIs ranging from 19 to 66% (mean, 36% ± 16%) (P < 0.001). Of the four patients with suspected immune reactivation, the AIs ranged from 79 to 100% (mean, 88% ± 10%). There was no statistical difference in avidity index between past infection and immune reactivation. For vaccinated people, the AIs ranged from 20 to 71% (mean, 44% ± 17%), regardless of the date of vaccination.
FIG. 2.
IgG anti-HAV AI according to clinical data.
Sixty unselected sera with HAV IgM-positive results.
The AIs were grouped according to the range of results (Table 1). Avidity could not be calculated for 19 patients (31%) due to IgG titers below the cutoff of the assay. Of these, HAV RNA could be tested in 15 patients and was positive in 13.
TABLE 1.
Age and HAV RNA results according to range of AI in 60 unselected HAV IgM-positive patients
| AI (%) | Mean age (yr [range]) | No. of patients (%) | HAV RNA (no. of patients)
|
||
|---|---|---|---|---|---|
| Positive | Negative | Not available | |||
| < Cutoff | 26 (6-53) | 19 (31) | 13 | 2 | 4 |
| <50 | 25 (8-61) | 16 (27) | 15 | 0 | 1 |
| 50-70 | 50 (12-93) | 9 (15) | 2 | 4 | 3 |
| >70 | 50 (19-78) | 16 (27) | 0 | 14 | 2 |
Sixteen of sixty patients (27%) had AIs of >70%. Among them, 14 were available for HAV RNA testing and were all found to be negative. In contrast, of the 25 samples with a calculable AI of <70%, 17 of the 21 sera (81%) available for HAV RNA testing were found to be positive (P < 0.001).
Nine samples (15%) had AIs between 50 and 70%. Two of six available for HAV RNA testing were positive (AIs of 50 and 51%), and four were negative (AI > 56%).
The mean age of the 16 patients with an AI of >70% was 50 ± 16 years, whereas the mean age of patients with an AI of <50% was 26 ± 14 years (P < 0.001).
DISCUSSION
For a variety of viruses, the specific IgG AI is known to help distinguish class M antibodies produced during primary infection from those produced due to the polyclonal stimulation of the immune system. In the present study we clearly show a statistically significant difference in anti-HAV IgG avidity between documented acute infection (mean = 36%) and prior infection (mean = 86%) (P < 0.001). In patients with documented immune reactivation, the mean avidity was 88%, which was no different from that of patients with a history of prior infection. Since the timing of the samples in relation to clinical illness onset was specified, the kinetics of avidity increase could be estimated. In the early days of infection, avidity was not calculable in some cases due to very low levels of anti-HAV IgG. When calculable, the avidity remained at <50% during the first month. Values of >50% could be detected shortly afterward in patients who were still viremic. Thus, we feel that values of <50% may be interpreted as corresponding to a recent infection and results of >70% may be interpreted as evidence of a prior infection. Results ranging from 50 to 70% should be interpreted as a gray zone of the avidity assay, since a protracted course of a recent HAV infection is possible, as shown by HAV RNA positivity in some of these cases.
The value of the measurement of the anti-HAV IgG avidity in improving the accurate diagnosis of acute HAV infection was further supported by the results obtained with serum samples from a routine laboratory. In this group, 27% of patients had an AI of >70%, suggesting a past infection, which is further supported by HAV RNA negativity. However, we have no idea why HAV IgM detection had been prescribed. In the absence of clinical data, a protracted course of recent HAV infection cannot be totally ruled out despite the negative viremia, but these cases probably correspond to immune reactivations. In contrast, all of the patients with AIs of <50% tested HAV RNA positive, thus confirming the diagnosis of acute hepatitis A.
Most clinicians understand that positive HAV-IgM results are an indication of acute hepatitis A. We show here that HAV IgM detection could correspond to immune reactivations in some cases and that this phenomenon was related to the patient's age. The proportion of probable HAV misdiagnosis may appear to be extremely high in the present series, but our finding is consistent with a recent French study showing that most people over the age of 50 years (82%) had antibodies to HAV (6). However, the proportion of immune reactivations might have been smaller with a different HAV IgM assay. This point needs to be further studied.
As we have seen, avidity may not be calculable in the early days of infection. Another shortcoming of the avidity assay is that interpretation of a low index is applicable only to natural infection. Indeed, in some vaccinated people, avidity remained low for a very long time after vaccination. This feature of vaccine-induced antibodies has already been shown for the rubella vaccine (13).
In conclusion, the hepatitis A IgG avidity assay we developed, if used along with conventional serological diagnosis, should help clinicians to diagnose cases of acute hepatitis A, especially in elderly patients.
Acknowledgments
We thank Richard Keros for revision of the English and Christine Hermet for technical assistance.
REFERENCES
- 1.Aalto, S. M., K. Linnavuori, H. Peltola, E. Vuori, B. Weissbrich, J. Schubert, L. Hedman, and K. Hedman. 1998. Immunoreactivation of Epstein-Barr virus due to cytomegalovirus primary infection. J. Med. Virol. 56:186-191. [PubMed] [Google Scholar]
- 2.Andersson, A., V. Vetter, L. Kreutzer, and G. Bauer. 1994. Avidities of IgG directed against viral capsid antigen or early antigen: useful markers for significant Epstein-Barr virus serology. J. Med. Virol. 43:238-244. [DOI] [PubMed] [Google Scholar]
- 3.Blackburn, N. K., T. G. Besselaar, B. D. Schoub, and K. F. O'Connell. 1991. Differentiation of primary cytomegalovirus infection from reactivation using the urea denaturation test for measuring antibody avidity. J. Med. Virol. 33:6-9. [DOI] [PubMed] [Google Scholar]
- 4.Bower, W. A., O. V. Nainan, X. Han, and H. S. Margolis. 2000. Duration of viremia in hepatitis A virus infection. J. Infect. Dis. 182:12-17. [DOI] [PubMed] [Google Scholar]
- 5.Castaneda-Ibarra, F., L. Ruiz-Maya, R. Campos-Rodriguez, and E. Garcia Latorre. 1991. Polyclonal activation of B lymphocytes in patients with amoebic hepatic abscess. Arch. Investig. Med. 22:13-17. [PubMed] [Google Scholar]
- 6.Denis, F., C. Delpeyroux, C. Debrock, S. Rogez, and S. Alain. 2003. Seroprevalence of hepatitis A in hospitalized patients in Limoges University Hospital. Gastroenterol. Clin. Biol. 27:727-731. (In French.) [PubMed] [Google Scholar]
- 7.Grangeot-Keros, L., M. J. Mayaux, P. Lebon, F. Freymuth, G. Eugene, R. Stricker, and E. Dussaix. 1997. Value of cytomegalovirus (CMV) IgG avidity index for the diagnosis of primary CMV infection in pregnant women. J. Infect. Dis. 175:944-946. [DOI] [PubMed] [Google Scholar]
- 8.Hedman, K., J. Hietala, A. Tiilikainen, A. L. Hartikainen-Sorri, K. Raiha, J. Suni, P. Vaananen, and M. Pietilainen. 1989. Maturation of immunoglobulin G avidity after rubella vaccination studied by an enzyme linked immunosorbent assay (avidity-ELISA) and by haemolysis typing. J. Med. Virol. 27:293-298. [DOI] [PubMed] [Google Scholar]
- 9.Hollinger, F., and J. Ticehurst. 1996. Hepatitis A virus, p. 735-782. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 10.Joussemet, M., P. Bourin, O. Lebot, G. Fabre, and R. Deloince. 1992. Evolution of hepatitis A antibodies prevalence in young French military recruits. Eur. J. Epidemiol. 8:289-291. [DOI] [PubMed] [Google Scholar]
- 11.Junker, A. K., and P. Tilley. 1994. Varicella-zoster virus antibody avidity and IgG-subclass patterns in children with recurrent chickenpox. J. Med. Virol. 43:119-124. [DOI] [PubMed] [Google Scholar]
- 12.Morgan-Capner, P., R. S. Tedder, and J. E. Mace. 1983. Reactivity for rubella-specific IgM in sera from patients with infectious mononucleosis. Lancet i:589. [DOI] [PubMed] [Google Scholar]
- 13.Nedeljkovic, J., T. Jovanovic, and C. Oker-Blom. 2001. Maturation of IgG avidity to individual rubella virus structural proteins. J. Clin. Virol. 22:47-54. [DOI] [PubMed] [Google Scholar]
- 14.Nobutoki, T., H. Hori, M. Higashigawa, E. Azuma, M. Sakurai, T. Yoshizumi, and T. Nunoue. 1996. A case of prolonged human parvovirus B19 DNA-emia associated with polyclonal B-cell activation. Acta Paediatr. Jpn. 38:348-351. [DOI] [PubMed] [Google Scholar]
- 15.Rezende, G., A. M. Roque-Afonso, D. Samuel, M. Gigou, E. Nicand, V. Ferre, E. Dussaix, H. Bismuth, and C. Feray. 2003. Viral and clinical factors associated with the fulminant course of hepatitis A infection. Hepatology 38:613-618. [DOI] [PubMed] [Google Scholar]
- 16.Soderlund, M., C. S. Brown, B. J. Cohen, and K. Hedman. 1995. Accurate serodiagnosis of B19 parvovirus infections by measurement of IgG avidity. J. Infect. Dis. 171:710-713. [DOI] [PubMed] [Google Scholar]
- 17.Stroffolini, T., R. D'Amelio, P. M. Matricardi, P. Chionne, A. Napoli, M. Rapicetta, S. Crateri, and P. Pasquini. 1993. The changing epidemiology of hepatitis A in Italy. Ital. J. Gastroenterol. 25:372-374. [PubMed] [Google Scholar]
- 18.Yang, N. Y., P. H. Yu, Z. X. Mao, N. L. Chen, S. A. Chai, and J. S. Mao. 1988. Inapparent infection of hepatitis A virus. Am. J. Epidemiol. 127:599-604. [PubMed] [Google Scholar]