Abstract
BK virus (BKV) load in urine alone or in combination with acute graft-versus-host disease (GVHD) was correlated to development of hemorrhagic cystitis (HC). BKV load in combination with acute GVHD discriminated the best, while BKV and viral load alone, but not GVHD, still showed predictive ability for HC.
BK virus (BKV) infection usually occurs in childhood and results in viral latency (11, 13, 20). BKV reactivation in hematopoietic stem cell transplant (SCT) patients has been suspected to be associated with late-onset (>2 weeks post-SCT) hemorrhagic cystitis (HC) (1, 2, 5). HC can occur as microscopic hematuria (grade I) or as gross hematuria with clots and urinary tract obstruction (grades II to IV) and may cause significant morbidity (6). Nevertheless, the incidence of HC in SCT patients is reported to be 5 to 40%, while BK viruria after SCT is detected in 50 to 100% of the patients (2, 4, 12, 17, 21, 23). Thus, BKV infection alone is not sufficient for inducing HC, and additional factors are necessary to predict patients at risk for HC. Acute graft-versus-host disease (GVHD), conditioning regimens, primary BKV infection, and specific BKV subtypes, including C→G mutations in the Sp1 transcription factor binding site, have been suggested to contribute to the development of HC (6, 9, 10, 15, 17, 18). However, it has been recently shown that SCT patients with HC excrete a significantly higher BK viral load in the urine than patients without HC (4, 7, 17). Nonetheless, wide individual variations in BKV load are observed among both HC and non-HC patients (19). In this pilot study, urine samples were collected from SCT patients at different time points after transplantation to determine the temporal relationship between HC and BK viruria, viral load, GVHD, and conditioning regimen. Information on the patients' clinical courses was collected retrospectively.
Thirty-one allogeneic hematopoietic SCT patients, 18 children and 13 adults, who consecutively received transplants at Huddinge University Hospital during 2002 and 2003 and who volunteered were included in this study (conducted according to ethical permission 433/98 from Karolinska Institutet). In total, 127 urine samples (1 to 28 samples/patient) were collected 1 week before to 12 months after SCT. Six patients had a documented episode of HC, and grading was applied according to the method of Bedi et al. (6). Patient diagnosis, sex, conditioning regimens, presence and grade of acute GVHD (22), and HC and BKV status were investigated. Urine samples were tested for BKV DNA by a nested PCR (8, 14), and all BKV-positive samples were tested for BK viral load (number of BKV copies per microliter of urine) by a quantitative real-time PCR (Q-RT-PCR) (19). Statistical calculations were made by chi-square exact testing.
BKV DNA was detected by nested PCR and Q-RT-PCR in the urine samples of 16 of 31 (52%) SCT patients, and 6 of these patients, all BKV positive, developed HC (Table 1). For one HC patient, samples were not available from the period before the onset of or during HC, but BKV DNA was detected in a sample taken after resolution of HC. All 5 of the remaining 5 HC patients had BK viruria before onset of HC, compared to 10 of 25 non-HC patients, indicating that BKV DNA (detected by nested PCR) in the urine of HC patients before the onset of HC is more common than BK viruria in non-HC patients (P = 0.04). However, detection of BKV in the urine of immunosuppressed patients is a common sign of BKV reactivation in both HC and non-HC patients and alone is not very useful as a predictive factor for HC development (2, 4, 6, 9, 10, 12, 15, 17, 18, 21).
TABLE 1.
HC, BKV, viral load, and GVHD after allogeneic SCT
| Patient group | No. of patients with indicated condition/ total no. of patients in group
|
||||
|---|---|---|---|---|---|
| Totala | BKV | >106 BKV copies/μl of urine | GVHD | >106 BKV copies/ μl of urine + GVHD I-IV | |
| HC | 6/31 | 6/6b | 4/5b | 6/6 | 4/5b |
| Non-HC | 25/31 | 10/25 | 3/25 | 12/25 | 2/25 |
| Total | 31/31 | 16/31 | 7/30b | 18/31 | 7/30b |
There were 9 patients with acute lymphoblastic leukemia, 7 patients with acute myeloid leukemia (AML), 4 patients with myelodisplastic syndrome/AML, 2 patients with chronic myelogenous leukemia, 2 patients with severe aplastic anemia, 2 patients with Fanconi anemia, and 1 patient each with paroxysmal nocturnal hemaglobinuria, Klatskin tumor in liver, liver cancer, kidney cancer, and prostate cancer.
For one HC patient, urine samples were available only after HC resolution.
A better predictive correlation was obtained by Q-RT-PCR, using an arbitrary BKV load of >106 BKV copies/μl of urine. Four of five patients excreted >106 BKV copies/μl of urine 2 to 13 days before HC onset compared to 3 of 25 without HC (P = 0.01) (Table 1). The fifth HC patient had two BKV-positive samples before HC onset, which not could be evaluated by Q-RT-PCR. The following sample collected during HC had >106 BKV copies/μl of urine. Thus, viral load may be more useful in clinical practice than only detection of BKV. This is in line with previous studies showing the frequent presence of a high BK viral load in HC patients (4, 7, 17, 19).
During the study period, acute GVHD grades II to IV were more common in HC patients (5 of 6) than in non-HC patients (5 of 25) (P = 0.008) or BKV-positive non-HC patients (3 of 10) (P = 0.014), but there was no correlation between HC and the development of GVHD grades I to IV (P = 0.18). A correlation between HC and more-severe GVHD has been described previously but has not been useful for HC prediction in clinical practice (16, 18).
Notably, as described elsewhere an overrepresentation (P = 0.028) of men in the HC group, 6 of 6 compared to 12 of 25 in the non-HC group, was observed (3).
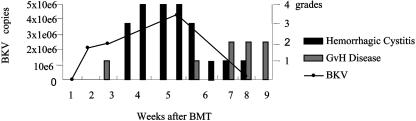
Considering the period before and the day of HC onset, the best correlation (P = 0.003) to the development of HC was found by comparing the combined occurrence of >106 BKV copies/μl of urine and acute GVHD I to IV in HC patients (4 of 5) and non-HC patients (2 of 25) (Table 1). This is illustrated for a typical HC patient in Fig. 1. Four of five HC patients had >106 BKV copies/μl of urine before HC onset, and three of these also had acute GVHD 5 to 19 days before HC onset, while the fourth HC patient presented GVHD grade II on the day of HC onset. The fifth HC patient, for whom the BKV load could not be determined before the onset of HC, developed acute GVHD grade II 1 week after the onset of HC.
FIG. 1.
BK viral load (number of BKV copies per microliter of urine), GVHD, and HC in one HC patient 0 to 9 weeks after SCT.
In addition, a predictive correlation to HC development was also obtained when high BKV load (>106 BKV copies/μl of urine) was present in combination with acute GVHD I to IV before the onset of HC (P = 0.02) in HC patients (3 of 5), in contrast to non-HC patients (2 of 25). Moreover, the occurrence of BKV independent of viral load with acute GVHD before the onset of HC differed significantly between HC patients (4 of 5) and non-HC patients (5 of 25; P = 0.02). It is possible that anti-GVHD treatment with immunosuppressive drugs allows for BKV reactivation and possibly also a large virus load. The conditioning regimen and the time to engraftment did not affect the occurrence of HC.
To conclude, this pilot study indicates that BKV DNA and particularly >106 BKV copies/μl of urine from SCT patients may have some predictive ability for HC. However, the best association to HC was achieved when a viral load of >106 BKV copies/μl of urine was present in combination with acute GVHD.
Acknowledgments
We thank the patients and the staff at the Centre of Allogeneic Stem Cell Transplantation, the Departments of Haematology and Paediatrics, and Bo Nilsson, Radiumhemmet, at the Karolinska for statistical calculations.
The Children's Cancer Foundation, the Swedish Cancer Foundation, the Stockholm Society for Cancer Research, the Stockholm County Council, and the Karolinska Institute are acknowledged for financial support.
REFERENCES
- 1.Apperley, J. F., S. J. Rice, J. A. Bishop, Y. C. Chia, T. Krausz, S. D. Gardner, and J. M. Goldman. 1987. Late-onset hemorrhagic cystitis associated with urinary excretion of polyomaviruses after bone marrow transplantation. Transplantation 43:108-112. [DOI] [PubMed] [Google Scholar]
- 2.Arthur, R. R., K. V. Shah, S. J. Baust, G. W. Santos, and R. Saral. 1986. Association of BK viruria with hemorrhagic cystitis in recipients of bone marrow transplants. N. Engl. J. Med. 315:230-234. [DOI] [PubMed] [Google Scholar]
- 3.Asano, Y., Y. Kanda, N. Ogawa, M. Sakata-Yanagimoto, M. Nakagawa, M. Kawazu, S. Goyama, K. Kandabashi, K. Izutsu, Y. Imai, A. Hangaishi, M. Kurokawa, S. Tsujino, S. Ogawa, K. Aoki, S. Chiba, T. Motokura, and H. Hirai. 2003. Male predominance among Japanese adult patients with late-onset hemorrhagic cystitis after hematopoietic stem cell transplantation. Bone Marrow Transplant. 32:1175-1179. [DOI] [PubMed] [Google Scholar]
- 4.Azzi, A., S. Cesaro, D. Laszlo, K. Zakrzewska, S. Ciappi, R. De Santis, R. Fanci, G. Pesavento, E. Calore, and A. Bosi. 1999. Human polyomavirus BK (BKV) load and haemorrhagic cystitis in bone marrow transplantation patients. J. Clin. Virol. 14:79-86. [DOI] [PubMed] [Google Scholar]
- 5.Azzi, A., R. Fanci, A. Bosi, S. Ciappi, K. Zakrzewska, R. de Santis, D. Laszlo, S. Guidi, R. Saccardi, A. M. Vannucchi, et al. 1994. Monitoring of polyomavirus BK viruria in bone marrow transplantation patients by DNA hybridization assay and by polymerase chain reaction: an approach to assess the relationship between BK viruria and hemorrhagic cystitis. Bone Marrow Transplant. 14:235-240. [PubMed] [Google Scholar]
- 6.Bedi, A., C. B. Miller, J. L. Hanson, S. Goodman, R. F. Ambinder, P. Charache, R. R. Arthur, and R. J. Jones. 1995. Association of BK virus with failure of prophylaxis against hemorrhagic cystitis following bone marrow transplantation. J. Clin. Oncol. 13:1103-1109. [DOI] [PubMed] [Google Scholar]
- 7.Biel, S. S., T. K. Held, O. Landt, M. Niedrig, H. R. Gelderblom, W. Siegert, and A. Nitsche. 2000. Rapid quantification and differentiation of human polyomavirus DNA in undiluted urine from patients after bone marrow transplantation. J. Clin. Microbiol. 38:3689-3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bogdanovic, G., M. Brytting, P. Cinque, M. Grandien, E. Fridell, P. Ljungman, B. Lönnqvist, and A.-L. Hammarin. 1994. Nested PCR for detection of BK virus and JV virus DNA. Clin. Diagn. Virol. 2:211-220. [DOI] [PubMed] [Google Scholar]
- 9.Bogdanovic, G., P. Ljungman, F. Wang, and T. Dalianis. 1996. Presence of human polyomavirus DNA in the peripheral circulation of bone marrow transplant patients with and without hemorrhagic cystitis. Bone Marrow Transplant. 17:573-576. [PubMed] [Google Scholar]
- 10.Bogdanovic, G., P. Priftakis, B. Taemmeraes, A. Gustafsson, T. Flaegstad, J. Winiarski, and T. Dalianis. 1998. Primary BK virus (BKV) infection due to possible BKV transmission during bone marrow transplantation is not the major cause of hemorrhagic cystitis in transplanted children. Pediatr. Transplant. 2:288-293. [PubMed] [Google Scholar]
- 11.Chesters, P. M., J. Heritage, and D. J. McCance. 1983. Persistence of DNA sequences of BK virus and JC virus in normal human tissues and in diseased tissues. J. Infect. Dis. 147:676-684. [DOI] [PubMed] [Google Scholar]
- 12.Childs, R., C. Sanchez, H. Engler, J. Preuss, S. Rosenfeld, C. Dunbar, F. van Rhee, M. Plante, S. Phang, and A. J. Barrett. 1998. High incidence of adeno- and polyomavirus-induced hemorrhagic cystitis in bone marrow allotransplantation for hematological malignancy following T cell depletion and cyclosporine. Bone Marrow Transplant. 22:889-893. [DOI] [PubMed] [Google Scholar]
- 13.Dorries, R., H. Imrich, A. Hein, S. Czub, and S. Schwender. 1994. The impact of the intracerebral antibody response on the clinical course of a virus-induced demyelination in a rat model system. J. Neurol. Neurosurg. Psychiatry 57(Suppl.):18-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hammarin, A. L., G. Bogdanovic, V. Svedhem, R. Pirskanen, L. Morfeldt, and M. Grandien. 1996. Analysis of PCR as a tool for detection of JC virus DNA in cerebrospinal fluid for diagnosis of progressive multifocal leukoencephalopathy. J. Clin. Microbiol. 34:2929-2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin, L., V. Pietropaolo, J. C. Booth, K. H. Ward, and D. W. Brown. 1995. Prevalence and distribution of BK virus subtypes in healthy people and immunocompromised patients detected by PCR-restriction enzyme. Clin. Diagn. Virol. 3:285-295. [DOI] [PubMed] [Google Scholar]
- 16.Leung, A. Y., R. Mak, A. K. Lie, K. Y. Yuen, V. C. Cheng, R. Liang, and Y. L. Kwong. 2002. Clinicopathological features and risk factors of clinically overt haemorrhagic cystitis complicating bone marrow transplantation. Bone Marrow Transplant. 29:509-513. [DOI] [PubMed] [Google Scholar]
- 17.Leung, A. Y., C. K. Suen, A. K. Lie, R. H. Liang, K. Y. Yuen, and Y. L. Kwong. 2001. Quantification of polyoma BK viruria in hemorrhagic cystitis complicating bone marrow transplantation. Blood 98:1971-1978. [DOI] [PubMed] [Google Scholar]
- 18.Ost, L., B. Lonnqvist, L. Eriksson, P. Ljungman, and O. Ringden. 1987. Hemorrhagic cystitis—a manifestation of graft versus host disease? Bone Marrow Transplant. 2:19-25. [PubMed] [Google Scholar]
- 19.Priftakis, P., G. Bogdanovic, P. Kokhaei, H. Mellstedt, and T. Dalianis. 2003. BK virus (BKV) quantification in urine samples of bone marrow transplanted patients is helpful for diagnosis of hemorrhagic cystitis, although wide individual variations exist. J. Clin. Virol. 26:71-77. [DOI] [PubMed] [Google Scholar]
- 20.Reploeg, M. D., G. A. Storch, and D. B. Clifford. 2001. BK virus: a clinical review. Clin. Infect. Dis. 33:191-202. [DOI] [PubMed] [Google Scholar]
- 21.Seber, A., X. O. Shu, T. Defor, S. Sencer, and N. Ramsay. 1999. Risk factors for severe hemorrhagic cystitis following BMT. Bone Marrow Transplant. 23:35-40. [DOI] [PubMed] [Google Scholar]
- 22.Thomas, E. D., R. Storb, R. A. Clift, A. Fefer, L. Johnson, P. E. Neiman, K. G. Lerner, H. Glucksberg, and C. D. Buckner. 1975. Bone-marrow transplantation (second of two parts). N. Engl. J. Med. 292:895-902. [DOI] [PubMed] [Google Scholar]
- 23.Vogeli, T. A., F. Peinemann, S. Burdach, and R. Ackermann. 1999. Urological treatment and clinical course of BK polyomavirus-associated hemorrhagic cystitis in children after bone marrow transplantation. Eur. Urol. 36:252-257. [DOI] [PubMed] [Google Scholar]