Abstract
Pseudomonas aeruginosa is a frequent cause of respiratory exacerbations in individuals with cystic fibrosis. An important virulence determinant of this pathogen is its type III protein secretion system. In this study, the type III secretion properties of 435 P. aeruginosa respiratory isolates from 56 chronically infected individuals with cystic fibrosis were investigated. Although it had been previously reported that 75 to 90% of P. aeruginosa isolates from patients with hospital-acquired pneumonia secreted type III proteins, only 12% of isolates from cystic fibrosis patients did so, with nearly all of these isolates secreting ExoS and ExoT but not ExoU. Despite the low overall prevalence of type III protein-secreting isolates, at least one secreting isolate was cultured from one-third of cystic fibrosis patients. Interestingly, the fraction of cystic fibrosis patient isolates capable of secreting type III proteins decreased with duration of infection. Although 90% of isolates from the environment, the presumed reservoir for the majority of P. aeruginosa strains that infect patients with cystic fibrosis, secreted type III proteins, only 49% of isolates from newly infected children, 18% of isolates from chronically infected children, and 4% of isolates from chronically infected adults with cystic fibrosis secreted these proteins. Within individual patients, isolates of clonal origin differed in their secretion phenotypes, indicating that as strains persisted in cystic fibrosis patient airways, their type III protein secretion properties changed. Together, these findings indicate that following infection of cystic fibrosis patient airways, P. aeruginosa strains gradually change from a type III protein secretion-positive phenotype to a secretion-negative phenotype.
The respiratory tracts of individuals with cystic fibrosis (CF) frequently become infected with Pseudomonas aeruginosa during childhood. By the time CF patients reach adulthood, greater than 80% are chronically infected with this pathogen (14). Infection has profound clinical implications, the most serious being a more rapid decline in pulmonary function (1, 34, 44, 45). Once established in the lower respiratory tracts of CF patients, P. aeruginosa is often not eradicated even by aggressive courses of antibiotic therapy, and strains may persist for the lifetime of the patient (4, 35, 48).
During respiratory infection in patients with CF, P. aeruginosa alters the expression of a number of virulence determinants as it adapts to the pulmonary environment. For example, mucoid variants of P. aeruginosa that overexpress the exopolysaccharide alginate typically emerge during chronic infection (21). Conversely, common virulence determinants, such as O antigen, pili, and flagella, are infrequently produced by isolates from CF patients (23, 37). Some of these phenotypic changes are not merely of microbiological interest but also have important prognostic implications; emergence of the mucoidy phenotype, for example, has been associated with poorer clinical outcomes in individuals with CF (10, 31, 43).
An important and recently recognized virulence determinant of P. aeruginosa is its type III protein secretion system (16). This system uses complex secretion and translocation machinery to inject a set of factors, called effector proteins, directly into the cytoplasm of eukaryotic cells. A large number of proteins, such as the Psc proteins, are thought to comprise the secretion apparatus (60), and at least three proteins, PopB, PopD, and PcrV, are essential for the translocation process (27, 59). In addition, three effector proteins have been particularly well studied and implicated in disease: ExoS, ExoT, and ExoU. ExoS and ExoT have GTPase-activating activity for Rho GTPases and ADP-ribosyltransferase activity (7, 18-20, 30, 36, 41, 42, 47, 56, 57). ExoU is a phospholipase that rapidly kills a variety of mammalian cell types in vitro, including macrophages, epithelial cells, and fibroblasts (6, 13, 15, 25, 26, 56). Strains of P. aeruginosa are heterogeneous with regard to type III secretion. Although all strains harbor type III protein secretion genes (12), only some strains are capable of secreting type III proteins when they are grown under secretion-inducing conditions in vitro (24, 50). In addition, secreting strains differ in the complements of effector proteins that they transport (24, 50).
The type III secretion properties of P. aeruginosa strains causing acute infections, such as hospital-acquired pneumonia and bacteremia, have been well characterized. From 75 to 90% of such strains have functional type III secretion systems (24, 50), and approximately one-third of these secrete ExoU, two-thirds secrete ExoS, and nearly all secrete ExoT (24). A growing body of evidence suggests that the specific type III protein secretion properties of strains causing acute infections are clinically relevant in that they dictate the type of cell damage that occurs and the severity of disease. For example, clinical isolates that secrete ExoU are associated with increased cytotoxicity in vitro and increased virulence in a mouse model of acute pneumonia (50, 52). In addition, infection with secreting strains is associated with poorer clinical outcomes in patients with ventilator-associated pneumonia (24).
In contrast to the significant literature on the type III protein secretion properties of P. aeruginosa isolates from acute infections, relatively little is known about the secretion properties of isolates from CF patients. Several reports suggest that functional type III secretion is far less common in CF patient isolates than in isolates from patients with acute infections (9, 50). In this study, the type III secretion properties of a large collection of P. aeruginosa respiratory isolates from children and adults with CF were characterized. This information will be crucial for future studies designed to determine whether this secretion system plays a role in pulmonary injury in CF patients.
MATERIALS AND METHODS
Bacterial isolates.
To determine the prevalence of type III protein-secreting isolates of P. aeruginosa in chronically infected CF patients, respiratory samples (sputum, throat swab, or bronchoalveolar lavage specimens) were prospectively collected from individuals attending the CF clinic at Children's Memorial Hospital (hereafter referred to as “children”) and those attending the CF clinic at Northwestern Memorial Hospital (hereafter referred to as “adults”) in Chicago, Ill., from January 2003 to August 2003. For the purposes of this study, chronically infected patients were defined as those who had a respiratory culture that grew P. aeruginosa within the preceding year. All such patients were invited to enroll in the study. Respiratory samples were collected from consecutive consenting patients at both clinics. Samples were processed by the microbiology laboratories at both facilities, and P. aeruginosa colonies were identified using criteria approved by the National Committee for Clinical Laboratory Standards. One or two respiratory samples were collected from each patient over the interval of the study. Five P. aeruginosa colonies from each sample were randomly selected for further analysis.
Newly infected CF patients were defined as those who grew P. aeruginosa from a respiratory culture after being culture-negative for a long time. Surveillance respiratory cultures had been routinely obtained every 3 months from these patients and had not grown P. aeruginosa in the preceding 2 years. These isolates were prospectively obtained from children attending the CF clinic at Children's Memorial Hospital between August 2003 and December 2003. Five P. aeruginosa colonies from each sample were randomly selected for further analysis.
For longitudinal assessment of the type III secretion phenotypes of individual P. aeruginosa strains, isolates were retrospectively chosen from an archived collection of strains cultured from CF patients at Northwestern Memorial Hospital between January 2000 and March 2002. From each respiratory sample, a single colony representative of each morphotype was analyzed.
Twenty environmental isolates of P. aeruginosa were obtained as previously described (12). Six of these strains were initially cultured from vegetables, four were from rivers, six were from soil, two were from lakes, and two were from well water.
This study was approved by the institutional review boards of Northwestern University and Children's Memorial Hospital.
Immunoblot analysis.
Bacteria were grown under low-calcium conditions known to induce type III protein secretion (55) in either Luria-Bertani broth supplemented with 1 mM EGTA or in MINS medium (33, 39). Bacteria were grown at 37°C for approximately 17 h with vigorous shaking. Supernatants were collected from 5-ml cultures by centrifugation at 6,000 × g at 4°C for 20 min and concentrated and partially purified as previously described (24). Immunoblot analysis using a mixture of antisera against the effector proteins ExoS, ExoT, and ExoU, as well as the secreted translocation proteins PopB and PopD, was performed as previously described (24).
PCR genotyping of strains.
The clonality of P. aeruginosa isolates was determined using randomly amplified polymorphic DNA (RAPD) PCR genotyping as previously described (12).
Pulsed-field gel electrophoresis.
Pulsed-field gel electrophoresis was performed by the Molecular Epidemiology Laboratory at Northwestern Memorial Hospital. Bacteria were grown for 17 h at 37°C on blood agar plates (tryptic soy agar with sheep blood; REMEL, Lenexa, Kans.). Cells were resuspended in cell suspension buffer (10 mM Tris [pH 7.2], 50 mM EDTA, 2 mM NaCl). Next, 20 μl of a lysozyme-lysostaphin mix (GenePath enzyme module group 1; Bio-Rad, Hercules, Calif.) was added to the suspension mix, which was then incubated at 37°C for 10 min. Bacterial lysates were gently mixed with 15 μl of 50-mg/ml proteinase K and 150 μl of 1.2% embedding agarose, which was allowed to solidify to a plug. Plugs were incubated at 50°C for 2 h in a proteinase K suspension (500 μl of proteinase K buffer, 10 μl of 50-mg/ml proteinase K). After the plugs were washed five times at 50°C for 15 min with wash buffer (20 mM Tris [pH 8.0], 50 mM EDTA), the plugs were digested with SpeI for 2 h at 37°C. The plugs were electrophoresed through a 1.0% agarose gel at 6 V/cm for 19.5 h with pulse switch times ranked from 5.3 to 34.9 s with a GenePath strain typing system (Bio-Rad). The criteria of Tenover et al. were used to distinguish clonal isolates from nonclonal isolates (54).
Statistical methods.
Comparisons between children and adults were made using a t test for continuous variables and the chi-square test for categorical variables. To compare the proportions of secreting isolates from adults, chronically infected children, newly infected children, and the environment, a repeated-measures logistic regression model that accounted for the clustering effect within multiple samples from each child or adult was used. Similar methods were used to compare the proportions of CF patient and environmental isolates that secreted ExoU and ExoS. The Bonferroni correction was used to adjust for multiple comparisons, and an odds ratio with a 95% confidence interval (CI) was computed to express the likelihood of secreting isolates in newly infected children versus chronically infected children and in chronically infected children versus adults. All conclusions were made at a 5% level of significance.
RESULTS
Stability of type III secretion phenotypes in vitro.
Before rigorously determining the secretion phenotypes of a large population of P. aeruginosa isolates, we first wished to determine whether secretion properties measured in vitro were relatively stable and therefore likely to reflect secretion potential in vivo. P. aeruginosa isolates were grown in vitro under conditions that induced type III protein secretion, and secreted type III proteins were detected by immunoblot analysis. Isolates that secreted type III proteins were referred to as type III protein secretion positive (TTS+) isolates, and isolates that did not secrete these proteins were referred to as type III protein secretion negative (TTS−) isolates. To determine whether these phenotypes were relatively stable, three TTS+ isolates and three TTS− isolates from patients with CF were serially passaged in vitro on Luria-Bertani agar. After five passages, the secretion properties of three colonies from each isolate were again measured using immunoblot analysis. In each case, the secretion phenotype was unchanged from the initial phenotype (data not shown). An additional 10 passages were then performed on all isolates, and the immunoblot analyses were repeated. Again, in each case the secretion phenotype was unchanged (data not shown). Thus, the type III secretion phenotype of P. aeruginosa isolates from CF patients is relatively stable in vitro, at least over a period of several weeks, and is likely to reflect secretion potential immediately prior to isolation.
CF patients chronically infected with P. aeruginosa.
To determine the prevalence of TTS+ isolates of P. aeruginosa in individuals with CF, respiratory samples were prospectively collected from 56 chronically infected patients: 32 children and 24 adults (Table 1). The mean ages of the children and adults were 12 and 27 years, respectively. As expected, the adult patients had been infected with P. aeruginosa for significantly longer periods of time than the children (13.5 versus 6.5 years, respectively; P < 0.0001). Both groups of patients were similar with regard to their CFTR genotype and the prevalence of pancreatic insufficiency and diabetes mellitus. They did, however, differ in the chronic use of macrolides, and there was a trend towards a difference in the use of inhaled tobramycin (P = 0.10), with more children than adults receiving these antibiotics.
TABLE 1.
Demographics of children and adults with CF from whom P. aeruginosa isolates were cultured
| Characteristic | Value for:
|
|
|---|---|---|
| Children (n = 32) | Adults (n = 24) | |
| Mean age (yr) (SD)a | 12 (5.1) | 27 (5.8) |
| No. (%) of males | 17 (53) | 11 (46) |
| Mean duration of infection with P. aeruginosa (yr) (SD)b,c | 6.5 (5.4) | 13.5 (5.5) |
| No. (%) with ΔF508 genotypeb | 26 of 28 (93) | 14 of 14 (100) |
| No. (%) with pancreatic insufficiencyb | 29 of 31 (94) | 22 (92) |
| No. (%) with diabetes mellitusb | 4 of 31 (13) | 6 (25) |
| No. (%) chronically using a macrolided | 26 (81) | 11 (46) |
| No. (%) chronically using inhaled tobramycin | 26 (81) | 14 (58) |
The difference between values for children and adults was statistically significant (P < 0.0001).
Duration of infection, genotype, pancreatic insufficiency, and diabetes mellitus information was not available for all patients.
The difference between children and adults was statistically significant (P = 0.0005).
The difference between values for children and adults was statistically significant (P ≤ 0.01).
Type III secretion phenotypes of isolates from chronically infected patients.
The type III secretion properties of the P. aeruginosa strains from chronically infected patients were determined. Respiratory cultures were collected from each patient, and five colonies of P. aeruginosa per culture were randomly chosen to ensure a representative sampling of the strains present. Bacteria from each colony were checked for the ability to secrete type III proteins under inducing conditions in vitro by immunoblot analysis. As expected, some isolates secreted type III proteins whereas others did not (Fig. 1), but in general, secretion was rare. Of the 435 isolates from patients with CF, 387 (88% after adjustment for the effect of clustering) did not secrete any of the examined type III proteins. Each of the remaining 48 isolates (12% after adjustment for the effect of clustering) secreted two or more of the examined type III proteins. Thus, the proportion of TTS+ isolates from patients with CF was considerably less than that observed in previously examined collections of P. aeruginosa isolates from non-CF patients (3, 24, 50).
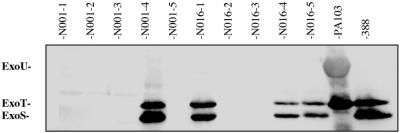
FIG. 1.
Immunoblot analysis showing the type III protein secretion phenotypes of representative P. aeruginosa isolates from CF patients. Individual isolates were grown under conditions that induced type III protein secretion. Immunoblot analysis was then performed on culture supernatants using a mixture of antisera against type III proteins. The migrations of the effector proteins ExoU, ExoT, and ExoS are shown to the left of the panel. P. aeruginosa isolate designations are listed above the membrane. The samples shown are five individual colonies from a single respiratory sample from patient N001 and five individual colonies from a single respiratory sample from patient N016. PA103 is a control strain that secretes ExoU and ExoT. Strain 388 is a control strain that secretes ExoT and ExoS. CF patient isolates differed in their type III secretion phenotypes.
Patients varied with regard to the type III secretion properties of the P. aeruginosa strains they harbored. Overall, 18 (32%) of the 56 enrolled patients had at least one TTS+ isolate. The vast majority (16 [89%]) of these 18 patients were infected with a mixture of TTS+ and TTS− isolates rather than all TTS+ isolates.
Type III secretion properties of environmental isolates.
Since the majority of individuals with CF are thought to acquire their P. aeruginosa strains from the environment (49), the proportion of TTS+ strains present in the environment was next determined. P. aeruginosa strains initially cultured from soil, vegetables, lakes, rivers, and well water were tested for the ability to secrete type III proteins. All together, 18 (90%) of 20 examined strains secreted type III proteins (Fig. 2). Thus, the TTS+ phenotype is significantly more common in environmental strains than in strains from patients with CF (P ≤ 0.001).
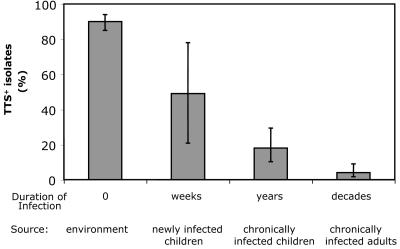
FIG. 2.
Percentage of isolates from CF patients and the environment that secreted type III proteins. “Environment” refers to isolates obtained from vegetables, rivers, soil, lakes, and well water. “Newly infected children” refers to isolates from children with CF who grew P. aeruginosa from a respiratory culture for the first time in 2 years. “Chronically infected children” refers to isolates from children with CF who had at least one respiratory culture within the preceding year that grew P. aeruginosa. “Chronically infected adults” refers to isolates from adults with CF, all of whom had P. aeruginosa grow from multiple prior respiratory cultures. “Duration of Infection” refers to the approximate mean duration of infection associated with each of these groups. Error bars represent 95% CIs. The prevalence of TTS+ isolates decreased with duration of infection of the CF patient airways.
Comparison of isolates from chronically infected children and adults with CF.
The observation that the fraction of P. aeruginosa isolates from CF patients that secreted type III proteins was much smaller than that of isolates from the environment, the reservoir from which the majority of CF patient isolates are thought to originate, suggested that isolates changed from TTS+ to TTS− during infection of the CF patient airways. If this is indeed the case, one would expect to culture a higher proportion of TTS+ isolates from children with CF than from adults with CF, since most children have been infected with P. aeruginosa for shorter periods than adults. To investigate this possibility, the percentages of TTS+ isolates chronically infecting children and adults were compared (Fig. 2). Overall, 40 (18% when adjusted for the effect of clustering) of 235 isolates cultured from children with CF were TTS+, and 8 (4%) of 200 isolates cultured from adults with CF were TTS+, a statistically significant difference (P = 0.0019). Isolates from chronically infected children were 5.8 times more likely to secrete type III proteins than isolates from chronically infected adults (95% CIs for odds ratio, 1.9 and 17.6). TTS+ isolates were cultured from 13 (41%) of the 32 children and 5 (21%) of the 24 adults enrolled in the study (P = 0.20). Therefore, whereas nearly all environmental isolates were TTS+, a relatively small proportion of isolates from chronically infected children with CF were TTS+, and an even smaller proportion of isolates from chronically infected adults with CF were TTS+. These findings suggest that P. aeruginosa strains gradually change from TTS+ to TTS− during infection of CF patient airways.
Type III secretion properties of newly infected CF patients.
If P. aeruginosa strains were indeed losing the ability to secrete type III proteins as they infected the CF patient airways for longer and longer periods of time, then TTS+ isolates should be more common in newly infected patients than in chronically infected individuals. To assess this possibility, 35 P. aeruginosa isolates from seven newly infected children with CF were prospectively collected and analyzed. In contrast to the 18% of TTS+ isolates observed in chronically infected children, 49% (17 of 35) of isolates from newly infected children with CF were TTS+ (P < 0.05) (Fig. 2). Isolates from acutely infected children were 4.33 times more likely to secrete type III proteins than isolates from chronically infected children (95% CIs for odds ratio, 1.02 and 18.39). Thus, the prevalence of TTS+ isolates decreased from 90% of environmental strains to 49% of isolates from newly infected children, to 18% of isolates chronically infecting children, and finally to 4% of isolates chronically infecting adults (Fig. 2). These trends persisted when the analysis was repeated with only sputum isolates to exclude a possible bias due to higher numbers of throat swab and bronchoalveolar lavage specimen isolates from children than from adults (data not shown). This conclusion is consistent with the model in which P. aeruginosa strains gradually change from TTS+ to TTS− during infection of CF patient airways.
Secretion of individual effector proteins.
The type III effector proteins secreted by CF patient isolates were compared to those secreted by environmental strains (Table 2). Of the 65 TTS+ CF patient isolates, 60 (92%) secreted ExoS, 65 (100%) secreted ExoT, and 2 (3%) secreted ExoU. In contrast, 13 (72%) of the 18 secreting environmental isolates secreted ExoS, 18 (100%) secreted ExoT, and 3 (17%) secreted ExoU. Therefore, of the TTS+ CF patient isolates, a larger proportion secreted ExoS and a smaller proportion secreted ExoU than the proportions of environmental strains secreting these proteins, although these differences were not statistically significant (P = 0.27 and P = 0.63, respectively). Interestingly, even among isolates from newly infected CF patients, ExoU secretion was rare (Table 2). All TTS+ isolates, regardless of source, secreted ExoT.
TABLE 2.
Type III effector proteins secreted by isolates from CF patients and the environment
| Isolate source | No. of TTS+ isolates | No. (%) of isolates that secreted the following:
|
||||
|---|---|---|---|---|---|---|
| ExoS | ExoT | ExoU | PopB | PopD | ||
| CF patients | 65 | 60 (92) | 65 (100) | 2 (3) | 52 (80) | 57 (88) |
| Adults | 8 | 8 (100) | 8 (100) | 0 (0) | 5 (63) | 7 (88) |
| Chronically infected children | 40 | 35 (88) | 40 (100) | 2 (5) | 33 (83) | 33 (83) |
| Newly infected children | 17 | 17 (100) | 17 (100) | 0 (0) | 14 (82) | 17 (100) |
| Environment | 18 | 13 (72) | 18 (100) | 3 (17) | 14 (78) | 15 (83) |
Change in type III secretion phenotypes of CF patient strains over time.
To more directly examine whether strains were indeed changing their type III secretion phenotypes, PCR genotyping was employed to monitor over time the secretion patterns of individual P. aeruginosa strains infecting CF patients. A panel of archived P. aeruginosa isolates from serial respiratory cultures collected over a 3-year period was used for this purpose. Immunoblot analyses were performed to identify nine individuals from whom both TTS+ and TTS− isolates had been cultured (data not shown). RAPD PCR genotyping was then performed on these isolates so that individual strains from each patient could be monitored over time.
In each of five patients, all P. aeruginosa isolates had similar genotyping patterns, indicating that these isolates constituted a single strain (Fig. 3 A). Interestingly, in each case, some isolates of the strain were TTS+, whereas others were TTS−. For example, patient CFN26 had nine P. aeruginosa isolates grown from a total of four different respiratory samples (Fig. 3 A). All nine of these isolates had the same PCR genotyping pattern, indicating that they were all the same strain and had originated from the same bacterium. Yet two of the nine isolates were TTS+, whereas the remaining seven were TTS−. Since the progenitor of these isolates was either TTS+ or TTS− at the time of initial infection, some of these isolates must have changed their type III secretion phenotype following infection of the airways of this patient.
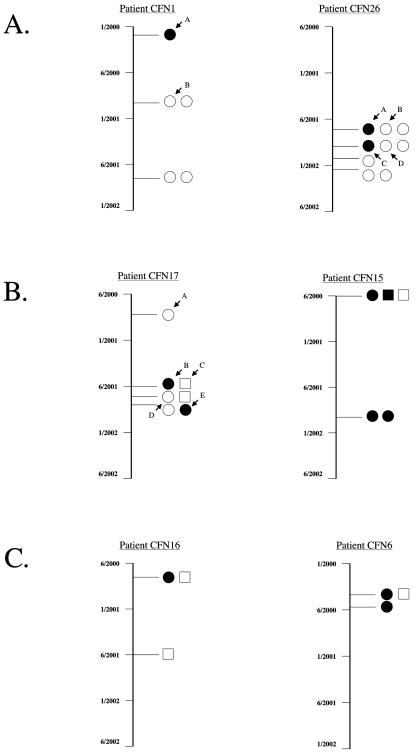
FIG. 3.
Type III protein secretion phenotypes and clonality of P. aeruginosa isolates from representative patients with CF. RAPD PCR genotyping was used to assess the clonality of isolates obtained over time from individual patients with CF, and immunoblot analysis was used to determine type III secretion phenotype. The date on which the culture was obtained is indicated on the left. Individual isolates are represented by a circle or a square. All isolates with similar or identical RAPD PCR genotyping patterns from a single patient are represented by the same geometric shape. TTS+ isolates are shown in black, whereas TTS− isolates are shown in white. Isolates that were chosen for further analysis by pulsed-field gel electrophoresis are indicated by arrows with letters. (A) Representative patients who were infected with both TTS+ and TTS− isolates that originated from a single clonal strain. (B) Patients who were infected by two unrelated strains, one of which consisted of both TTS+ and TTS− isolates. (C) Patients who were infected with a TTS+ strain and an unrelated TTS− strain.
In contrast to what was found for the isolates from these five patients, two distinct PCR genotyping patterns were observed in isolates from the remaining four patients, suggesting that each of these patients was infected by two distinct strains of P. aeruginosa. For two of the four patients, the secretion phenotype varied within one of the two strains but remained constant in the other (Fig. 3B). This finding again indicates that type III secretion phenotype switching had occurred following infection of the CF patient airways. From the two remaining patients, one strain was TTS+, whereas the other strain was TTS− (Fig. 3C). Thus, in these two patients, the isolation of both TTS+ and TTS− strains merely reflected infection with two unrelated strains, one of which secreted type III proteins and one of which did not.
To verify that both TTS+ and TTS− bacteria had indeed originated from a single P. aeruginosa strain, pulsed-field gel electrophoresis was performed on representative isolates (Fig. 3 and 4). DNA digestion patterns confirmed the results of the RAPD analysis. In some instances, a TTS+ isolate and a TTS− isolate had identical SpeI digestion patterns (Fig. 4, patient CFN17, lanes A and B). In other instances, a small number of bands differed between a TTS+ and a TTS− isolate (Fig. 4, patient CFN1, lanes A and B), but these differences were relatively minor and did not meet criteria for designating the isolates as nonclonal (54). In still other patients, TTS+ and TTS− isolates were unrelated to one another (Fig. 4, patient CFN17, lanes B and C). Together, these results indicated that within individual patients different type III protein secretion profiles represented either the presence of one clonal strain manifesting multiple secretion phenotypes or the presence of multiple strains. This in turn implied that in some cases the type III secretion phenotypes of P. aeruginosa bacteria had changed during infection of the CF patient airways.
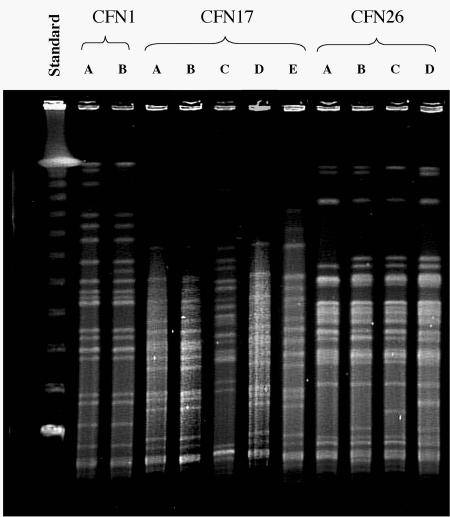
FIG. 4.
Pulsed-field gel electrophoresis analysis of P. aeruginosa isolates from representative CF patients. Isolates from three patients, designated CFN1, CFN17, and CFN26, were chosen for characterization. The examined isolates correspond to those marked by arrows and letters in Fig. 3. Using the criteria of Tenover et al. (54), it was determined that isolates A and B of patient CFN1 are closely related; isolates A, B, D, and E of patient CFN17 are closely related or identical, whereas isolate C is distinct; and isolates A, B, C, and D of patient CFN26 are all closely related to one another.
DISCUSSION
This is the most extensive study to date of the type III protein secretion properties of P. aeruginosa isolates from CF patients, encompassing nearly 500 isolates. Our analysis demonstrated that a relatively small proportion of isolates infecting these patients secreted type III proteins and that this proportion decreased with duration of infection. Furthermore, bacteria within the airways of these patients changed their secretion phenotype over time. Although other explanations are possible, these results are consistent with a model in which P. aeruginosa strains gradually change from a TTS+ phenotype to a TTS− phenotype while infecting the CF patient airways.
Overall, only 12% of isolates from chronically infected CF patients were TTS+ in vitro. This value agrees with those of other investigators, who also noted that most examined CF patient isolates were TTS− (9, 50). Dacheux et al. found that only 8 (29%) of 28 isolates from CF patients in France secreted type III proteins (9). Roy-Burman et al. noted that 15 (41%) of 37 isolates from CF patients in San Francisco, Calif., secreted at least one type III protein (50). Although the percentage of TTS+ isolates in these studies may differ somewhat from our own because of the different type III proteins that were measured and dissimilar patient demographics, all the studies noted that a minority of isolates secreted type III proteins. The relatively low prevalence of TTS+ CF patient isolates was in sharp contrast to the high percentage of TTS+ isolates from other sources. For example, in the present study 90% of environmental isolates secreted type III proteins. Likewise, 77% of isolates from patients with hospital-acquired pneumonia (24), 89% of isolates from respiratory and blood specimens from patients with acute respiratory infection (50), and 80% of isolates from patients with bacteremia (3) secreted these proteins. Thus, the type III secretion properties of P. aeruginosa isolates infecting the CF patient lung differed dramatically from those causing acute infections, including acute respiratory infections.
Examination of multiple P. aeruginosa isolates from each CF patient allowed us to assess the heterogeneity of isolates within individuals. Interestingly, in many cases individual patients were infected with both TTS+ and TTS− isolates. A consequence of this finding is that prior investigations, which examined one or two P. aeruginosa isolates per CF patient, (9, 50), may have underestimated the proportion of CF patients that harbor TTS+ strains. By examining 5 to 10 isolates from each of the patients enrolled in our study, we determined that at least 32% were infected with TTS+ isolates. If more than 5 to 10 isolates per patient had been examined, it is likely that additional patients would have been shown to be infected with at least one isolate that secreted type III proteins. Thus, the actual proportion of patients infected with TTS+ isolates is likely to exceed one-third.
Interestingly, even among isolates cultured from CF patients, the proportion of TTS+ P. aeruginosa isolates differed depending upon the duration of infection. Although 90% of P. aeruginosa isolates from the environment, the reservoir from which the majority of individuals with CF are thought to acquire their P. aeruginosa strains (49), secreted type III proteins, only 49% of isolates from newly infected CF patients did so. In chronically infected children with CF, this prevalence decreased to 18%, and in chronically infected adults, who are expected to have harbored P. aeruginosa strains for the longest times, only 4% of isolates secreted type III proteins. This trend was not merely due to an inability of mucoid isolates, which are more common in chronically infected individuals, to secrete type III proteins, as numerous TTS+ and TTS− isolates were observed among both mucoidy and nonmucoidy strains (data not shown). These results suggest a model whereby P. aeruginosa strains gradually lose the ability to secrete type III proteins as length of time in the CF patient airways increases. We could not, however, rule out the possibility that differences in levels of secretion between isolates from adults and children resulted from differences in the therapeutic practices applied to these two groups of patients, such as chronic use of antibiotics.
If P. aeruginosa isolates indeed switch from a TTS+ phenotype to a TTS− phenotype while in CF patient airways, it should be possible to identify both TTS+ and TTS− isolates of the same strain within an individual. In other words, a single bacterial clone should yield some progeny that secrete type III proteins and other progeny that do not. This was indeed the case. TTS+ and TTS− CF patient isolates with the same PCR genotyping patterns were identified from the same source patients.
A possible explanation for the decreased prevalence of TTS+ isolates of P. aeruginosa in CF patients is that there is selection against such strains or selection for TTS− strains. Individuals with CF mount an antibody response against type III proteins (2, 38). The presence of antibodies against at least one of these type III proteins, PcrV, is protective and results in clearance of P. aeruginosa during acute pulmonary infections (11, 17, 51, 53). Therefore, it is conceivable that over time TTS+ strains are cleared from CF patients by an antibody response against type III proteins but that TTS− strains are not. Also, secretion of ExoU and ExoS leads to host cell death (13, 26, 32, 41, 46, 50), tissue destruction, and, as a result, an increased inflammatory response. Such effects may lead to more-severe disease in patients with acute infections, such as hospital-acquired pneumonia (24), but may ultimately result in clearance of the organism and may not be suitable for long-term persistence in CF patient airways. Interestingly, other virulence determinants, such as type IV pili and flagella, are also infrequently produced by CF patient isolates of P. aeruginosa (37), indicating that this may be a general approach used by P. aeruginosa to persist in the CF patient pulmonary environment for prolonged periods.
Also of interest was the fact that TTS+ CF patient isolates in general produced different type III effector proteins than isolates from acute infections. Although approximately one-third of TTS+ isolates from patients with acute infections, such as hospital-acquired pneumonia, secreted ExoU (24, 50), only 3% of TTS+ CF patient isolates secreted this toxin. Among TTS+ isolates, ExoU secretion is usually inversely related to ExoS secretion (12, 24). Therefore, it was not surprising that nearly all TTS+ CF patient isolates secreted ExoS, compared to two-thirds of TTS+ isolates from patients with acute infections (24, 50). These results are consistent with previous reports that P. aeruginosa isolates containing the ExoU-encoding gene are significantly less common in patients with CF than in patients with hospital-acquired pneumonia (12). Likewise, isolates containing the ExoS-encoding gene are significantly more common in CF patient isolates (12). Possible interpretations of these observations are that ExoU-secreting strains are selected against or that ExoS-secreting strains are selected for in CF patient airways.
The mechanism by which P. aeruginosa strains change their type III secretion phenotypes is unknown, but it is clear that nearly all TTS− CF patient isolates harbor at least some type III protein secretion genes and thus may have secreted type III proteins at one time (9, 12). The environment of the CF patient lung may provide a selective advantage to spontaneously arising mutants that fail to secrete type III proteins. This advantage may result from the absence of type III proteins themselves or from changes in the production of factors that are coregulated with type III protein secretion. For example, hyperpiliated small-colony variants of P. aeruginosa have been shown to arise in the airways of CF patients (28, 29). These variants differ from wild-type P. aeruginosa strains in their ability to form biofilms and in their secretion of a large number of factors, including type III proteins (58). Changes in motility, metabolism, and antibiotic resistance have also been linked to the development of a hypermutable state in some P. aeruginosa isolates following infection of the airways of a CF patient (40). Whether hypermutability also results in changes in type III protein secretion remains to be determined. Alternatively, a more specific and directed regulatory process may be responsible. For example, P. aeruginosa may have regulatory systems that respond to the environmental conditions found in CF patient airways by specifically altering the expression or secretion of type III proteins. In this regard, it is interesting that overexpression of ExsA, a transcriptional activator of the type III secretion system, restores the expression of type III proteins in some TTS− P. aeruginosa isolates from CF patients (8). This finding suggests that at least in some TTS− isolates, the type III protein secretion regulon remains largely intact.
That P. aeruginosa bacteria change their type III secretion phenotypes during infection of individuals with CF may have important implications. Since type III secretion factors have a number of effects on host tissues, the expression or lack of expression of these virulence determinants may influence the clinical outcomes of patients with CF. Such is the case for alginate, a P. aeruginosa factor that causes the mucoidy phenotype and, when overexpressed in individuals with CF, has prognostic significance (10, 31, 43). The role of type III protein secretion or its absence in disease progression in infected individuals with CF is unclear but worthy of exploration. If, like the mucoidy phenotype, a particular type III secretion phenotype is associated with more rapid clinical deterioration, testing for type III protein secretion strains may have prognostic utility. Likewise, developing compounds that modulate changes in type III secretion phenotypes may be beneficial. Also, it is now appreciated that individuals with CF may acquire P. aeruginosa from either the natural environment or from close contact with infected individuals with CF (5, 22, 49). Our findings indicate that strains from these two reservoirs differ significantly in their type III protein secretion properties. These differences may affect the rate of infection, persistence of infection, or clinical deterioration in individuals who acquire P. aeruginosa from one or the other of these two reservoirs.
Acknowledgments
This work was supported by the following: grant RR-00048 from the National Center for Research Resources (M.J.), grants RO1-AI053674 (A.R.H.) and KO8-AI01524 (A.R.H.) from the National Institutes of Health, and an unrestricted gift from the Chiron Corporation.
We thank Susan Collins and Xiaotian Zheng for assistance in identifying and collecting P. aeruginosa isolates, Jenny Vergara and Eileen Potter for assistance with the enrollment of patients and collection of clinical data, and Michael Malczynski for performing the pulsed-field gel electrophoresis experiments.
REFERENCES
- 1.Abman, S. H., J. W. Ogle, R. J. Harbeck, N. Butler-Simon, K. B. Hammond, and F. J. Accurso. 1991. Early bacteriologic, immunologic, and clinical courses of young infants with cystic fibrosis identified by neonatal screening. J. Pediatr. 119:211-217. [DOI] [PubMed] [Google Scholar]
- 2.Banwart, B., M. L. Splaingard, P. M. Farrell, M. J. Rock, P. L. Havens, J. Moss, M. E. Ehrmantraut, D. W. Frank, and J. T. Barbieri. 2002. Children with cystic fibrosis produce an immune response against exoenzyme S, a type III cytotoxin of Pseudomonas aeruginosa. J. Infect. Dis. 185:269-270. [DOI] [PubMed] [Google Scholar]
- 3.Berthelot, P., I. Attree, P. Plesiat, J. Chabert, S. de Bentzmann, B. Pozzetto, and F. Grattard. 2003. Genotypic and phenotypic analysis of type III secretion system in a cohort of Pseudomonas aeruginosa bacteremia isolates: evidence for a possible association between O serotypes and exo genes. J. Infect. Dis. 188:512-518. [DOI] [PubMed] [Google Scholar]
- 4.Bryan, L. E. 1989. Microbial persistence or phenotypic adaptation to antimicrobial agents: cystic fibrosis as an illustrative case, p. 411-420. In L. E. Bryan (ed.), Microbial resistance to drugs. Springer-Verlag, Berlin, Germany.
- 5.Cheng, K., R. L. Smyth, J. R. Govan, C. Doherty, C. Winstanley, N. Denning, D. P. Heaf, H. van Saene, and C. A. Hart. 1996. Spread of beta-lactam-resistant Pseudomonas aeruginosa in a cystic fibrosis clinic. Lancet 348:639-642. [DOI] [PubMed] [Google Scholar]
- 6.Coburn, J., and D. Frank. 1999. Macrophages and epithelial cells respond differently to the Pseudomonas aeruginosa type III secretion system. Infect. Immun. 67:3151-3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cowell, B. A., D. Y. Chen, D. W. Frank, A. J. Vallis, and S. M. J. Fleiszig. 2000. ExoT of cytotoxic Pseudomonas aeruginosa prevents uptake by corneal epithelial cells. Infect. Immun. 68:403-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dacheux, D., I. Attree, and B. Toussaint. 2001. Expression of ExsA in trans confers type III secretion system-dependent cytotoxicity on noncytotoxic Pseudomonas aeruginosa cystic fibrosis isolates. Infect. Immun. 69:538-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dacheux, D., B. Toussaint, M. Richard, G. Brochier, J. Croize, and I. Attree. 2000. Pseudomonas aeruginosa cystic fibrosis isolates induce rapid, type III secretion-dependent, but ExoU-independent, oncosis of macrophages and polymorphonuclear neutrophils. Infect. Immun. 68:2916-2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demko, C. A., P. J. Byard, and P. B. Davis. 1995. Gender differences in cystic fibrosis: Pseudomonas aeruginosa infection. J. Clin. Epidemiol. 48:1041-1049. [DOI] [PubMed] [Google Scholar]
- 11.Faure, K., J. Fujimoto, D. W. Shimabukuro, T. Ajayi, N. Shime, K. Moriyama, E. G. Spack, J. P. Wiener-Kronish, and T. Sawa. 2003. Effects of monoclonal anti-PcrV antibody on Pseudomonas aeruginosa-induced acute lung injury in a rat model. J. Immune Based Ther. Vaccines 1:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feltman, H., G. Schulert, S. Khan, M. Jain, L. Peterson, and A. R. Hauser. 2001. Prevalence of type III secretion genes in clinical and environmental isolates of Pseudomonas aeruginosa. Microbiology 147:2659-2669. [DOI] [PubMed] [Google Scholar]
- 13.Finck-Barbançon, V., J. Goranson, L. Zhu, T. Sawa, J. P. Wiener-Kronish, S. M. J. Fleiszig, C. Wu, L. Mende-Mueller, and D. Frank. 1997. ExoU expression by Pseudomonas aeruginosa correlates with acute cytotoxicity and epithelial injury. Mol. Microbiol. 25:547-557. [DOI] [PubMed] [Google Scholar]
- 14.Fitzsimmons, S. C. 1993. The changing epidemiology of cystic fibrosis. J. Pediatr. 122:1-9. [DOI] [PubMed] [Google Scholar]
- 15.Fleiszig, S. M. J., J. P. Wiener-Kronish, H. Miyazaki, V. Vallas, K. Mostov, D. Kanada, T. Sawa, T. S. B. Yen, and D. Frank. 1997. Pseudomonas aeruginosa-mediated cytotoxicity and invasion correlate with distinct genotypes at the loci encoding exoenzyme S. Infect. Immun. 65:579-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frank, D. 1997. The exoenzyme S regulon of Pseudomonas aeruginosa. Mol. Microbiol. 26:621-629. [DOI] [PubMed] [Google Scholar]
- 17.Frank, D. W., A. Vallis, J. W. Wiener-Kronish, A. Roy-Burman, E. G. Spack, B. P. Mullaney, M. Megdoud, J. D. Marks, R. Fritz, and T. Sawa. 2002. Generation and characterization of a protective monoclonal antibody to Pseudomonas aeruginosa PcrV. J. Infect. Dis. 186:64-73. [DOI] [PubMed] [Google Scholar]
- 18.Frithz-Lindsten, E., Y. Du, R. Rosqvist, and A. Forsberg. 1997. Intracellular targeting of exoenzyme S of Pseudomonas aeruginosa via type III-dependent translocation induces phagocytosis resistance, cytotoxicity and disruption of actin microfilaments. Mol. Microbiol. 25:1125-1139. [DOI] [PubMed] [Google Scholar]
- 19.Garrity-Ryan, L., B. Kazmierczak, R. Kowal, J. Comolli, A. Hauser, and J. N. Engel. 2000. The arginine finger domain of ExoT contributes to actin cytoskeleton disruption and inhibition of internalization of Pseudomonas aeruginosa by epithelial cells and macrophages. Infect. Immun. 68:7100-7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goehring, U.-M., G. Schmidt, K. J. Pederson, K. Aktories, and J. T. Barbieri. 1999. The N-terminal domain of Pseudomonas aeruginosa exoenzyme S is a GTPase-activating protein for Rho GTPases. J. Biol. Chem. 274:36369-36372. [DOI] [PubMed] [Google Scholar]
- 21.Govan, J. R. W., and V. Deretic. 1996. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol. Rev. 60:539-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grothues, D., U. Koopmann, H. von der Hardt, and B. Tummler. 1988. Genome fingerprinting of Pseudomonas aeruginosa indicates colonization of cystic fibrosis siblings with closely related strains. J. Clin. Microbiol. 26:1973-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hancock, R. E., L. M. Mutharia, L. Chan, R. P. Darveau, D. P. Speert, and G. B. Pier. 1983. Pseudomonas aeruginosa isolates from patients with cystic fibrosis: a class of serum-sensitive, nontypable strains deficient in lipopolysaccharide O side chains. Infect Immun. 42:170-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hauser, A. R., E. Cobb, M. Bodí, D. Mariscal, J. Vallés, J. N. Engel, and J. Rello. 2002. Type III protein secretion is associated with poor clinical outcomes in patients with ventilator-associated pneumonia caused by Pseudomonas aeruginosa. Crit. Care Med. 30:521-528. [DOI] [PubMed] [Google Scholar]
- 25.Hauser, A. R., and J. N. Engel. 1999. Pseudomonas aeruginosa induces type III secretion-mediated apoptosis of macrophages and epithelial cells. Infect. Immun. 67:5530-5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hauser, A. R., P. J. Kang, and J. Engel. 1998. PepA, a novel secreted protein of Pseudomonas aeruginosa, is necessary for cytotoxicity and virulence. Mol. Microbiol. 27:807-818. [DOI] [PubMed] [Google Scholar]
- 27.Hauser, A. R., P. J. Kang, S. J. M. Fleiszig, K. Mostov, and J. Engel. 1998. Defects in type III secretion correlate with internalization of Pseudomonas aeruginosa by epithelial cells. Infect. Immun. 66:1413-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haussler, S., B. Tummler, H. Weissbrodt, M. Rohde, and I. Steinmetz. 1999. Small-colony variants of Pseudomonas aeruginosa in cystic fibrosis. Clin. Infect. Dis. 29:621-625. [DOI] [PubMed] [Google Scholar]
- 29.Haussler, S., I. Ziegler, A. Lottel, F. von Gotz, M. Rohde, D. Wehmhohner, S. Saravanamuthu, B. Tummler, and I. Steinmetz. 2003. Highly adherent small-colony variants of Pseudomonas aeruginosa in cystic fibrosis lung infection. J. Med. Microbiol. 52:295-301. [DOI] [PubMed] [Google Scholar]
- 30.Henriksson, M. L., R. Rosqvist, M. Telepnev, H. Wolf-Watz, and B. Hallberg. 2000. Ras effector pathway activation by epidermal growth factor is inhibited in vivo by exoenzyme S ADP-ribosylation of Ras. Biochem. J. 347:217-222. [PMC free article] [PubMed] [Google Scholar]
- 31.Henry, R. L., C. M. Mellis, and L. Petrovic. 1992. Mucoid Pseudomonas aeruginosa is a marker of poor survival in cystic fibrosis. Pediatr. Pulmonol. 12:158-161. [DOI] [PubMed] [Google Scholar]
- 32.Hirakata, Y., B. B. Finlay, D. A. Simpson, S. Kohno, S. Kamihira, and D. P. Speert. 2000. Penetration of clinical isolates of Pseudomonas aeruginosa through MDCK epithelial cell monolayers. J. Infect. Dis. 181:765-769. [DOI] [PubMed] [Google Scholar]
- 33.Kang, P. J., A. R. Hauser, G. Apodaca, S. Fleiszig, J. Wiener-Kronish, K. Mostov, and J. N. Engel. 1997. Identification of Pseudomonas aeruginosa genes required for epithelial cell injury. Mol. Microbiol. 24:1249-1262. [DOI] [PubMed] [Google Scholar]
- 34.Kerem, E., M. Corey, R. Gold, and H. Levison. 1990. Pulmonary function and clinical course in patients with cystic fibrosis after pulmonary colonization with Pseudomonas aeruginosa. J. Pediatr. 116:714-719. [DOI] [PubMed] [Google Scholar]
- 35.Kersulyte, D., M. J. Struelens, A. Deplano, and D. E. Berg. 1995. Comparison of arbitrary primed PCR and macrorestriction (pulsed-field gel electrophoresis) typing of Pseudomonas aeruginosa strains from cystic fibrosis patients. J. Clin. Microbiol. 33:2216-2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krall, R., G. Schmidt, K. Aktories, and J. T. Barbieri. 2000. Pseudomonas aeruginosa ExoT is a Rho GTPase-activating protein. Infect. Immun. 68:6066-6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mahenthiralingam, E., M. E. Campbell, and D. P. Speert. 1994. Nonmotility and phagocytic resistance of Pseudomonas aeruginosa isolates from chronically colonized patients with cystic fibrosis. Infect. Immun. 62:596-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moss, J., M. E. Ehrmantraut, B. D. Banwart, D. W. Frank, and J. T. Barbieri. 2001. Sera from adult patients with cystic fibrosis contain antibodies to Pseudomonas aeruginosa type III apparatus. Infect. Immun. 69:1185-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nicas, T. I., and B. H. Iglewski. 1984. Isolation and characterization of transposon-induced mutants of Pseudomonas aeruginosa deficient in production of exoenzyme S. Infect. Immun. 45:470-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oliver, A., R. Canton, P. Campo, F. Baquero, and J. Blazquez. 2000. High frequency of hypermutable Pseudomonas aeruginosa in cystic fibrosis lung infection. Science 288:1251-1254. [DOI] [PubMed] [Google Scholar]
- 41.Olson, J. C., J. E. Fraylick, E. M. McGuffie, K. M. Dolan, T. L. Yahr, D. W. Frank, and T. S. Vincent. 1999. Interruption of multiple cellular processes in HT-29 epithelial cells by Pseudomonas aeruginosa exoenzyme S. Infect. Immun. 67:2847-2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olson, J. C., E. M. McGuffie, and D. W. Frank. 1997. Effects of differential expression of the 49-kilodalton exoenzyme S by Pseudomonas aeruginosa on cultured eukaryotic cells. Infect. Immun. 65:248-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parad, R. B., C. J. Gerard, D. Zurakowski, D. P. Nichols, and G. B. Pier. 1999. Pulmonary outcome in cystic fibrosis is influenced primarily by mucoid Pseudomonas aeruginosa infection and immune status and only modestly by genotype. Infect. Immun. 67:4744-4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pedersen, S. S. 1992. Lung infection with alginate-producing, mucoid Pseudomonas aeruginosa in cystic fibrosis. APMIS 100:1-79. [PubMed] [Google Scholar]
- 45.Pedersen, S. S., N. Hoiby, F. Espersen, and C. Koch. 1992. Role of alginate in infection with mucoid Pseudomonas aeruginosa in cystic fibrosis. Thorax 47:6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pederson, K. J., and J. T. Barbieri. 1998. Intracellular expression of the ADP-ribosyltransferase domain of Pseudomonas aeruginosa exoenzyme S is cytotoxic to eukaryotic cells. Mol. Microbiol. 30:751-759. [DOI] [PubMed] [Google Scholar]
- 47.Pederson, K. J., A. J. Vallis, K. Aktories, D. W. Frank, and J. T. Barbieri. 1999. The amino-terminal domain of Pseudomonas aeruginosa ExoS disrupts actin filaments via small-molecular-weight GTP-binding proteins. Mol. Microbiol. 32:393-401. [DOI] [PubMed] [Google Scholar]
- 48.Romling, U., B. Fiedler, J. Bosshammer, D. Grothues, J. Greipel, H. von der Hardt, and B. Tummler. 1994. Epidemiology of chronic Pseudomonas aeruginosa infection in cystic fibrosis. J. Infect. Dis. 170:1616-1621. [DOI] [PubMed] [Google Scholar]
- 49.Romling, U., J. Wingender, H. Muller, and B. Tummler. 1994. A major Pseudomonas aeruginosa clone common to patients and aquatic habitats. Appl. Environ. Microbiol. 60:1734-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roy-Burman, A., R. H. Savel, S. Racine, B. L. Swanson, N. S. Revadigar, J. Fujimoto, T. Sawa, D. W. Frank, and J. P. Wiener-Kronish. 2001. Type III protein secretion is associated with death in lower respiratory and systemic Pseudomonas aeruginosa infections. J. Infect. Dis. 183:1767-1774. [DOI] [PubMed] [Google Scholar]
- 51.Sawa, T., T. Yahr, M. Ohara, K. Kurahashi, M. A. Gropper, J. P. Wiener-Kronish, and D. W. Frank. 1999. Active and passive immunization with the Pseudomonas V antigen protects against type III intoxication and lung injury. Nature Med. 5:392-398. [DOI] [PubMed] [Google Scholar]
- 52.Schulert, G. S., H. Feltman, S. D. P. Rabin, C. G. Martin, S. E. Battle, J. Rello, and A. R. Hauser. 2003. Secretion of the toxin ExoU is a marker for highly virulent Pseudomonas aeruginosa isolates obtained from patients with hospital-acquired pneumonia. J. Infect. Dis. 188:1695-1706. [DOI] [PubMed] [Google Scholar]
- 53.Shime, N., T. Sawa, J. Fujimoto, K. Faure, L. R. Allmond, T. Karaca, B. L. Swanson, E. G. Spack, and J. P. Wiener-Kronish. 2001. Therapeutic administration of anti-PcrV F(ab′)2 in sepsis associated with Pseudomonas aeruginosa. J. Immunol. 167:5880-5886. [DOI] [PubMed] [Google Scholar]
- 54.Tenover, F. C., R. D. Arbert, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thompson, M. R., M. J. Bjorn, P. A. Sokol, J. D. Lile, and B. H. Iglewski. 1980. Exoenzyme S: an ADP-ribosyl transferase produced by Pseudomonas aeruginosa, p. 425-433. In M. Smulson and T. Sugimura (ed.), Novel ADP-ribosylation of regulatory enzymes and proteins. Elsevier/North-Holland Inc., Amsterdam, The Netherlands.
- 56.Vallis, A. J., V. Finck-Barbancon, T. L. Yahr, and D. W. Frank. 1999. Biological effects of Pseudomonas aeruginosa type III-secreted proteins on CHO cells. Infect. Immun. 67:2040-2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vincent, T. S., J. E. Fraylick, E. M. McGuffie, and J. C. Olson. 1999. ADP-ribosylation of oncogenic Ras proteins by Pseudomonas aeruginosa exoenzyme S in vivo. Mol. Microbiol. 32:1054-1064. [DOI] [PubMed] [Google Scholar]
- 58.Wehmhoner, D., S. Haussler, B. Tummler, L. Jansch, F. Bredenbruch, J. Wehland, and I. Steinmetz. 2003. Inter- and intraclonal diversity of the Pseudomonas aeruginosa proteome manifests within the secretome. J. Bacteriol. 185:5807-5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yahr, T., L. M. Mende-Mueller, M. B. Friese, and D. W. Frank. 1997. Identification of type III secreted products of the Pseudomonas aeruginosa exoenzyme S regulon. J. Bacteriol. 179:7165-7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yahr, T. L., J. Goranson, and D. W. Frank. 1996. Exoenzyme S of Pseudomonas aeruginosa is secreted by a type III secretion pathway. Mol. Microbiol. 22:991-1003. [DOI] [PubMed] [Google Scholar]