Abstract
A sensitive NO2− biosensor that is based on bacterial reduction of NO2− to N2O and subsequent detection of the N2O by a built-in electrochemical N2O sensor was developed. Four different denitrifying organisms lacking NO3− reductase activity were assessed for use in the biosensor. The relevant physiological aspects examined included denitrifying characteristics, growth rate, NO2− tolerance, and temperature and salinity effects on the growth rate. Two organisms were successfully used in the biosensor. The preferred organism was Stenotrophomonas nitritireducens, which is an organism with a denitrifying pathway deficient in both NO3− and N2O reductases. Alternatively Alcaligenes faecalis could be used when acetylene was added to inhibit its N2O reductase. The macroscale biosensors constructed exhibited a linear NO2− response at concentrations up to 1 to 2 mM. The detection limit was around 1 μM NO2−, and the 90% response time was 0.5 to 3 min. The sensor signal was specific for NO2−, and interference was observed only with NH2OH, NO, N2O, and H2S. The sensor signal was affected by changes in temperature and salinity, and calibration had to be performed in a system with a temperature and an ionic strength comparable to those of the medium analyzed. A broad range of water bodies could be analyzed with the biosensor, including freshwater systems, marine systems, and oxic-anoxic wastewaters. The NO2− biosensor was successfully used for long-term online monitoring in wastewater. Microscale versions of the NO2− biosensor were constructed and used to measure NO2− profiles in marine sediment.
Nitrite (NO2−) has a central position in the global nitrogen (N) cycle and is involved in many important biological N transformations. With an intermediate oxidation state, NO2− acts as an electron donor in nitrification and serves as an electron acceptor in denitrification, dissimilative nitrite reduction to ammonium, and anammox.
In natural ecosystems NO2− is of interest because of its toxicity for microorganisms and higher organisms (20, 37). NO2− concentrations in natural bulk water bodies are usually very low, and in freshwater systems the average worldwide NO2− concentration has been estimated to be about 1 μg of N liter−1 (∼0.07 μM NO2−) (28). Recently, however, there have been several reports of NO2− accumulation to concentrations of 5 to 140 μM in European eutrophic rivers and estuaries (3, 8, 15, 19, 44). Studies have indicated that NO2− accumulations can be related to imbalances in NO2− production and consumption rates for either aerobic sediment processes (nitrification) or anaerobic sediment processes (denitrification and dissimilatory nitrate reduction to ammonia). In marine systems NO2− concentrations are normally negligible, but concentrations of 0.3 to 2.5 μM have been reported near the oxic-anoxic boundary of stratified marine water bodies (5, 22). The distribution of NO2− in sediments is largely uncharacterized, except for a few studies that have documented NO2− accumulations in freshwater sediments (19, 38, 39, 40). The recent discovery of high anammox rates in marine sediments (6) has caused increased interest in NO2− in sediments.
Generally, relatively little attention has been directed towards descriptions of NO2− concentrations in wastewaters; the main emphasis has been on the status of ammonium (NH4+) and nitrate (NO3−). In wastewater treatment N removal is traditionally performed by degradation of the organic compounds, followed by oxidation (nitrification) of the liberated NH4+ to NO3− and subsequent reduction (denitrification) of the NO3− produced to free nitrogen (N2). During this type of oxic-anoxic cycling, transient NO2− accumulations have been found and were recognized to be a result of imbalanced nitrification and denitrification processes (29). Inhibitory effects of high NO2− concentrations have been observed for different reactor processes, like nitrification, denitrification, and biological phosphate uptake (2, 13, 27, 43). Accumulation of NO2− is also presumed to promote release of the greenhouse gas nitrous oxide (N2O) from wastewaters (12, 16). Recently, the research focus has been on development and control of partial nitrification to NO2− (14, 33, 42) instead of full nitrification to NO3−. Compared to full nitrification-denitrification processes, the implementation of partial nitrification-anammox processes is expected to significantly decrease energetic and economic costs of N removal from high-ammonium wastewaters (17, 36).
Several different types of NO2− sensors have been developed. Examples include a liquid ion-exchange sensor (7) and a sensor based on an immobilized enzyme (35). However, as tools for online measurement of NO2− in wastewater or microscale analysis of NO2− in marine sediment, these types of sensors have distinct limitations. A specific NOx− biosensor for environmental analysis, including wastewater monitoring, has been described previously (24, 26, 29); this biosensor is based on bacterial reduction of NO2− and NO3− to N2O, followed by electrochemical N2O detection. The strain used in the NOx− biosensor has a truncated denitrifying pathway with termination at N2O instead of N2. If we could identify a suitable bacterium that also lacks a nitrate reductase, it should be possible to make an NO2− biosensor. Apparently, very few bacterial strains have such a restricted denitrifying pathway, and only three such isolates have been described (10). In this paper we describe a new NO2− biosensor based on one of these strains and compare this sensor with a sensor based on Alcaligenes faecalis, which may be used when acetylene is added to block its N2O reductase (45).
MATERIALS AND METHODS
Macroscale biosensor construction.
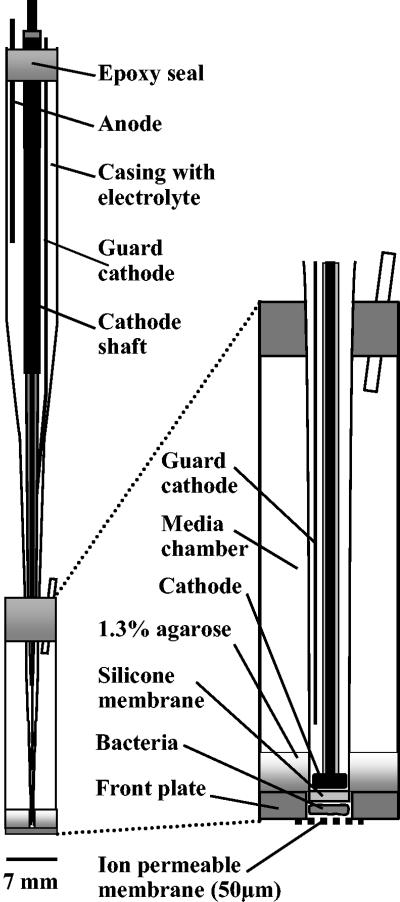
The macroscale NO2− biosensor functioned according to the same principles as those previously described for a microscale NOx− biosensor (23) and was in most respects identical to the commercially available NOx− biosensor (Unisense, Aarhus, Denmark). The sensor consisted of two major compartments (Fig. 1). A Clark-type N2O sensor (Unisense) with a tip diameter of about 0.275 mm was positioned behind a reaction chamber with an inner diameter of 0.3 mm. The bacterial chamber was drilled in a 0.35-mm-thick polyester plate that was subsequently glued onto a 6-mm-inside-diameter glass tube. The two compartments were glued together so that the N2O sensor partly protruded into the hole of the plate, creating a small, 0.15- to 0.3-mm deep (∼0.01- to 0.02-mm3) reaction chamber in front of the sensor tip. Bacterial biomass for the sensor was obtained by aerobic growth on a tryptic soy broth (TSB) agar plate. The culture was collected from the plate with a sterile glass rod and subsequently inoculated into the reaction chamber from the front of the sensor through a very thin plastic tube (diameter, 0.1 to 0.2 mm) made by heat drawing a plastic syringe (125-μl Distritip syringe; Gilson). To help retain the culture in the reaction chamber, a 1.3% agarose solution was placed behind the bacterial biomass. To separate the bacterial biomass from the outside environment, a 50-μm-thick ion-permeable membrane (Unisense) was attached to the front plate by using double-adhesive tape. A large medium reservoir (∼1,000 mm3) supplied the culture with all essential growth constituents except the electron acceptor. The standard growth medium inside the sensor contained 4 g of sodium acetate liter−1, 5 g of sodium citrate · 2H2O liter−1, 0.5 g of TSB (Difco) liter−1, 0.1 g of (NH4)2SO4 liter−1, and 3.3 g of Na2WO4 liter−1 (∼10 mM). The pH was adjusted to 6.9. Tetracycline (20 mg liter−1) was added to the growth medium when Stenotrophomonas nitritireducens was used. Acetylene-saturated growth medium was used when the organism was A. faecalis. A sterilization procedure in which propylene oxide was the sterilizing agent was used to minimize the frequency of contamination with other bacteria, as follows: (i) droplets of propylene oxide were added to the bacterial chamber and allowed to evaporate; (ii) pieces of ion-permeable membrane were bathed in a solution of 10% propylene oxide in water for 24 h before use; and (iii) the glue surface of the sensor tip was exposed to a 10% propylene oxide solution for 4 to 6 h while it was coated with a 5-μm-thick polyethylene film. All work with the highly toxic and volatile compound propylene oxide was performed in a fume hood.
FIG. 1.
Macroscale NO2− biosensor based on bacterial reduction of NO2− to N2O and subsequent detection by a built-in electrochemical N2O sensor. The entire sensor is shown on the left. An enlargement of the sensor tip region is shown on the right.
Microscale biosensors.
Microscale biosensors were constructed as described by Larsen et al. (23) and were made with tips as large as 60 μm to enable analysis of low NO2− concentrations (<5 μM). They were operated with electrophoretic sensitivity control (ESC) (21), which means that a positive tip potential of 0.5 V versus a standard calomel reference electrode was applied to facilitate entry of the negatively charged NO2− ions into the sensor. The bacterial chambers of the microscale biosensors were not sterilized, and the sensors usually functioned for only a few days.
Sensor principle.
The detection of NO2− by the biosensor is based on bacterial reduction of NO2− to N2O and subsequent detection of this N2O by the built-in electrochemical N2O sensor. NO2− diffuses freely through the ion-permeable membrane into the bacterial biomass of the reaction chamber, where it is denitrified to N2O and subsequently reduced to N2 at the cathode of the N2O sensor. There is a linear correlation between the amount of NO2− reduced and the magnitude of the sensor signal. The N2O sensor is sensitive to O2, but bacterial respiration in the outer oxic parts of the chamber prevents O2 from reaching the cathode. Bacterial biomass in the deeper, anoxic parts of the chamber is responsible for the reduction of NO2− to N2O, and the denitrifying capacity of the biomass determines the linear range of the sensor.
Physiological characteristics of bacterial strains.
Three organisms (S. nitritireducens, Luteimonas mephitis, and Pseudoxanthomonas broegbernensis) that can be obtained from Deutsche Sammlung von Mikroorganismen und Zellkulturen are all characterized by a denitrifying pathway deficient in both NO3− and N2O reductases (10). These three organisms were tested for use in an NO2− biosensor together with the denitrifying organism A. faecalis, which is deficient in an NO3− reductase but has an active N2O reductase (31, 32). Batch cultures with complex media containing 10 g of TSB liter−1 plus 1 mM NO3− and batch cultures with 10 g of TSB liter−1 plus 1 mM NO2− incubated under anoxic conditions were used to test the capacity for NO3− and N2O reduction, respectively, in order to confirm previously described denitrifying pathways. An N2O sensor (1) was used to measure accumulations of N2O in the batch cultures. NO2− tolerance was examined by anoxic incubation of batch cultures (10 g of TSB liter−1) in the presence of 0, 2, 5, and 10 mM NO2−. The pH growth range was evaluated by oxic incubation on agar plates at pH 5 to 9. Detailed physiological studies of growth-temperature and growth-salinity relationships were performed with S. nitritireducens and A. faecalis. The growth rate was examined at a temperature range of 15 to 42.5°C and at a salinity range of 0 to 90 g of NaCl liter−1 (in addition to the salts present in TSB). The growth rates of L. mephitis and P. broegbernensis were determined only at 30°C and with no NaCl. For each growth condition three replicates of 500-ml Erlenmeyer bottles containing 200 ml of complex medium (10 g of TSB liter−1) were used. After addition of a small inoculum, the bottles were placed in an incubator shaker (Innova 4330 refrigerated incubator shaker; New Brunswick Scientific) and kept at a constant temperature with continuous stirring (120 rpm). The bacterial density (optical density at 600 nm) was measured regularly to determine the growth rate. Prior to salinity experiments the cultures used for inocula were acclimatized to the experimental conditions to prevent a long lag phase due to osmotic stress.
Sensor characteristics.
Macrosensor characteristics and function were examined most extensively for biosensors with S. nitritireducens, but most characteristics were also tested for sensors constructed with A. faecalis. An experimental setup in which several biosensors could be tested simultaneously was used. The setup consisted of a plastic container holding 2 liters of water with control of the temperature, O2 level, turbulence, and concentrations of various chemical species. Calibrations were performed to determine the linearity, linear range, response time, and sensitivity. The effects of various parameters on the sensor signal were evaluated; these parameters included temperature, O2 level, salinity, turbulence, and pH. Interference with the NO2− signal was tested for a range of environmentally relevant compounds, including NO3−, NO, N2O, NH4+, NH2OH, and H2S. Except for a linearity check, no investigations of microsensor performance were conducted as similar characteristics were expected.
Biosensor method versus standard analytical method.
Activated sludge from a municipal wastewater treatment plant was placed in a 2-liter laboratory-scale reactor and exposed to oxic-anoxic cycles. Two NO2− biosensors, one NOx− biosensor (Unisense), and one Clark-type O2 minisensor (Unisense) supplied online information about chemical species in the sludge. Sensor signals were recorded continuously by using a data logger (ADC-16; Pico Technology) connected to a laptop computer. Calibrations of NO2− and NOx− biosensors were performed in 100 ml of reactor water that was previously separated from the biomass fraction by centrifugation and filtration. NO2− and NO3− were added from 100 mM stock solutions to final concentrations of 100 μM. During the oxic-anoxic cycle, sludge samples were obtained from the reactor at 5-min intervals, rapidly filtered through 0.22-μm-pore-size filters (CAMEO 25AS; Osmonics), and immediately frozen. NO2− concentrations in the samples were subsequently determined with a biosensor and also by spectrophotometry (30). Sensor measurements of NO2− levels were obtained at a controlled temperature in a small glass container with constant stirring. To increase the small volumes of filtrate obtained, 2-ml samples were mixed with 2 ml of a 1-g liter−1 NaCl solution. Sensor calibration was performed at a similar salinity by using 2 ml of sterile filtered wastewater (without NO2−) and 2 ml of an NaCl solution.
Environmental monitoring.
Macroscale NO2− biosensors were used to monitor NO2− concentrations in a 1,000-liter pilot-scale wastewater treatment plant with activated sludge during a 3-week period. The plant was operated with intermittent oxic-anoxic cycles. Microscale versions of the NO2− biosensor were used to analyze the NO2− distribution in sediment from the Wadden Sea (Germany) in a laboratory setup.
RESULTS AND DISCUSSION
Our research goal was to develop a long-term stable macroscale NO2− biosensor with a lifetime on the order of months that is suitable for laboratory analysis and online monitoring of wastewater. In addition, we also wanted to use the technology for construction of an NO2− microscale biosensor for profiling biofilms and sediments. A successful outcome of our efforts was expected due to the availability of a stable electrochemical N2O sensor (1) that has previously been used in well-functioning NOx− biosensors.
Physiological characteristics of bacterial strains.
In a preliminary evaluation of the four bacterial strains available for the NO2− biosensor, the two most important properties were denitrification characteristics and growth rate. The objective was to identify the most suitable strain, ideally a strain with a very restricted denitrifying pathway limited to the reduction of NO2− to N2O. Alternatively, successful use of a strain with N2O reductase activity should also have been possible when acetylene was added to the sensor interior to block N2O reductase activity (45). Tests with batch cultures supplemented with NO2− or NO3− confirmed previously described denitrification pathways (Table 1). A high respiration rate of the culture used was equally important to ensure complete consumption of O2 and to give the sensor a wide linear range for NO2−. S. nitritireducens and A. faecalis (Table 1), which were characterized by growth rates that were significantly higher than those of the other two organisms, were selected as the most promising candidates for the biosensor. The linear range for similar NOx− biosensors does not exceed 2 mM at room temperature, so the NO2− tolerance of up to 5 mM observed for S. nitritireducens was more than sufficient for any relevant measuring range. A. faecalis exhibited even higher NO2− tolerance.
TABLE 1.
Physiological characteristics of four bacterial strains that were tested for the NO2− biosensor
| Characteristic | Pseudoxanthomonas broegbernensis | Luteimonas mephitis | Stenotrophomonas nitritireducens | Alcaligenes faecalis |
|---|---|---|---|---|
| NO3− → NO2− | − | − | − | − |
| NO2− → N2O | + | + | + | + |
| N2O → N2 | − | − | − | + |
| μmax (h−1)a | 0.30 ± 0.01 | 0.33 ± 0.02 | 0.74 ± 0.03 | 0.79 ± 0.03 |
| pH range | 6-9 | 6-8.5 | 5-9 | 5.5-9 |
| NO2− tolerance (mM) | 0-2 | 0-2 | 0-5 | 0-10 |
μmax, growth rate. The values are means ± standard deviations for three replicates.
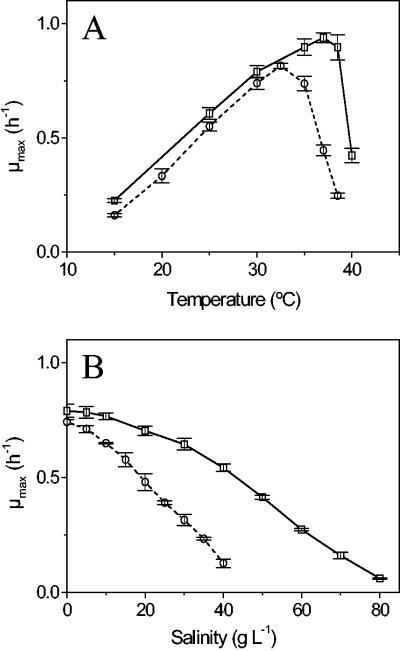
Temperature and salinity effects on the growth rate were critical aspects of the bacterial physiology, as these two key parameters limit the range of environmental applications of a biosensor. The growth constants that were obtained showed that both S. nitritireducens and A. faecalis are typical mesophilic organisms (Fig. 2A), with temperature optima around 33 and 37°C, respectively. The growth range was broader in the case of A. faecalis, which had higher upper and lower temperature limits for growth. The salinity-growth curves showed that both organisms had the highest growth rate under no-salt conditions, but there was a significantly higher salt inhibition factor for S. nitritireducens than for A. faecalis. At a sea strength concentration (34 g liter−1) the growth rate of S. nitritireducens was reduced to less than one-third the maximum rate when no salts were added, and the upper limit for growth was about 45 g liter−1. A. faecalis showed only a 20% reduction in the growth rate at a sea strength concentration, and the upper limit for growth was about 80 g liter−1.
FIG. 2.
Temperature-growth curves (A) and salinity-growth curves (B) for S. nitritireducens (○) and A. faecalis (□). The growth rates (μmax) are the means for three replicates, and the error bars indicate standard deviations.
In summary, both of the organisms characterized could grow under a broad range of conditions. However, the physiological data obtained also indicated that under certain environmental conditions, biosensor application would be suitable only when A. faecalis was used. In particular, for S. nitritireducens a comparatively low growth rate (and respiration rate) at a low temperature and high salinity might limit the applicability of sensors made with this organism.
Sensor characteristics.
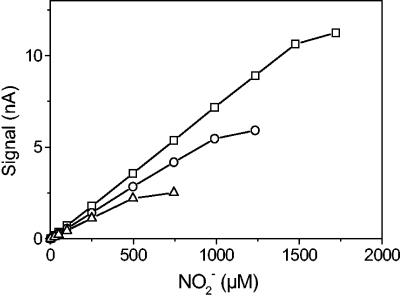
The sensor characteristics described below refer to macroscale biosensors containing S. nitritireducens, but most characteristics were also observed for sensors with A. faecalis. The biosensor had a linear response to NO2− (Fig. 3), and depending on individual sensor characteristics the linear range extended to between 0.5 and 2 mM NO2− at 20°C. A negative correlation between the linear range and the sensor response time was found, with 90% response times ranging from 0.5 to 3 min (20°C), indicating that the sensors with the longest diffusion path between the tip membrane and the internal electrochemical N2O sensor and thus the largest active biovolume had the largest measuring range. The NO2− response was about 4 to 6 pA for 1 μM NO2−, and the sensor zero signal ranged from 100 to 300 pA (20°C). The detection limit was around 1 μM NO2− and depended primarily on the stability of the zero signal from the N2O sensor
FIG. 3.
Calibration curves for an NO2− biosensor (S. nitritireducens) at three temperatures, 10°C (▵), 20°C (○), and 30°C (□).
The effects of temperature, salinity, turbulence, pH, and O2 level on the sensor characteristics were investigated. There was a substantial temperature effect as a result of the temperature influence on diffusion coefficients and the bacterial respiration rate (Fig. 3). Several sensor characteristics were affected, including the linear range, the slope of the calibration curve, the sensor zero signal, and the response time. In the temperature range from 10 to 37°C the linear range of the sensor increased with an increase in the temperature. At temperatures below 8 to 9°C several sensors ceased to work and became sensitive to O2 due to an insufficient respiration rate. This observation correlated well with the temperature effect on the growth rate found for S. nitritireducens in batch culture (Fig. 2A). A significant increase in the NO2− response corresponding to an increase in the slope of the calibration curve of ∼2.5% per degree Celsius corresponded well to the calculated increase in diffusion coefficients (4, 25) at 20°C, which was 2.7% per degree Celsius. Higher temperatures also increased the zero signal, presumably by increased hydrogen production at the cathode surface by electrolysis of water. The response time decreased with increasing temperature as a result of the increased diffusion speed, and the 90% response time for a typical sensor thus decreased from about 115 s at 15°C to about 80 s at 30°C. This is in agreement with the theoretical change in response time calculated by the Einstein-Smulochowski equation (18), which states that the diffusion time is inversely proportional to the diffusion coefficient. According to this equation there should be a 1.46-fold increase in the response time when the temperature is lowered from 30 to 15°C.
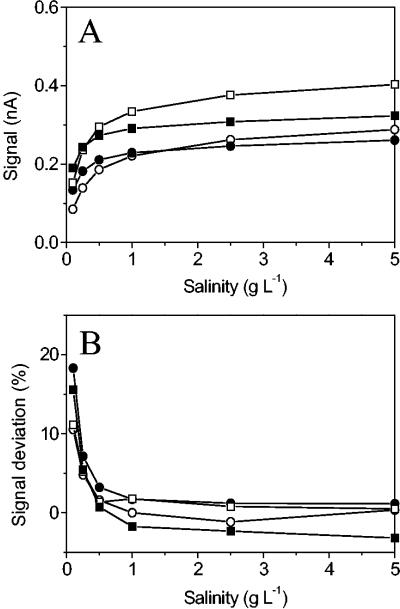
The sensor signal was influenced by changes in the salinity and turbulence of the medium analyzed (Fig. 4). An increased NO2− signal was observed with an increased salt concentration (Fig. 4A). This effect was most pronounced at low salt concentrations, and at salinities above about 0.5 to 1.0 g liter−1 the relative signal change due to moderate variations in salinity was small. However, the salinity effect was also affected by the ionic composition of the internal sensor medium. Addition of extra salt (NaCl) to the internal medium reduced the sensitivity to variations in the external salinity, particularly in the lower-salinity range (Fig. 4A). Both external salinity and internal salinity also affected the sensitivity to turbulence in the external medium. Generally, sensors exhibited the greatest sensitivity to turbulence at a low external salinity (Fig. 4B), and at an NaCl concentration of 0.1 g liter−1 the signal in stagnant medium was up to 18% higher than the signal in vigorously stirred medium. At salinities higher than 0.5 g liter−1, the sensitivity to stirring was negligible (±2%). The signal difference between low turbulence and high turbulence was much less than the difference between totally stagnant and vigorously stirred conditions illustrated in Fig. 4B.
FIG. 4.
Responses of two NO2− biosensors (sensor 1 [□ and ▪] and sensor 2 [○ and •]) to variations in salinity (A) and water turbulence (B) of a medium containing 100 μM NO2−. Data are shown for two types of internal sensor media that differed in ionic strength, standard medium (open symbols) and standard medium containing 10 g of NaCl liter−1 (solid symbols). The turbulence effects (B) are expressed as the relative difference between the sensor signals under stagnant and stirred conditions.
No marked effect of either pH or O2 level on sensor characteristics was observed. Variation in the external pH did not affect the sensor signal in the range from pH 5 to 9. However, a shift from oxic to anoxic conditions caused a small decrease in the sensor zero signal that for the sensors tested corresponded to an NO2− concentration of 0.1 to 4 μM. This deviation can be explained by the relatively higher CO2 production in the bacterial biomass during oxic conditions that affected the N2O sensor signal. High CO2 concentrations have previously been shown to elevate the zero signals from N2O sensors (1). Furthermore, a slight increase (∼1%) in the relative NO2− response was observed under anoxic conditions. This effect may be explained as a change in the location of the denitrifying zone inside the reaction chamber in the absence of O2. Unexpectedly, no effect of O2 status on the linear range for NO2− was observed. A broader range was expected under anoxic conditions due to a potentially larger NO2−-respiring biomass, and a wider range has previously been observed for NOx− biosensors (23).
Interference.
Ideally, the biosensor developed should respond only to NO2−, and it was thus important to examine interference with the NO2− signal by relevant chemical species. As expected, the sensor responded to any N2O present in the system. Theoretically, equal concentrations of N2O and NO2− should result in a 2.5-fold-higher signal for N2O as it takes two NO2− molecules to produce one N2O molecule and the diffusion coefficient for NO2− is about 0.8 times that of N2O (4, 25). However, variations in the tip potential of the sensor may change this value, and for several sensors tested a factor of about 3 was observed. For NO, which was reduced to N2O by the bacteria inside the sensor, the equivalent factor was about 1.5. Low to moderate (100 μM) concentrations of hydrogen sulfide (H2S) at pH 7.2 had no effect on the sensor zero signal, but only 1 μM H2S caused a 15% decrease in the NO2− sensitivity, and at a concentration of 100 μM less than 5% of the signal remained. Fortunately, the decrease in sensitivity caused by H2S is reversible (1)
Interference from NH4+ and NH2OH was expected for sensors based on A. faecalis because this organism has been described as a heterotrophic ammonium oxidizer and also is known to oxidize NH2OH with production of N2O (31). However, NH4+ interference was not observed for sensors containing either of the two organisms. Interference from NH2OH was observed when both organisms were used, and the interfering signal was further enhanced by a high NH4+ concentration. The relative response to NH2OH was highly variable, corresponding to 1 to 10% of the signal for an equivalent concentration of NO2−, and the response was much slower than the responses to NO2−, N2O, and NO. The effect was most pronounced with biosensors containing S. nitritireducens. No interference from NO3− was observed with freshly prepared sensors, but during the first 1.5 years of our work with the NO2− biosensor NO3− interference developed after periods varying from hours to weeks. Analysis of biomass from NO3−-sensitive sensors confirmed the presence of NO3−-reducing contaminants. Addition of tungstate (Na2WO4) to the internal growth medium at a final concentration of 10 mM alleviated the contamination problem with sensors containing S. nitritireducens. Sensors made with A. faecalis have not been tested with tungstate. Tungstate is placed in the NO3− reductase of contaminants instead of molybdenum, resulting in a nonfunctional enzyme (9). Not all bacterial NO3− reductases are completely inhibited by even 10 mM tungstate (11), but for the present application the inhibitory effect seemed to be sufficient.
The most relevant interfering agent is probably N2O. There have been several reports which described release of significant amounts of N2O from wastewaters, and the rate of N2O production has been correlated with various parameters, like low pH, high NO2− levels, and low chemical oxygen demand/N ratios (12, 16, 41). We have, however, never observed detectable N2O or NO (>0.5 μM) during normal operation with nitrifying-denitrifying activated sludge, nor have we detected the oxidized nitrogen gases (concentrations, >0.5 μM) in marine sediments. By use of microsensors it is very easy to check for the presence of N2O plus NO, as a negative ESC potential of 0.5 V prevents entry of NO2− plus NO3− into the sensor (21) and a signal can then be assumed to be due to N2O plus NO. We are currently trying to develop membranes that allow use of ESC also with a macroscale sensor so that similar interference tests (and zero control) can be performed. H2S may be a problem in some systems, but the low concentrations present in, for example, activated sludge have not affected the sensor response. For most field applications, changes in sensitivity and zero current caused by changes in temperature are a much larger source of error than chemical interference.
Stability of NO2− biosensor.
Once bacterial biomass was successfully introduced, the biosensor immediately responded to NO2−. The stability of the sensor signal was examined during online monitoring in a pilot-scale wastewater treatment plant and in different laboratory setups. Generally, the characteristics of the biosensors constructed changed gradually, and there was an observed increase in the sensor zero current (0 to 100%) and also a decrease in the relative NO2− response (0 to 30%) during a 2-month period in a laboratory setup. For most sensors the linear NO2− range remained unchanged without a loss of bacterial activity. It is likely that sensors may monitor NO2− for up to 3 months, as this is the case for the equivalent NOx− biosensors, but due to previous problems with contamination of cultures within biosensors we have not tested NO2− biosensors in wastewater for more than 6 weeks. None of four biosensors constructed with a tungstate-containing inner medium that were used in wastewater for 3 weeks showed any sign of deterioration of characteristics in this period.
Although addition of tungstate seems to be efficient in terms of avoiding NO3− reduction to NO2− within the sensor, contamination should be kept at a low level to minimize the risk of contamination with an N2O-reducing bacterium that would render the sensor insensitive to all nitrogen species. Three routes of contamination were recognized: (i) proliferation of bacteria already present on various sensor parts, (ii) introduction of contaminants during the inoculation procedure, and (iii) contamination with bacteria from the external environment caused by insufficient sealing at the glue-membrane interface. Sterilization of various sensor parts, including the interior of the reaction chamber, the membrane, and the glue surface, by using propylene oxide as the sterilization agent proved to be very efficient. Addition of tetracycline at a concentration of 20 mg liter−1 to the growth medium was used for sensors containing S. nitritireducens to limit proliferation of contaminants that might enter along the glue-membrane interface. However, like S. nitritireducens, many other bacterial strains are resistant to tetracycline (34). Analysis of biomass from several contaminated sensors to which tetracycline was added revealed resistance for all contaminants examined.
Biosensors containing A. faecalis.
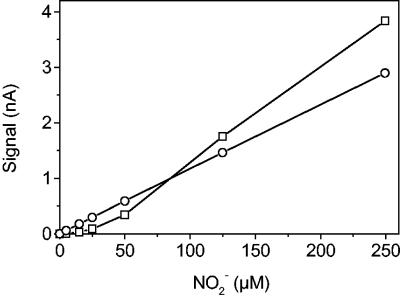
Functional biosensors were successfully constructed by using A. faecalis, although this organism has a denitrifying pathway with termination at N2 instead of N2O. It was possible to solve this problem through saturation of the growth medium inside the sensor with acetylene, which inhibited the activity of N2O reductase (45). However, in many cases the sensor response to low NO2− concentrations was completely absent (Fig. 5), presumably due to inadequate acetylene saturation in some anoxic parts of the reaction chamber. Apparently, a relatively loose fit between the sensor tip and the reaction chamber was critical to ensure sufficient diffusive flux of acetylene from the medium reservoir to the reaction chamber. Acetylene inhibition was improved if an N2O sensor with a smaller tip diameter was used and when the usual 0.35-mm front plate was replaced with a 0.2-mm plate. However, such sensors suffered from slower response due to diffusion of N2O in the gap between the N2O sensor and the front plate wall; when acetylene could be supplied to the tip, N2O could also escape to the bulk medium reservoir. In agreement with the growth-versus-temperature relationships (Fig. 2A) biosensors constructed with A. faecalis functioned well at temperatures lower than the temperatures at which sensors containing S. nitritireducens functioned. At 5°C, the A. faecalis-based sensors were still working and had a linear range to about 0.5 mM NO2−.
FIG. 5.
Calibration curves for two NO2− biosensors containing the bacterium A. faecalis, showing examples of complete (○) and incomplete (□) inhibition of N2O reductase activity by acetylene.
Biosensor versus standard analytical method.
An experiment was conducted to examine the performance of the NO2− biosensor during online monitoring of wastewater. Fast process dynamics made it difficult to make an unbiased comparison of online biosensor readings for the sludge with a standard analysis of NO2− in filtered samples, and for comparison we therefore also analyzed NO2− in the samples with a biosensor.
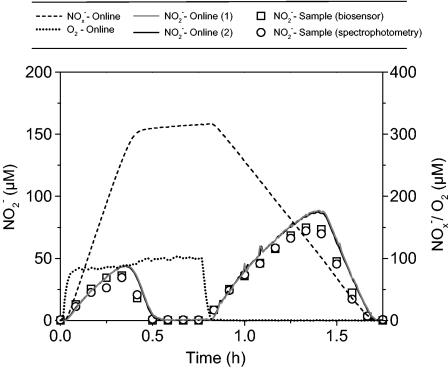
A very good correlation was found between online signals from the two NO2− biosensors, which showed almost identical NO2− concentrations during the complete cycle (Fig. 6). Furthermore, NO2− analysis of a filtered sample showed that there was good agreement between conventional analysis and sensor analysis. There was, however, a marked difference between the data from the online sensor analysis and the concentrations analyzed in the filtered samples. This discrepancy could be explained by either the 1- to 2-min response time of the sensors or the nitrogen conversions that occurred during processing of the filtered samples.
FIG. 6.
Calibrated online sensor outputs for an O2 sensor, an NOx− biosensor, and two NO2− biosensors during one cycle consisting of 0.75 h of oxic conditions and 1 h of anoxic conditions in a 2-liter laboratory-scale reactor with activated sludge. The graph includes comparable data for NO2− concentrations in filtered samples analyzed with a biosensor or by spectrophotometry.
Environmental monitoring.
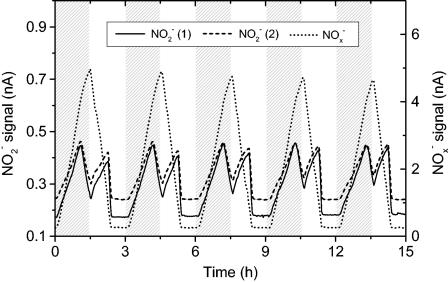
Online monitoring of NO2−, NOx−, and O2 in activated sludge exposed to oxic-anoxic cycles (Fig. 7) demonstrated that NO2− accumulated to concentrations of about 65 μM during operation. NO2− accumulated in the early part of the oxic phase due to higher rates of NO2− production (ammonia oxidation) than of NO2− consumption (nitrite oxidation). A second NO2− peak was observed during the anoxic phase, which was caused by imbalanced denitrification with higher rates of NO3− reduction than of NO2− reduction.
FIG. 7.
Online sensor outputs for two NO2− biosensors [NO2− (1) NO2− (2)] and one NOx− biosensor (NOx−) during cycles consisting of 1.5 h of oxic conditions and 1.5 h of anoxic conditions in a pilot-scale wastewater treatment plant with activated sludge. The shaded areas indicate oxygenated periods. Calibrated sensor signals correspond to concentration peaks of about 65 μM NO2− and 600 μM NOx−. The sensor signals for 100 μM NO2− and 100 μM NOx− were as follows: biosensor NO2− (1), 0.42 nA; biosensor NO2− (2), 0.33 nA; and biosensor NOx−, 0.79 nA.
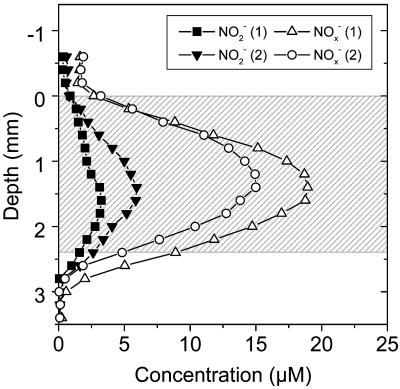
Microscale analysis of NO2−, NOx−, and O2 in a marine sediment (Fig. 8) showed that there was NO2− accumulation to concentrations of about 3 to 6 μM in the oxic part of the sediment. The NO2− peak was located at a depth of about 1.5 mm and was found at approximately the same depth as the NOx− peak (about 15 to 19 μM). The NO2− accumulation was most likely due to a high ammonia oxidation rate in the oxic part of the sediment. The NO2− concentration gradients indicated that there was a flux down to the deeper anoxic layers (∼2.4 to 3.2 mm) and that there was also net release of NO2− to the overlying water phase.
FIG. 8.
Concentration profiles for NO2− and NOx− in a marine sediment (Wadden Sea) measured during incubation in the dark in a laboratory setup. The shaded area indicates the location of the oxic zone in the sediment.
Perspectives.
The new biosensor may be used for sensitive analysis of NO2− at high spatial and temporal resolution. This sensor can be used in basic research on processes, but it may also be a valuable tool for online monitoring and feedback control in wastewater treatment. The preferred organism for the biosensor is S. nitritireducens. However, for some purposes it maybe more suitable to use sensors containing A. faecalis, due to this organism's higher tolerance to extremes of temperature and salinity. We are currently trying to isolate psychrotrophic bacteria that enable NO2− analysis at temperatures down to those of freezing seawater, so that we can perform analyses under most relevant environmental conditions.
Acknowledgments
This study was performed as part of the Icon project under the Fifth Framework Programme of the European Commission (EVK1-CT-2000-00054).
REFERENCES
- 1.Andersen, K., T. Kjær, and N. P. Revsbech. 2001. An oxygen insensitive microsensor for nitrous oxide. Sens. Actuators 81:42-48. [Google Scholar]
- 2.Anthonisen, A. C., R. C. Loehr, T. B. S. Prakasam, and E. G. Srinath. 1976. Inhibition of nitrification by ammonia and nitrous acid. J. Water Pollut. Control Fed. 48:835-852. [PubMed] [Google Scholar]
- 3.Bellos, D., T. Sawidis, and I. Tsekos. 2004. Nutrient chemistry of River Pinios. Environ. Int. 30:105-115. [DOI] [PubMed] [Google Scholar]
- 4.Broecker, W. S., and T.-H. Peng. 1974. Gas exchange rates between air and sea. Tellus 26:21-35. [Google Scholar]
- 5.Dalsgaard, T., D. E. Canfield, J. Petersen, B. Thamdrup, and J. Acuña-González. 2003. N2 production by the anammox reaction in the anoxic water column of Golfo Dulce, Costa Rica. Nature 422:606-608. [DOI] [PubMed] [Google Scholar]
- 6.Dalsgaard, T., and B. Thamdrup. 2002. Factors controlling anaerobic ammonium oxidation with nitrite in marine sediments. Appl. Environ. Microbiol. 68:3802-3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Beer, D., A. Schramm, C. M. Santegoeds, and M. Kühl. 1997. A nitrite microsensor for profiling environmental biofilms. Appl. Environ. Microbiol. 63:973-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong, L. F., D. B. Nedwell, G. J. C. Underwood, D. C. O. Thornton, and I. Rusmana. 2002. Nitrous oxide formation in the Colne estuary, England: the central role of nitrite. Appl. Environ. Microbiol. 68:1240-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Enoch, H. G., and R. L. Lester. 1972. Effects of molybdate, tungstate, and selenium compounds on formate dehydrogenase and other enzymes in Escherichia coli. J. Bacteriol. 110:1032-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finkmann, W., K. Altendorf, E. Stackebrandt, and A. Lipski. 2000. Characterization of N2O-producing Xanthomonas-like isolates from biofilters as Stenotrophomonas nitritireducens sp. nov., Luteimonas mephitis gen. nov., sp. nov. and Pseudoxanthomonas broegbernensis gen. nov., sp. nov. Int. J. Syst. Evol. Microbiol. 50:273-282. [DOI] [PubMed] [Google Scholar]
- 11.Gates, A. J., R. O. Huges, S. R. Sharp, P. D. Millington, A. Nilavongse, J. A. Cole, E.-R. Leach, B. Jepson, D. J. Richardson, and C. S. Butler. 2003. Properties of the periplasmic nitrate reductase from Paracoccus pantotrophus and Escherichia coli after growth in tungsten-supplemented media. FEMS Microbiol. Lett. 220:261-269. [DOI] [PubMed] [Google Scholar]
- 12.Gejlsbjerg, B., L. Frette, and P. Westermann. 1998. Dynamics of N2O production from activated sludge. Water Res. 32:2113-2121. [Google Scholar]
- 13.Glass, C., J. Silverstein, and J. Oh. 1997. Inhibition of denitrification in activated sludge by nitrite. Water Environ.Res. 69:1086-1093. [Google Scholar]
- 14.Hellinga, C., A. A. J. C. Schellen, J. W. Mulder, M. C. M. van Loosdrect, and J. J. Heijnen. 1998. The SHARON process: an innovative method for nitrogen removal from ammonium-rich wastewater. Water Sci.Technol. 37:135-142. [Google Scholar]
- 15.House, W. A., and M. S. Warwick. 1998. Intensive measurements of nutrient dynamics in the River Swale. Sci. Total Environ. 210/211:111-137. [Google Scholar]
- 16.Itokawa, H., K. Hanaki, and T. Matsuo. 2001. Nitrous oxide production in high-loading biological nitrogen removal process under low COD/N ratio condition. Water Res. 35:657-664. [DOI] [PubMed] [Google Scholar]
- 17.Jetten, M. S. M., S. J. Horn, and M. C. M. van Loosdrecht. 1997. Towards a more sustainable municipal wastewater treatment system. Water Sci. Technol. 9:171-180. [Google Scholar]
- 18.Jost, W. 1952. Diffusion in solids, liquids, and gasses. In E. Hutchinson (ed.), Physical chemistry. Academic Press, New York, N.Y.
- 19.Kelso, B. H. L., R. V. Smith, R. J. Laughlin, and S. D. Lennox. 1997. Dissimilatory nitrate reduction in anaerobic sediments leading to river nitrite accumulation. Appl. Environ. Microbiol. 63:4679-4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelso, B. H. L., D. M. Glass, and R. V. Smith. 1999. Toxicity of nitrite to freshwater invertebrates, p. 175-188. In W. S. Wilson, A. S. Ball, and R. H. Hinton (ed.), Managing risk of nitrates to humans and the environment. Royal Society of Chemistry, Cambridge, United Kingdom.
- 21.Kjær, T., L. H. Larsen, and N. P. Revsbech. 1999. Sensitivity control of ion-selective biosensors by electrophoretically mediated analyte transport. Anal. Chim. Acta 391:57-63. [Google Scholar]
- 22.Kuypers, M. M. M., A. O. Sliekers, G. Lavik, M. Schmid, B. B. Jørgensen, J. G. Kuenen, J. S. Sinninghe Damsté, M. Strous, and M. S. M. Jetten. 2003. Anaerobic ammonium oxidation by anammox bacteria in the Black Sea. Nature 422:608-611. [DOI] [PubMed] [Google Scholar]
- 23.Larsen, L. H., T. Kjær, and N. P. Revsbech. 1997. A microscale NO3− biosensor for environmental applications. Anal. Chem. 69:3527-3531. [DOI] [PubMed] [Google Scholar]
- 24.Larsen, L. H., L. R. Damgaard, T. Kjær, T. Stenstrøm, A. Lynggard-Jensen, and N. P. Revsbech. 2000. Fast responding biosensor for on-line determination of nitrate/nitrite in activated sludge. Water Res. 9:2463-2468. [Google Scholar]
- 25.Li, Y.-H., and S. Gregory. 1974. Diffusion of ions in sea water and in deep-sea sediments. Geochim. Cosmochim. Acta 38:703-714. [Google Scholar]
- 26.Lorenzen, J., L. H. Larsen, T. Kjaer, and N. P. Revsbech. 1998. Biosensor determination of the microscale distribution of nitrate, nitrate assimilation, nitrification, and denitrification in a diatom-inhabited freshwater sediment. Appl. Environ. Microbiol. 64:3264-3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meinhold, J., E. Arnold, and S. Isaacs. 1999. Effect of nitrite on anoxic phosphate uptake in biological phosphorus removal in activated sludge. Water Res. 33:1871-1883. [Google Scholar]
- 28.Meybeck, M. 1982. Carbon, nitrogen and phosphorus transport by world rivers. Am. J. Sci. 282:401-450. [Google Scholar]
- 29.Nielsen, M., N. P. Revsbech, L. H. Larsen, and A. Lynggaard. 2002. On-line determination of nitrite in wastewater treatment by use of a biosensor. Water Sci. Technol. 45:69-76. [PubMed] [Google Scholar]
- 30.Norwitz, G., and P. N. Keliher. 1984. Spectrophotometric determination of nitrite with composite reagents containing sulfanilamide, sulfanilic acid or 4-nitroaniline as the diazotizable aromatic amine and N-(1-naphthyl)ethylenediamine as the coupling agent. Analyst 109:1281-1286. [Google Scholar]
- 31.Otte, S., J. Schalk, J. G. Kuenen, and M. S. M. Jetten. 1999. Hydroxylamine oxidation and subsequent nitrous oxide production by the heterotrophic ammonia oxidizer Alcaligenes faecalis. Appl. Microbiol. Biotechnol. 51:255-261. [DOI] [PubMed] [Google Scholar]
- 32.Otte, S., N. G. Grobben, L. A. Robertson, M. S. M. Jetten, and J. G. Kuenen. 1996. Nitrous oxide production by Alcaligenes faecalis under transient and dynamic aerobic and anaerobic conditions. Appl. Environ. Microbiol. 62:2421-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pollice, A., V. Tandoi, and C. Lestingi. 2002. Influence of aeration and sludge retention time on ammonium oxidation to nitrite and nitrate. Water Res. 36:2541-2546. [DOI] [PubMed] [Google Scholar]
- 34.Roberts, M. C. 1996. Tetracycline resistance determinants: mechanisms of action, regulation of expression, genetic mobility, and distribution. FEMS Microbiol. Rev. 19:1-24. [DOI] [PubMed] [Google Scholar]
- 35.Sasaki, S., I. Karube, N. Hirota, Y. Arikawa, M. Nishiyama, M. Kukimoto, S. Horinouchi, and T. Beppu. 1998. Application of nitrite reductase from Alcaligenes faecalis S-6 for nitrite measurement. Biosens. Bioelectron. 13:1-5. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt, I., O. Sliekers, M. Schmid, E. Bock, J. Fuerst, J. G. Kuenen, M. S. M. Jetten, and M. Strous. 2003. New concepts of microbial treatment processes for the nitrogen removal in wastewater. FEMS Microbiol. Rev. 27:481-492. [DOI] [PubMed] [Google Scholar]
- 37.Smith, R. V., L. C. Burns, S. D. Lennox, B. H. L. Kelso, R. H. Foy, and R. J. Stevens. 1997. Free ammonia inhibition of nitrification in river sediments leading to nitrite accumulation. J. Environ. Qual. 26:1049-1055. [Google Scholar]
- 38.Stief, P., and D. Neumann. 1998. Nitrite formation in sediment cores from nitrate-enriched running waters. Arch. Hydrobiol. 142:153-169. [Google Scholar]
- 39.Stief, P. 2001. Influence of sediment and pore-water composition on nitrite accumulation in a nitrate-perfused freshwater sediment. Water Res. 35:2811-2818. [DOI] [PubMed] [Google Scholar]
- 40.Stief, P., D. De Beer, and D. Neumann. 2002. Small-scale distribution of interstitial nitrite in freshwater sediment microcosms: the role of nitrate and oxygen availability, and sediment permeability. Microb. Ecol. 43:367-378. [DOI] [PubMed] [Google Scholar]
- 41.Thörn, M., and F. Sörensson. 1996. Variation of nitrous oxide formation in the denitrification basin in a wastewater treatment plant with nitrogen removal. Water Res. 30:1543-1547. [Google Scholar]
- 42.Tseng, C.-C., T. G. Potter, and B. Koopman. 1998. Effect of influent chemical oxygen demand to nitrogen ratio on partial nitrification/complete denitrification process. Water Res. 32:65-173. [Google Scholar]
- 43.Weon, S.-Y., C.-W. Lee, S.-I. Lee, and B. Koopman. 2002. Nitrite inhibition of aerobic growth of Acinetobacter sp. Water Res. 36:4471-4476. [DOI] [PubMed] [Google Scholar]
- 44.Wiesche, M. V. D., and A. Wetzel. 1998. Temporal and spatial dynamics of nitrite accumulation in the River Lahn. Water Res. 32:1653-1661. [Google Scholar]
- 45.Yoshinari, T., and R. Knowles. 1976. Acetylene inhibition of nitrous oxide reduction by denitrifying bacteria. Biochem. Biophys. Res. Commun. 69:705-710. [DOI] [PubMed] [Google Scholar]