Abstract
Staphylococcus intermedius isolates from dogs (n = 44) and pigeons (n = 62) were categorized into 12 types by intergenic ribosomal DNA spacer polymorphism analysis. All isolates from pigeons were lukS positive and all isolates from dogs were lukS and lukF positive by dot blot analysis. The mean leukotoxicity titer for dog isolates was at least 129-fold higher than that for pigeon isolates.
Staphylococcus aureus strains produce several toxins, including single-component α-hemolysin and the bicomponent leukotoxins Panton-Valentine leucocidin (PVL) and γ-hemolysin (4). PVL is cytotoxic to human and rabbit polymorphonuclear cells, monocytes, and macrophages, and γ-hemolysin is cytolytic to mammalian erythrocytes (4, 7). PVL-producing S. aureus is strongly associated with skin infections, such as furuncles (14), and with lethal necrotizing pneumonia in young immunocompetent patients (6). An S. intermedius leukotoxin known as Luk-I has also been identified (15). Characterization and sequence analysis have shown that, similar to PVL, Luk-I is encoded as a lukI operon with two cotranscribed genes, lukS and lukF (referred to elsewhere as lukS-I and lukF-I, respectively), encoding LukS and LukF (15). Luk-I shows a strong leukotoxicity on various polymorphonuclear cells, but only a slight hemolytic activity on rabbit erythrocytes (15).
It has been shown by various genotyping methods, such as 16S-23S intergenic ribosomal DNA spacer polymorphism analysis (ITS-PCR), EcoRI ribotyping, and SmaI pulsed-field gel electrophoresis, that S. intermedius strains are diverse and that the genotypes of S. intermedius isolates from dogs are distinct from those from pigeons (2, 3, 18). The enterotoxins and hemolysins are more prevalent among S. intermedius isolates from dogs than among those from pigeons (5, 17). The prevalence of leukotoxin in S. intermedius isolates from dogs and pigeons, however, has yet to be investigated.
Here, we have typed S. intermedius isolates from dogs and pigeons by ITS-PCR and have investigated the prevalence of the lukI operon by dot blot hybridization and the leukotoxic activity of the isolates. We also report the identification of a new leukotoxin gene, i.e., a lukS ortholog, in S. intermedius isolates from pigeons.
The study was carried out with 106 S. intermedius isolates recovered from healthy skin or infected sites of dogs and pigeons from four different prefectures in Japan (Chiba, Kanagawa, Saitama, and Tokyo). Included were 44 isolates from dogs (8 healthy dogs, 23 dogs with pyoderma, and 13 dogs with otitis externa) and 62 isolates from pigeons (5 pigeons from a zoo, 10 domesticated pigeons, and 47 wild pigeons). Isolation and identification of S. intermedius isolates were done as described previously (5). An S. intermedius type strain from pigeons, JCM2422T (8), was used as the quality control strain.
Genomic DNA preparation from S. intermedius and genotyping by ITS-PCR were done as described by Matsuhashi et al. (11) and Bes et al. (2), respectively. Probes used to detect lukS and lukF in S. intermedius isolates were prepared by PCR using genomic DNA of a dog isolate, S. intermedius AV8004. The primers used were 5′-TGTAAGCAGCAGAAAATGGGG-3′ and 5′-GCCCGATAGGACTTCTTACAA-3′ for lukS and 5′-CCTGTCTATGCCGCTAATCAA-3′ and 5′-AGGTCATGGAAGCTATCTCGA-3′ for lukF. DNA amplifications were performed for 35 cycles of 1 min at 94°C, 1 min at 57°C, and 1 min at 72°C. Sequences of PCR products, such as lukS (503 bp) and lukF (572 bp), were confirmed with published sequences in a database (GenBank accession number X79188). Dot blot hybridization was done as described previously (10) by using the PCR probes, bacterial genomic DNA, and a DIG DNA labeling and detection kit (Roche).
The assay for leukotoxic activity was performed as described previously by Rainard et al. (16), with some modifications. The culture supernatants of bacteria grown overnight in brain heart infusion broth (Difco) were collected and stored frozen at −20°C until used. Freezing did not have a significant effect on the leukotoxic activity of the samples examined. Freshly isolated rabbit leukocytes were suspended in phosphate-buffered saline containing 0.5% gelatin to get a concentration of 2.0 × 105 cells/20 μl. Serial twofold dilutions (20 μl each) of the culture supernatant in phosphate-buffered saline containing 0.5% gelatin were done in a 96-well microtiter plate and were mixed with 20 μl (each) of leukocyte suspension and incubated at 37°C for 10 min in a moisturized chamber (12). The last dilution that induced the flattening of cells, a feature of cytotoxicity, in 95% of leukocytes was determined under a phase-contrast microscope and confirmed by Giemsa staining. The leukotoxicity titer was the inverse of the last dilution (16).
S. intermedius isolates (n = 106) were categorized into 12 ITS-PCR types (A to L); isolates from dogs and pigeons were distributed into types A to G and types H to L, respectively (Table 1). The observed heterogeneity among our S. intermedius isolates is in agreement with the report of Bes et al. (2). Isolates from zoo pigeons and from domesticated pigeons were restricted to ITS-PCR types H and I, respectively. Isolates from infected and healthy dogs were genotypically not distinguishable by ITS-PCR typing (data not shown), similar to previous findings (1, 9, 13).
TABLE 1.
Genotypic and phenotypic characteristics of S. intermedius isolates from dogs and pigeons
| Source of isolate | ITS-PCR type | No. of isolates | No. of isolates positive for:
|
Leukotoxicity titera
|
||
|---|---|---|---|---|---|---|
| lukS | lukF | Range | Meanb | |||
| Dogs | A | 14 | 14 | 14 | 256-512 | 441 |
| B | 16 | 16 | 16 | 256-1024 | 450 | |
| C | 4 | 4 | 4 | 512 | 512 | |
| D | 6 | 6 | 6 | 256-1024 | 512 | |
| E | 2 | 2 | 2 | 512-1024 | 724 | |
| F | 1 | 1 | 1 | 512 | 512 | |
| G | 1 | 1 | 1 | 256 | 256 | |
| Total | 44 | 44 | 44 | 256-1024 | 466 | |
| Pigeons | H | 5 | 5c | 0 | <2 | <2 |
| I | 11 | 11c | 0 | 8-32 | 12.4 | |
| J | 1 | 1c | 0 | <2 | <2 | |
| K | 5 | 5c | 0 | <2 | <2 | |
| L | 40 | 40c | 0 | <2-8 | <3 | |
| Total | 62 | 62 | 0 | <2-32 | <3.6 | |
Leukotoxicity titer is the inverse of the last dilution that induced the flattening of cells in 95% of leukocytes.
Calculated after logarithmic transformation.
Weak reaction as depicted in Fig. 1.
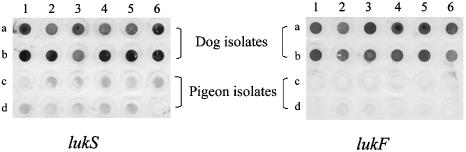
All 44 S. intermedius isolates from dogs (ITS-PCR types A to G) were lukS and lukF positive and exhibited very high cytotoxic activity on rabbit leukocytes, with a mean leukotoxicity titer of 466 (Table 1). Some randomly selected dog isolates (n = 11) also had similar levels of activity on human leukocytes (data not shown). There was no significant difference in leukotoxic activity between the dog isolates belonging to ITS-PCR types A to E or to different sources (healthy dogs, dogs with pyoderma, and dogs with otitis externa) (P = 0.52 or P = 0.77, respectively; Tukey-Kramer test). In contrast, 62 isolates from pigeons (ITS-PCR types H to L) were positive only for lukS by dot blot analysis (Fig. 1). Dot blot results were confirmed by PCR (data not shown). Furthermore, the mean leukotoxicity titer for pigeon isolates was <3.6, which was significantly lower than that for dog isolates (P < 0.0001; t test).
FIG. 1.
Dot blot analysis of genomic DNA from S. intermedius isolates with lukS and lukF probes. Spots a1 and a2, ITS-PCR type A; a3 and a4, ITS-PCR type B; a5 and a6, ITS-PCR type C; b1 and b2, ITS-PCR type D; b3 and b4, ITS-PCR type E; b5, ITS-PCR type F; b6, ITS-PCR type G; c1 and c2, ITS-PCR type H; c3 and c4, ITS-PCR type I; c5, ITS-PCR type J; c6 and d1, ITS-PCR type K; d2 to d5, ITS-PCR type L; and d6, negative control (Salmonella enterica serovar Typhimurium). All S. intermedius strains were included in dot blot hybridization analysis, but only two representative, randomly selected strains for each ITS-PCR type, with the exception of four strains for ITS-PCR type L and one strain each for ITS-PCR types F and G, are shown.
Sequence analysis of PCR-amplified lukS products from representative isolates from each ITS-PCR type showed that lukS of pigeon isolates had a lower homology (75 to 86% identity at the amino acid level), compared with that of dog isolates (98 to 100% identity), to the lukS probe from the dog isolate AV8004 used in dot blot hybridization. Accordingly, we considered the leukotoxin gene amplifiable by lukS primers in pigeon isolates to be a new ortholog. This low homology may explain the weak reactions observed for dot blot analysis of pigeon isolates (Fig. 1). From these observations, it is conceivable that the apparent difference in leukotoxicity between S. intermedius strains from dogs and from pigeons in this study is contributed by lukF and that the presence of both lukF and the lukS ortholog is required for maximal leukotoxic activity. However, our results do not eliminate the possibility that a gene for the second component was present in pigeon isolates but not detected in this study.
Summarizing, our results demonstrated that there was a significant difference in the leukotoxic activity between S. intermedius strains from dogs and from pigeons, with at least 129-fold-higher activity in strains from dogs, and that the S. intermedius strains recovered from infected dogs were not distinct from those from healthy dogs with regard to leukotoxin production and genotype by ITS-PCR typing.
Nucleotide sequence accession number.
The lukS ortholog was assigned GenBank accession number AB185109.
REFERENCES
- 1.Barrs, V. R., D. Briscoe, R. Malik, and D. N. Love. 2000. Use of multilocus enzyme electrophoresis to distinguish clinically important strains of Staphylococcus intermedius from the skin of dogs. Aust. Vet. J. 78:267-272. [DOI] [PubMed] [Google Scholar]
- 2.Bes, M., L. S. Slim, F. Becharnia, H. Meugnier, F. Vandenesch, J. Etienne, and J. Freney. 2002. Population diversity of Staphylococcus intermedius isolates from various host species: typing by 16S-23S intergenic ribosomal DNA spacer polymorphism analysis. J. Clin. Microbiol. 40:2275-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chesneau, O., A. Morvan, S. Aubert, and N. E. Solh. 2000. The value of rRNA gene restriction site polymorphism analysis for delineating taxa in the genus Staphylococcus. Int. J. Syst. Evol. Microbiol. 50:689-697. [DOI] [PubMed] [Google Scholar]
- 4.Dinges, M. M., P. M. Orwin, and P. M. Schlievert. 2000. Exotoxins of Staphylococcus aureus. Clin. Microbiol. Rev. 13:16-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Futagawa-Saito, K., M. Suzuki, M. Ohsawa, S. Ohshima, N. Sakurai, W. Ba-Thein, and T. Fukuyasu. 2004. Identification and prevalence of an enterotoxin-related gene, se-int, in Staphylococcus intermedius isolates from dogs and pigeons. J. Appl. Microbiol. 96:1361-1366. [DOI] [PubMed] [Google Scholar]
- 6.Gillet, Y., B. Issartel, P. Vanhems, J.-C. Fournet, G. Lina, M. Bes, F. Vandenesch, Y. Piémont, N. Brousse, D. Floret, and J. Etienne. 2002. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet 359:753-759. [DOI] [PubMed] [Google Scholar]
- 7.Gladstone, G. P., and W. E. Heyningen. 1957. Staphylococcal leucocidins. Br. J. Exp. Pathol. 38:123-137. [PMC free article] [PubMed] [Google Scholar]
- 8.Hajek, V. 1976. Staphylococcus intermedius, a new species isolated from animals. Int. J. Syst. Bacteriol. 26:401-408. [Google Scholar]
- 9.Hesselbarth, J., and S. Schwarz. 1995. Comparative ribotyping of Staphylococcus intermedius from dogs, pigeons, horses and mink. Vet. Microbiol. 45:11-17. [DOI] [PubMed] [Google Scholar]
- 10.Holeckova, B., E. Holoda, M. Fotta, V. Kalinacova, J. Gondol', and J. Grolmus. 2002. Occurrence of enterotoxigenic Staphylococcus aureus in food. Ann. Agric. Environ. Med. 9:179-182. [PubMed] [Google Scholar]
- 11.Matsuhashi, M., M. D. Song, F. Ishino, M. Wachi, M. Doi, M. Inoue, K. Ubukata, N. Yamashita, and M. Konno. 1986. Molecular cloning of the gene of a penicillin-binding protein supposed to cause high resistance to β-lactam antibiotics in Staphylococcus aureus. J. Bacteriol. 167:975-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morinaga, N., Y. Kaihou, and M. Noda. 2003. Purification, cloning and characterization of variant LukE-LukD with strong leukocidal activity of staphylococcal bi-component leukotoxin family. Microbiol. Immunol. 47:81-90. [DOI] [PubMed] [Google Scholar]
- 13.Overturf, G. D., D. A. Talan, K. Singer, N. Anderson, J. I. Miller, R. T. Greene, and S. Froman. 1991. Phage typing of Staphylococcus intermedius. J. Clin. Microbiol. 29:373-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prevost, G., P. Couppie, P. Prevost, S. Gayet, P. Petiau, B. Cribier, H. Monteil, and Y. Piemont. 1995. Epidemiological data on Staphylococcus aureus strains producing synergohymenotropic toxins. J. Med. Microbiol. 42:237-245. [DOI] [PubMed] [Google Scholar]
- 15.Prevost, G., T. Bouakham, Y. Piemont, and H. Monteil. 1995. Characterisation of a synergohymenotropic toxin produced by Staphylococcus intermedius. FEBS Lett. 376:135-140. [DOI] [PubMed] [Google Scholar]
- 16.Rainard, P., J. C. Corrales, M. B. Barrio, T. Cochard, and B. Poutrel. 2003. Leucotoxic activities of Staphylococcus aureus strains isolated from cows, ewes, and goats with mastitis: importance of LukM/LukF′-PV leukotoxin. Clin. Diagn. Lab. Immunol. 10:272-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shimizu, A., J. Kawano, and S. Kimura. 1986. Biotyping of coagulase-positive Staphylococcus aureus and Staphylococcus intermedius strains isolated from various animals in Japan. Jpn. J. Vet. Sci. 48:1227-1235. [DOI] [PubMed] [Google Scholar]
- 18.Wakita, Y., A. Shimizu, V. Hajek, J. Kawano, and K. Yamashita. 2002. Characterization of Staphylococcus intermedius from pigeons, dogs, foxes, mink, and horses by pulsed-field gel electrophoresis. J. Vet. Med. Sci. 64:237-243. [DOI] [PubMed] [Google Scholar]