Abstract
The opportunistic pathogens Rhizomucor pusillus and Rhizomucor miehei may be agents of frequently fatal mycotic diseases. In the present study, the susceptibilities of 27 clinical and environmental isolates of R. miehei and R. pusillus to lovastatin under different culturing conditions were investigated. Most of the R. miehei strains grew at lovastatin concentrations as high as 64 to 128 μg/ml. In contrast, the inhibitory effect of lovastatin on all of the R. pusillus strains was evident at lovastatin concentrations as low as 1 to 2 μg/ml. A simple and reliable method for species-level differentiation, based on the significantly higher sensitivity of R. pusillus to lovastatin than that of R. miehei, was elaborated. According this, on malt extract agar containing 6 μg of lovastatin/ml, R. pusillus is not able to produce colonies, while R. miehei will form compact colonies.
A growing number of susceptible hosts form a high-risk population for opportunistic fungal infections. Among these, zygomycosis comprises a diverse group of increasingly recognized mycotic diseases caused by some members of the orders Entomophthorales and Mucorales (e.g., Absidia, Mortierella, Rhizomucor, and Rhizopus [5]). In spite of the fact that these mycoses are in most cases limited to patients with a challenged and weakened immune system, they are attracting even more attention because of their frequently fatal outcome. The limited success of the current therapies mainly stems from the rapidly progressive nature of these infections and the low number of antimycotics that can be applied (3, 5, 10).
An expanding body of data is becoming available that demonstrates the opportunistic pathogenic role of the members of the genus Rhizomucor (1, 2, 4, 5, 9, 11, 16). This genus involves two ubiquitous species: R. pusillus and R. miehei. Their thermophilic nature and characteristic morphological features, such as the presence of stolons, rhizoids, and repeatedly branched sporangiophores, clearly distinguish them from most members of the Mucorales (7). At the same time, the identification of Rhizomucor isolates at a species level seems to be problematic, frequently resulting in misidentifications, or the species names remain to be determined.
There are several approaches that may be of help in the identification of Rhizomucor species, but all of these methodologies involve unresolved problems and limitations. Determination of the number of nuclei in the sporangiospores (12) or morphological characterization of the zygospores (the diameter is <50 μm and >50 μm for R. miehei and R. pusillus, respectively) (7, 15) has some potential in providing characters for species delimitation, but the results are not clear-cut in most cases. It is also known that homo- and heterothallic Rhizomucor strains do not group according to species: R. miehei involves only homothallic strains, but both hetero- and (rarely) homothallic R. pusillus isolates are known (7). Assimilation differences can also be used to differentiate these organisms: an inability to assimilate sucrose, glycine, phenylalanine, and β-alanine and the stimulative effect of thiamine on growth are known to be characteristic of R. miehei: however, these differences may be evaluated only with reference strains for comparison of the growth. Analyses of isoenzyme (7, 8, 13) and randomly amplified polymorphic DNA patterns (14) also provide markers for species identification, but they need multistep protocols requiring specialized equipment and expert knowledge.
Lovastatin (Chemical Abstracts Service registry no. 75330-75-5) is known to be a competitive inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A reductase, the key enzyme of the acetate mevalonate pathway. Formation of mevalonic acid is necessary for the biosynthesis of isoprenoid compounds (e.g., sterols and ubiquinone) and also the mevalonate-derived prenyl groups. The inhibition of sterol (and carotenoid) biosynthesis in fungi is accompanied by ultrastructural changes in the hyphal cells or inhibition of growth, depending on the lovastatin concentration (6).
In the present study, the susceptibilities of R. miehei and R. pusillus to lovastatin under different culturing conditions were investigated, and a simple and reliable method for the unambiguous differentiation of Rhizomucor species was devised.
The Rhizomucor strains used in this study, derived from single spores of clinical and environmental isolates (genetically homogenous strains), were subcultured on antimicrobial agent-free media (malt extract agar [MEA]) at 37°C for 3 days to ensure purity and viability. The strains were maintained on MEA slants at 4°C. The 27 strains examined involved 9 R. miehei isolates and 18 R. pusillus isolates (among them both homothallic and heterothallic isolates). The accuracy of the initial species identifications was checked rigorously via examination of morphological traits and physiological features (7) and by isoenzyme and randomly amplified polymorphic DNA analysis (13, 14). Spore counts were made in a hemocytometer, and the resulting suspensions were adjusted to the required sporangiospore number per milliliter (spores were suspended in sterile water during all of the study).
Lovastatin (Mevacor) was obtained from Merck Sharp Dohme (Haarlem, The Netherlands), and 200 mg of stock/ml was prepared in methanol. This was used to prepare a dilution plate series with MEA purchased from Fluka (Buchs, Germany). All chemicals were of analytical grade. Solidified media were air dried and inoculated with 3 μl of sporangiospore suspension as a small drop. Fungal growth was checked visually after incubation for 2 to 5 days at 37°C. The MIC corresponds to complete (100%) growth inhibition. Each test was carried out at least three times, performed in parallel.
In preliminary experiments, the effects of the culturing conditions were determined. Selected R. miehei and R. pusillus strains were grown under various pH conditions (pH range, 4 to 8) on different media containing lovastatin. The pH of the medium was adjusted with lactic acid. Under all conditions tested, the R. pusillus isolates proved to be more sensitive to lovastatin than the R. miehei isolates. It was shown that the inhibitory effect of lovastatin is stronger at lower pHs, and the inhibitory effect depends on the size of the inoculum: MICs were lower at lower sporangiospore concentrations (results not shown).
On the basis of these preliminary results, the 9 R. miehei strains and 18 R. pusillus strains were tested on MEA plates containing 0.2% lactic acid (pH 4) and lovastatin in the range of 1 to 128 μg/ml. MICs were determined after 2 days of incubation. Sporangiospores were inoculated in 3-μl droplets from spore suspensions with different concentrations (e.g., 106, 105, or 104 sporangiospores per ml). It was observed that with a 3-μl inoculum from a suspension of 106 sporangiospores per ml, most of the R. miehei strains grew at lovastatin concentrations as high as 64 to 128 μg/ml (depending on the strain), and all of them grew well at 8 μg of lovastatin/ml (Fig. 1). In contrast, the inhibitory effect of lovastatin on all of the R. pusillus strains was evident at concentrations as low as 1 to 2 μg/ml, and they hardly grew at all at 4 μg of lovastatin/ml. The MICs were significantly lower with a smaller inoculum size; however, the difference in sensitivities to lovastatin between the two species could be detected. When 3 μl of 104 sporangiospores per ml was inoculated, none of the R. pusillus isolates grew at a lovastatin concentration of >2 μg/ml, while only one R. miehei isolate apparently did not grow at a lovastatin concentration of 6 μg/ml (Fig. 2).
FIG. 1.
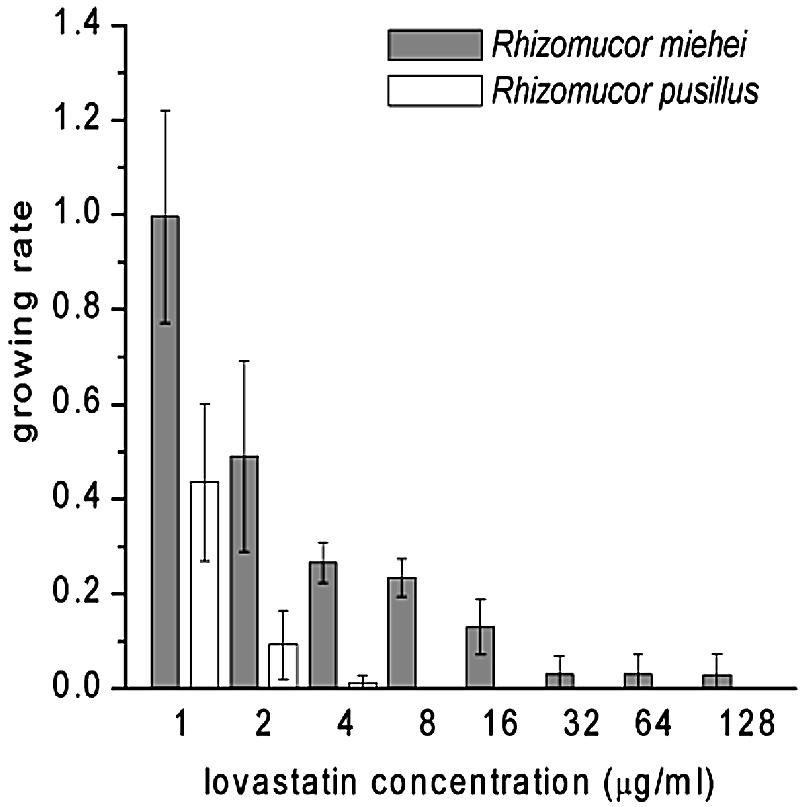
Graphical representation of the growth of R. miehei and R. pusillus on MEA containing different concentrations of lovastatin. Sporangiospores were inoculated in 3-μl droplets from suspensions of 106 sporangiospores per ml. Growth of both R. miehei and R. pusillus on MEA without lovastatin was considered to be a growing rate of 1.
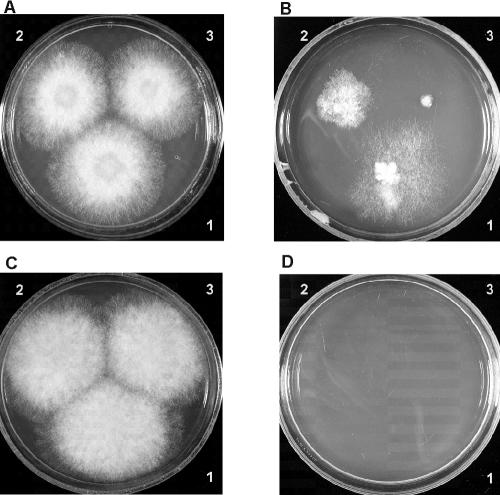
FIG. 2.
Growth of R. miehei and R. pusillus on MEA (plates A and C, respectively) and MEA supplemented with 6 μg of lovastatin/ml (plates B and D, respectively). Sporangiospores were inoculated in 3-μl droplets from a suspension of 106 (1), 105 (2), or 104 (3) sporangiospores per ml.
Accordingly, the recommended method for differentiation of the two species is as follows. After the inoculation of sporangiospores (in a 3-μl drop from a suspension of 106 sporangiospores per ml) on MEA containing 6 μg of lovastatin (pH 4; adjusted with lactic acid)/ml, the fungus is cultivated on 37°C for 2 days. R. miehei will produce more or less compact colonies, while the growth of R. pusillus will be blocked completely; evaluation is based on the presence or absence of colony formation. As a control, the sample should also be inoculated on MEA without lovastatin. Each of the 27 strains tested could be clearly identified by using this method. The difference in sensitivity to lovastatin provides a reliable method for the accurate differentiation of R. miehei from R. pusillus. The great advantage of this approach is its simplicity. With the usage of an R. pusillus strain and an R. miehei strain as reference strains (for example, ATCC 46342 and ATCC 16457, the type strains of R. pusillus and R. miehei, respectively), even the effect of the occasionally different qualities of the available lovastatin products might be excluded.
The molecular background of the different sensitivities of the two species is not yet known, but it could be rooted in the different copy numbers of the 3-hydroxy-3-methylglutaryl-coenzyme A reductase gene in their genomes. Experiments have been started in connection with this question.
Acknowledgments
This research was supported in part by grants from the Hungarian Scientific Research Fund (OTKA T37471 and F046658).
REFERENCES
- 1.Abbas, A. A. H., C. J. H. Fryer, and S. K. Felimban. 2002. Fatal rhinocerebral mucormycosis in a leukaemic child presenting with sudden loss of vision: a case report. Haema 5:341-344. [Google Scholar]
- 2.Björkholm, M., G. Runarsson, F. Celsing, M. Kalin, B. Petrini, and P. Engervall. 2001. Liposomal amphotericin B and surgery in the successful treatment of invasive pulmonary mucormycosis in a patient with acute T-lymphoblastic leukemia. Scand. J. Infect. Dis. 33:316-319. [DOI] [PubMed] [Google Scholar]
- 3.Eucker, J., O. Sezer, B. Graf, and K. Possinger. 2001. Mucormycoses. Mycoses 44:253-260. [PubMed] [Google Scholar]
- 4.Goldstein, M. F., D. J. Dvorin, E. H. Dunsky, R. W. Lesser, P. J. Heuman, and J. H. Loose. 1992. Allergic Rhizomucor sinusitis. J. Allergy Clin. Immunol. 90:394-404. [DOI] [PubMed] [Google Scholar]
- 5.Ribes, J. A., C. L. Vanover-Sams, and D. J. Baker. 2000. Zygomycetes in human disease. Clin. Microb. Rev. 13:236-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roze, L. V., and J. E. Linz. 1998. Lovastatin triggers an apoptosis-like cell death process in the fungus Mucor racemosus. Fungal Genet. Biol. 25:119-133. [DOI] [PubMed] [Google Scholar]
- 7.Schipper, M. A. A. 1978. On the genera Rhizomucor and Parasitella. Stud. Mycol. 17:53-71. [Google Scholar]
- 8.Scholer, H. J., E. Müller, and M. A. A. Schipper. 1983. Mucorales, p. 9-59. In D. H. Howard (ed.), Fungi pathogenic for humans and animals, part A: biology. Marcel Dekker, New York, N.Y.
- 9.Severo, L. C., F. Job, and T. C. Mattos. 1991. Systemic zygomycosis: nosocomial infection by Rhizomucor pusillus. Mycopathologia 113:79-80. [DOI] [PubMed] [Google Scholar]
- 10.Smitherman, K. O., and J. E. Peacock, Jr. 1995. Infectious emergencies in patients with diabetes mellitus. Med. Clin. N. Am. 79:53-77. [DOI] [PubMed] [Google Scholar]
- 11.St-Germain, G., A. Robert, M. Ishak, C. Tremblay, and S. Claveau. 1993. Infection due to Rhizomucor pusillus: report of four cases in patients with leukemia and review. Clin. Infect. Dis. 16:640-645. [DOI] [PubMed] [Google Scholar]
- 12.Tansey, M. R., S. M. Kamel, and R. Shamsai. 1984. The number of nuclei in sporangiospores of Rhizomucor species: taxonomic and biological significance. Mycologia 76:1089-1094. [Google Scholar]
- 13.Vastag, M., T. Papp, Zs. Kasza, and Cs. Vágvölgyi. 1998. Differentiation of Rhizomucor species by carbon source utilization and isoenzyme analysis. J. Clin. Microbiol. 36:2153-2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vastag, M., T. Papp, Zs. Kasza, and Cs. Vágvölgyi. 2000. Intraspecific variation in two species of Rhizomucor assessed by random amplified polymorphic DNA analysis. J. Basic Microbiol. 40:269-277. [DOI] [PubMed] [Google Scholar]
- 15.Weitzman, I., S. Whittier, J. C. McKitrick, and P. Della-Latta. 1995. Zygospores: the last word in identification of rare or atypical Zygomycetes isolated from clinical specimens. J. Clin. Microbiol. 33:781-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wickline, C. L., T. G. Cornitius, and T. Butler. 1989. Cellulitis caused by Rhizomucor pusillus in a diabetic patient receiving continuous insulin infusion pump therapy. S. Med. J. 82:1432-1434. [DOI] [PubMed] [Google Scholar]