Abstract
Glucose, enzymatically released from pulp fiber sludge, was combined with inorganic salts and used as a growth medium for Alcaligenes eutrophus, a gram-negative strain producing poly(3-hydroxybutyrate) (PHB). By controlling the concentrations of the inorganic salts in the growth medium, almost 78% of the cell mass was converted to pure PHB. Efforts were made to find conditions for bacterial growth in the form of a biofilm on a cheap and reusable carrier. A number of positively charged carriers were tested, and the anion exchanger DEAE-Sephadex A-25 was chosen as a microcarrier for packed-bed biofilm cultures of A. eutrophus. Conditions for attachment, growth, and detachment were established. Biofilm formation on the microcarrier is strongly dependent on the ionic strength of the attachment medium. In order to achieve formation of the biofilm and its recovery from the microcarrier, the ionic strengths of the attachment and the detachment media were varied. Low ionic strength was tested for attachment, and high ionic strength was tested for detachment. Although biofilm formation in the packed-bed reactor is limited, the volumetric yield of cells based on the void volume of the packed bed is comparable with the batch culture yield.
Polyhydroxyalkanoates (PHAs) are hydroxyacid polyesters that are synthesized and accumulated as intracellular granules by a wide variety of bacteria (15). Generally, production of PHA is enhanced when a suitable carbon source is in excess but cellular growth is limited by another nutrient, e.g., nitrogen or phosphorus (3). Of the big family of PHAs, a homopolymer of 3-hydroxybutyrate, poly(3-hydroxybutyrate) (PHB), is the most widespread and the best characterized. PHB has aroused much interest in industry and research as a biocompatible, biodegradable, thermoplastic, and piezoelectric polymer with potential applications in medical, agricultural, and marine fields (19). Among the factors restricting the economy of PHB production is the cost of the carbon source (3). A glucose-utilizing strain of Alcaligenes eutrophus is the most widely used because it can produce enhanced amounts of PHB in a simple defined medium (13). The pathway and regulation of PHB synthesis in A. eutrophus have been studied in detail (8, 9).
One of the natural physiological growth forms for a microorganism is a biofilm, in which the microbial community is attached to a solid surface. From the biotechnological point of view, it is interesting to use a microbial film immobilized on a microcarrier surface for the production of a wide variety of biochemicals that can be utilized for different purposes (22, 24).
For biotechnology purposes, the packed-bed reactor system is one of the widely used operating modes of biofilm culture. Such a system has several advantages, such as yielding high cell densities and large amounts of target metabolites. Other advantages are protection from mechanical shear, the possibility for easy scale-up, and the facilitation of medium exchange, continuous removal of inhibitory metabolites, and product separation (6). Among the disadvantages are the inability to access cells directly during cultivation and the heterogeneity of the packed bed, i.e., the concentrations and cell gradients over the fixed bed height and within the carriers (5). Finally, the thickness of the biofilm is regulated by the available dissolved oxygen (10). Understanding the behavior of the biofilm is crucial for its control and management. The key factors for production and recovery of the biofilm are the steps involving attachment, growth, and detachment.
In the present study, PHB was synthesized by A. eutrophus using glucose recovered by enzymatic hydrolysis of pulp fiber sludge as a carbon source. The microorganism was grown on a selected microcarrier to produce PHB in the form of a biofilm. The conditions for biofilm attachment, growth, and detachment were studied.
MATERIALS AND METHODS
Cultivation conditions.
A. eutrophus (NCIMB 11599) from the National Collections of Industrial, Marine and Food Bacteria, Aberdeen, United Kingdom, was kept as a frozen stock at −80°C in 15% glycerol and used for all experiments. Three kinds of media were used (Table 1). The first subculture was made from the frozen stock on a 1.5% agar plate consisting of medium no. 1 (21), incubated for 48 h at 30°C, and then transferred to a 1.5% agar plate of medium no. 2 (20) to grow for another 40 h. This agar plate was kept for further seed culture.
TABLE 1.
Medium composition for A. eutrophus
| Component | Amt in medium no. 1 (g liter−1) | Component | Amt in:
|
|
|---|---|---|---|---|
| Medium no. 2 (g liter−1) | Medium no. 3 (g liter−1) | |||
| Yeast extract | 10 | Glucose | 10 | 20 |
| Polypeptone | 10 | (NH4)2SO4 | 1.0 | 4.0 |
| Meat/beef extract | 5 | MgSO4 · 7H2O | 0.2 | 1.2 |
| (NH4)2SO4 | 5 | KH2PO4 | 1.5 | 13.3 |
| Na2HPO4 · 12H2O | 9.0 | |||
| Citric acid | 1.7 | |||
| Trace element solutiona | 1.0 ml liter−1 | 10 ml liter−1 | ||
Trace element solution has the following composition (per liter): FeSO4·7H2O, 10 g; ZnSO4·7H2O, 2.25 g; CuSO4·5H2O, 1 g; MnSO4·H2O, 0.35 g; CaCl2·2H2O, 2 g; Na2B4O7·10H2O, 0.23 g; (NH4)6Mo7O24, 0.1 g; HCl, 35%, 10 ml. The pH of the medium was adjusted to 6.8 to 7.0 with 1.0 M NaOH. To make solid medium, 15 g of agar liter−1 was used.
For the seed culture, a loopful of cells was added to 20 ml of liquid medium no. 2 and cultured on a rotary shaker (150 rpm) at 30°C for 16 to 20 h. This seed culture was then transferred either to 200 ml of undiluted medium no. 3 (13) or to 10-times-diluted medium no. 3 with undiluted glucose (referred to below as diluted medium no. 3).
Analytical procedure.
The biomass of the culture was determined by the attenuance at 600 nm (D600) (Hitachi U-3200 spectrophotometer) and by dry weight at regular intervals. For cell dry weight estimation, a 10-ml sample was centrifuged at 7,000 × g in a preweighed centrifuge tube, and the pellet was washed twice and finally freeze-dried. The centrifuge tube was weighed again to calculate the cell dry weight. The relationship between the D600 and the dried biomass concentration was roughly determined by simple linear correlation. The glucose concentration was determined by off-line measurements employing glucose oxidase and peroxidase (25). The phosphate and ammonium ion concentrations were analyzed using standard Merck Spectroquant test kits. The PHB concentration in the biomass was determined directly by acid hydrolysis of PHB to crotonic acid according to the method of Korotkova et al. (14). About 10 mg of lyophilized cells was treated with 1.0 ml of 96% H2SO4 at 100°C for 1 h to form crotonic acid. The reaction mixture was then cooled to room temperature. Five milliliters of distilled water was added to the hydrolysate. Insoluble particles were removed from the mixtures by centrifugation at 7,000 × g for 15 min, and the samples were diluted with water. The crotonic acid was determined by using a LaChrom high-performance liquid chromatography system (Merch HITACHI) on a LiChroCART Purospher C18 reversed-phase column (dimensions, 3 by 125 mm; particle diameter = 5 μm). For analysis, 20 μl of sample was injected (using an L-7200 auto sampler), and the crotonic acid was eluted isocratically with water-methanol (1:1 [vol/vol]) at a flow rate of 0.4 ml min−1. The elution peaks were monitored at 210 nm with an L-7455 diode array UV detector. The PHB content was calculated from the calibration curve for standards of commercial crotonic acid (Sigma) and of pure PHB standards (Aldrich) hydrolyzed with concentrated sulfuric acid.
Enzymatic hydrolysis of cellulose from pulp sludge.
The fiber sludge from the pulp production was obtained from Kemira Kemi AB (Helsinborg, Sweden). One hundred forty ml of sludge centrifuged at 14,000 × g for 15 min resulted in 32 ml of wet solids (∼23% of the raw sludge volume). The pH of the wet solids was adjusted to 4.6 with 1.0 M HCl, and two crude enzymes were added simultaneously: 250 μl of cellulase (Celluclast; 1.5 liters) and 250 μl of cellobiase (NOVOZYM 188), which act sequentially. The hydrolysis was carried out continuously for 15 h at 50°C. To measure the released glucose from the sludge during this hydrolysis period, 0.2 ml of supernatant was drawn from the reaction vessel at 0.5-h intervals and analyzed using the glucose oxidase-peroxidase test.
Biofilm formation in batch mode on different microcarriers.
The biomass to be used as the inoculum was grown in diluted medium no. 3 (Table 1) for 24 h at 30°C and 150 rpm. The cells were harvested by centrifugation, washed twice with 0.01 M phosphate buffer (pH 7.0), and finally resuspended in the phosphate buffer to a D600 of 0.83. Five types of commercial anion exchanger were tested as microcarriers for A. eutrophus attachment (Table 2). The microcarrier (0.1 g [wet weight]) was equilibrated with 0.01 M phosphate buffer (pH 7.0), mixed with 1.5 ml of bacterial suspension, and incubated at 30°C with mixing at 150 rpm for 4 h. Thereafter, the unattached bacteria were measured in the supernatant after 2 min, when the carrier with attached bacteria had settled.
TABLE 2.
Tested microcarrier attachment capacities (q) for A. eutrophus with initial cell concentration of 0.83 D600
| Name | Brand | Charge capacity | Functional group | q (g g−1) |
|---|---|---|---|---|
| DEAE-cellulose DE-32 | Whatmann | 1.0 meq g−1 | Diethyl aminoethyl | 0.0028 |
| DEAE-Sephadex A-25 | Pharmacia | 3.5 meq g−1 | Diethyl aminoethyl | 0.0026 |
| AG 1-X8 | Bio-Rad | 1.4 meq ml−1 | Quaternary ammonium | 0.0018 |
| AG 3-X4A | Bio-Rad | 1.9 meq ml−1 | Poly-alkylamine | 0.0010 |
| IRA958 Cl | AMBERLITE | 0.8 meq ml−1 | Quaternary ammonium | 0.0004 |
Microscopy was used to evaluate the biofilm formation on the tested microcarrier after bacterial attachment. The samples were washed once with 0.01 M phosphate buffer (pH 7.0), stained with 0.1% (wt/vol) safranin to detect the formation of biofilm (18), and viewed by microscope (Nikon, Tokyo, Japan). Images were captured using a Nikon digital camera.
Selection and determination of the best ionic strength for bacterial attachment to and detachment from DEAE-Sephadex A-25.
Attachment was tested at sodium phosphate buffer (pH 7.0) concentrations from 0.01 to 0.4 M. At pH 7.0, monohydrogen phosphate (HPO42−) and dihydrogen phosphate (H2PO4−) are the dominant ions, with the following dissociation equilibrium:
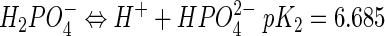 |
(1) |
Therefore, the concentrations of HPO42−, H2PO4−, and Na+ can be determined, and the corresponding ionic strengths (I) can be calculated from the following formula:
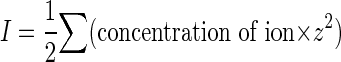 |
(2) |
where z is the charge for each ionic species.
DEAE-Sephadex A-25 was preswollen, autoclaved for 20 min at 120°C, and equilibrated in the same buffer in which bacteria would be suspended. This preequilibrated microcarrier of 0.2 g (wet weight) was then mixed with 5 ml of bacterial suspension (D600 = 1.44) in phosphate buffer with defined ionic strength. After 4 h of incubation, the further decreases in the D600 of the supernatant were negligible in all cases. The attachment was terminated at this point, the supernatant was removed, and 5 ml of 0.5 M NaCl in 0.01 M phosphate buffer solution was added to detach the adsorbed cells. The amount of detached cells (as D600) in the suspension after the microcarrier had settled was determined after 4 h of incubation.
To study the effect of the bacterial concentration on the attachment capacity, the bacterial suspensions, with D600s ranging from 0.26 to 3.7, were tested for attachment to 0.2 g of DEAE-Sephadex A-25 in 5 ml of 0.01 M phosphate buffer, pH 7.0.
Packed-bed biofilm reactor culture.
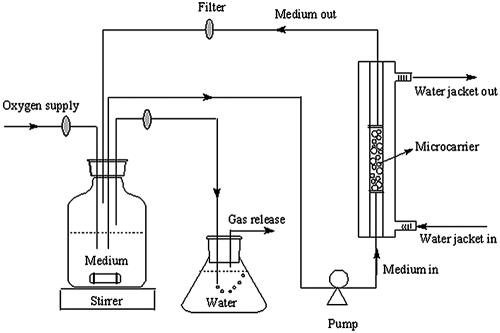
Phosphate buffer (0.01 M; pH 7.0) was used throughout the procedure. Inoculation of A. eutrophus onto the microcarrier was done in batch mode. The inoculum was prepared using cells cultured for 20 h, centrifuged, washed, and resuspended in 100 ml of phosphate buffer to a D600 of 1.50. The suspension was mixed with 20 g of wet DEAE-Sephadex A-25 and incubated for 4 h at 30°C with mixing at 150 rpm. To remove the unadsorbed cells, the microcarrier was allowed to settle, was separated from the supernatant, and was then washed with buffer, resuspended, and aseptically transferred to a chromatographic column (inner diameter, 17 mm; height, 350 mm; Pharmacia LKB no. 2137). The column, maintained at 30°C, formed part of a system that is illustrated in Fig. 1. Medium (100 ml of diluted medium no. 3 [Table 1]) was kept and stirred at room temperature. The effluent medium was circulated through a sterile filter before being returned to the medium reservoir to prevent any detached cells from reaching the medium container. The voidage volume (the volume between beads) of this DEAE-Sephadex A-25 gel column was determined using the method described by Zhang and Sun (26).
FIG. 1.
Schematic diagram of packed-bed biofilm reactor.
The biofilm growth in the column was initiated by feeding oxygen-saturated (bubbled at 16 ml min−1) medium at a flow rate of 0.28 ml min−1. Usually around the seventh day of growth, the biofilm became fully developed and the bacteria started to leak out of the column and block the filter. The biofilm was then detached by switching the medium to 0.01 M phosphate buffer (pH 7.0) containing 0.5 M NaCl, collected, and freeze-dried.
RESULTS
Enzymatic production of glucose from pulp fiber sludge.
The pH of the raw sludge was 7.4, and it contained negligible amounts of phosphate and ammonia, which had to be supplied as part of medium no. 3. Figure 2 shows the time profile of enzymatic glucose release from pulp sludge. The hydrolysis of the applied sludge was completed after 15 h. The remaining suspension was centrifuged, and the solid was discarded. The glucose concentration in the liquid phase (27 ml) was analyzed as 30 g liter−1 and then used as carbon source in the growth media. From 1 liter of the starting wet solids, the glucose produced by the enzymatic hydrolysis corresponded to 25 g.
FIG. 2.
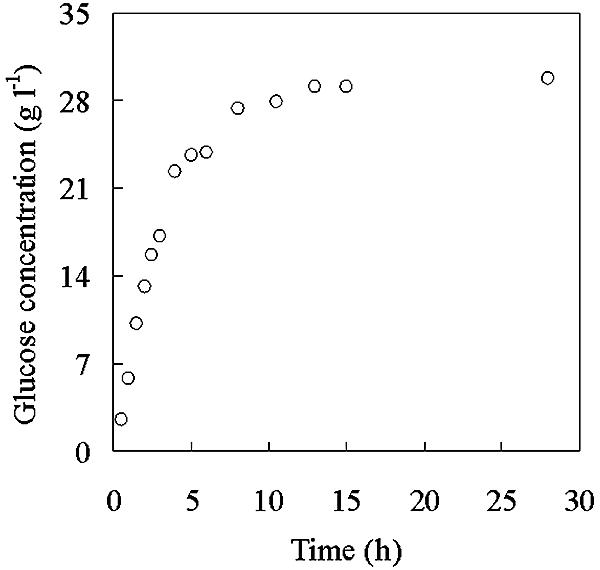
Time course of enzymatic glucose release from pulp fiber sludge.
Attachment of bacteria to different carriers.
Five tested ion exchangers with the positively charged functional group are listed in Table 2. The bacterial capacity for attachment to those micocarriers was evaluated as follows:
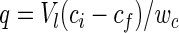 |
(3) |
where q (in grams of biomass per gram of microcarrier) is the cell attachment capacity, Vl (in liters) is the volume of bacterial solution, wc (in grams) is the wet weight of the microcarrier, and ci and cf (in grams liter−1) are the initial and final biomass concentrations in the solution before and after attachment, which can be roughly calculated according to the following correlating equation between the D600 and the dried biomass concentration:
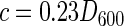 |
(4) |
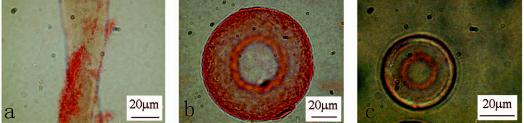
DEAE-cellulose had the highest adsorption capacity for the tested bacteria, in spite of the lower charge capacity of this carrier. Microscopic observation showed that most attached bacteria are unevenly distributed on the edge of the DEAE-cellulose carrier (Fig. 3), while AG 1-X8, AG3-X4A, and IRA-958Cl were unsatisfactory in both the capacity and the evenness of distribution of cell attachment. Both the microscopic evaluation and the cell attachment capacity were factors affecting the choice of DEAE-Sephadex A-25. It can be seen that there is no significant correlation between the carrier's charge density and the amount of attached bacteria (Table 2). Anion exchangers, such as the hydrophobic (polystyrene-divinylbenzene) Amberlite resin IR-45 (BDH Chemicals) and the hydrophilic, positively charged Amberlite IRA-958 Cl (Rohm & Haas), were also tested. These resins were unsuitable because their attachment capacities for bacteria were relatively low compared to that of the chosen resin (data not shown).
FIG. 3.
Photomicrographs of attached bacteria on microcarrier surfaces. The microcarriers (0.1 g) were incubated for 4 h with 1.5 ml of bacterial solution (D600 = 0.83) in 0.01 M phosphate buffer and then stained with 0.1% safranin. (a) DEAE = cellulose; (b) DEAE = Sephadex; (c) AG 1 = X8.
DEAE-Sephadex A-25.
A series of cell attachment experiments with DEAE-Sephadex A-25 in phosphate buffer (0.01 M; pH 7.0) were conducted with bacterial concentrations between 0.3 and 3.7D600. The maximal attachment capacity, q, was 0.0068 g of biomass per g of microcarrier when the initial cell concentration was ∼0.4 g liter−1 (Fig. 4). Further increases in the initial cell concentration did not enhance the attachment capacity. The experiments were carried out in triplicate, and the results shown in Fig. 4, 5, and 6 are expressed as the mean, with the standard error of the mean not exceeding ±6%.
FIG. 4.
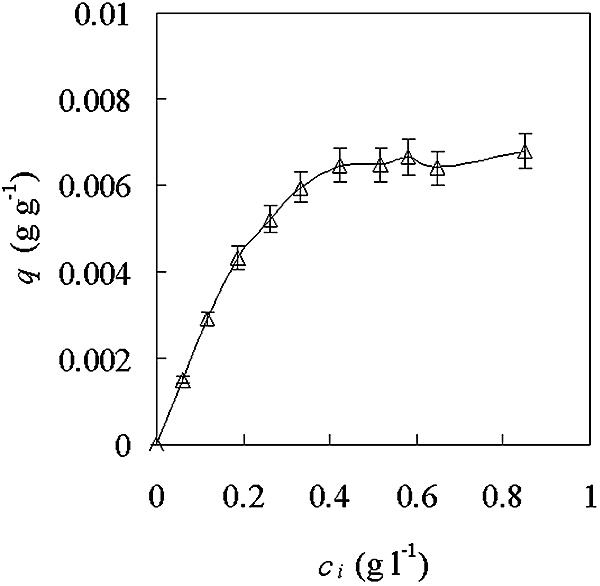
Saturation curve of A. eutrophus cell attachment to DEAE-Sephadex A-25. DEAE-Sephadex A-25 (0.2 g) was incubated with 5 ml of bacterial suspension with different initial D600s in 0.01 M phosphate buffer. q, attachment capacity; ci, starting concentration of bacteria. The error bars indicate standard errors of the mean.
FIG. 5.
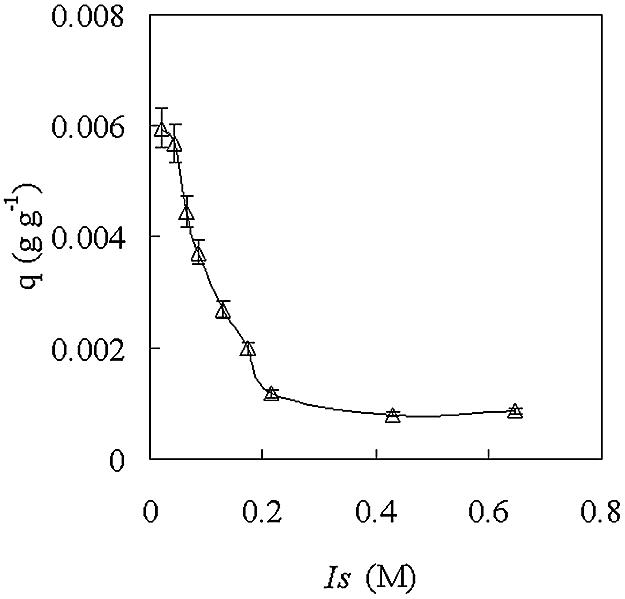
Effect of ionic strength on attachment of A. eutrophus cells to DEAE-Sephadex A-25. DEAE-Sephadex A-25 (0.2 g) was incubated with 5 ml of bacterial suspension (D600 = 1.44) in phosphate buffer with different ionic strengths. q, attachment capacity. Ionic strength was calculated from equations 1 and 2. The error bars indicate standard errors of the mean.
FIG. 6.
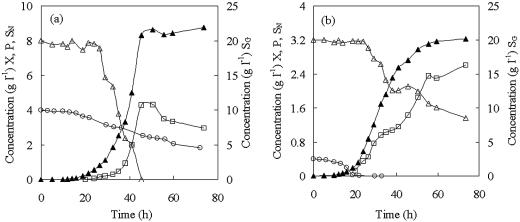
A. eutrophus batch growth and PHB biosynthesis in medium no 3. (a) Undiluted medium; (b) 10-times-diluted medium. SG, glucose concentration (g liter−1) (▵); SN, (NH4)2SO4 concentration (g liter−1) (○); P, PHB concentration (g liter−1) (□); X, biomass concentration (g liter−1) (▴).
Most gram-negative bacteria, like A. eutrophus, carry a negative charge (4, 16). Therefore, the attachment of A. eutrophus cells to DEAE-Sephadex A-25 is probably based on electrostatic interactions and dependent on the charge density of the carrier. Changes in the attachment conditions, such as ionic strength and pH, that would affect the charge “seen” by the bacteria could have significant effects. Figure 5 demonstrates strong dependence between the number of attached cells and the ionic strength of the equilibrium buffer used. Already at 0.04 M phosphate buffer (I = 0.086 M), the number of cells that can be attached is only 50% that obtained at 0.01 M phosphate buffer (I = 0.022 M) with the same type and amount of carrier.
The efficiency of cell recovery from DEAE-Sephadex A-25 using 0.5 M NaCl in 0.01 M phosphate buffer can be roughly estimated by following the biomass concentration changes in the liquid phase after detachment. About 90% of cells were recovered when the cells were attached under low ionic strength (0.01 M phosphate solution).
Comparison of cultures grown in modified media.
The optimal attachment of cells was achieved at 0.01 M phosphate buffer with a conductivity corresponding to 1.69 mS. Conductivity provides a rough indication of the total number of ions present in solution; it is related to ionic strength. To provide the appropriate ionic strength for cell attachment and growth, the ionic strength of the medium was adjusted by measuring conductivity. Growth medium no. 3 has high conductivity (16.4 mS); therefore, all the components except glucose were diluted 10 times to match the conductivity corresponding to 0.01 M phosphate buffer. The conductivity of this diluted medium no. 3 was 2.44 mS.
The growth of cells and the biosynthesis of PHB were tested in undiluted and diluted medium no. 3. In Fig. 6, the cell growth, PHB, glucose, and ammonia concentrations are shown. Phosphate was also measured, but no changes were observed, and therefore it is not indicated in the figure. After 20 h, ammonium in the growth medium was depleted while glucose was still in excess (Fig. 6b), and the concentration of PHB continued to increase. After 74 h of cultivation, the bacterial culture was stopped. The yield of the cells in undiluted medium no. 3 (8.78 g of dried cells liter−1) was twice as high as that obtained from the diluted medium no. 3 (3.22 g of dried cells liter−1). However, the specific PHB content of the 10-times-diluted medium was 78%, in contrast to 31.9% for the undiluted medium (Table 3).
TABLE 3.
PHB levels in cells grown in undiluted and diluted medium no. 3
| Medium | Dried cell concn (g liter−1) | PHB content in dried cells (%) | PHB concn (g liter−1) |
|---|---|---|---|
| Undiluted | 8.78 | 31.9 | 2.8 |
| Diluted | 3.22 | 77.8 | 2.5 |
Packed-bed biofilm culture.
For the packed-bed reactor setup in this study, 20 g (wet weight) of DEAE-Sephadex A-25 gave a packed volume of 31 ml and a voidage volume of 11.2 ml before biofilm culture. After 1 day of bacterial cultivation, a visible change of the carrier color from completely white to dark grey was observed at the bottom. With time, the grey zone spread all over the column. The culture growth was completed by the seventh day, and the biofilm was detached. The yield from the used packed-bed reactor was 0.064 g of freeze-dried cells.
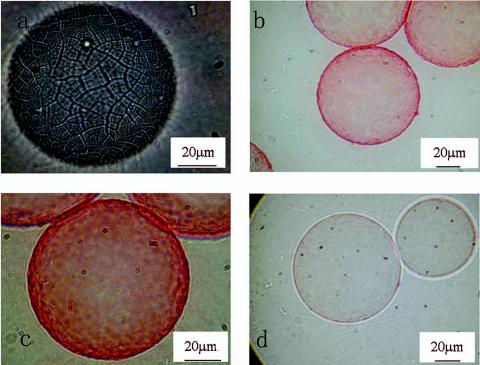
Microcarrier samples were taken before and after detachment of the biofilm from the top of the column, stained with safranin, and evaluated by microscope. The surface changes of the microcarrier before and after inoculation and with the grown and detached biofilm are shown in Fig. 7a, b, c, and d, respectively. Staining with safranin provided an easy and quick method to detect biofilm formation (18). For the original microcarrier, no stain remained on the surface (Fig. 7a), while for the microcarrier with developed biofilm, more stain was observed (Fig. 7b and c).
FIG. 7.
Photomicrographs illustrating the packed-bed biofilm culture of A. eutrophus on DEAE-Sephadex A-25. (a) Original microcarrier bead; (b) microcarrier after 4 h of incubation in 100 ml of bacterial solution with a D600 of 1.50 in 0.01 M phosphate buffer; (c) after 7 days of culture; (d) after detachment with 0.05 M NaCl. The microcarriers were stained with 0.1% safranin.
DISCUSSION
Sludge from pulp is very rich in cellulose of low fiber quality. Those cellulose fibers are usually discarded. It is known that the cost of raw materials for the production of PHB is one of the limiting economic factors in the scaling up of such processes. About 40% of the expenditure for PHB production is the cost of the carbon source (3). In the present study, the carbon source was recovered from fiber sludge by converting cellulose to glucose using cheap, commercially available enzymes, cellulase and cellobiase. The prices of these two kinds of crude enzymes were ∼€5 per liter. To obtain 1 kg of glucose from pulp fiber sludge, ∼0.3 liters of both enzymes, which cost only €3, are needed. This is much less than the price of glucose from commercial suppliers, e.g., €27.8 per kg in the Sigma catalogue. The use of sludge also minimizes the cost of paper wastewater treatment that is normally incurred.
The glucose released was used as a carbon source for the growth of the PHB-synthesizing bacterium A. eutrophus. The cells were grown on a cheap microcarrier as a biofilm to reduce the cost of harvesting. A. eutrophus is usually found in natural environments, such as soil (11). According to DeFlaun and Condee (4), bacteria isolated from soil possess a net negative charge, as revealed by electrokinetic transport. Determination of the surface charge of bacteria by colloid titration experiments gave the same conclusion that the surface charge of bacteria is always negative except below pH 2 (16). Also, A. eutrophus is speculated to have a net negative charge. In searching for a potential microcarrier, different brands of positively charged microcarriers (Table 2) were tested. At low ionic strength, there will be charge interaction between the positively charged microcarrier and the negatively charged bacteria. From the screened carriers, DEAE-Sephadex A-25 was selected due to its higher cell attachment capacity and even cell distribution on the carrier surface (Fig. 3). Also, the strong dependence of A. eutrophus attachment on ionic strength observed here (Fig. 5) supports the hypothesis about the electrostatic interactions between the cell and the ion exchanger. For each kind of cell and carrier with charges, there could be a different optimal charge density for attachment and growth, which has to be experimentally chosen (1). Furthermore, for a specific cell, the microcarrier properties (size, shape, roughness, and porosity) are also factors affecting attachment.
Keeping in mind the inhibitory effect of increased ionic strength on the maintenance and growth of the cells on the microcarrier surface, all the components except glucose in growth medium no. 3 were diluted 10 times to achieve a conductivity similar to that of the 0.01 M phosphate buffer. This diluted medium resulted in a significantly enhanced PHB content in the biomass (Table 3 and Fig. 6). From the point of view of metabolism, a shortage of the nitrogen source (ammonium) and an excess of the carbon source (glucose) switches the cell from growth mode to accumulation mode (PHB). Until now, the highest PHB productivity and content ever reported was also obtained with ammonia limitation and an excess of the carbon source (23). Although the amount of biomass production in diluted medium decreased, the amounts of PHB recovered were almost the same in both cases (Table 3). The separation of PHB granules from cells with increased PHB content requires reduced amounts of the digesting reagent and equipment-related costs (3). Additionally, the use of glucose recovered from pulp sludge greatly decreased the cost of the carbon source. In the case of undiluted medium no. 3, the yield of PHB was 0.14 g of PHB per g of glucose (Table 3). To produce 1 g of PHB, ∼0.28 liters of raw sludge solid, containing 6.2 g of glucose, needed to be digested.
Based on the previously described advantages of growing cells on a microcarrier (6), a mini-preparative-scale packed-bed reactor was set up to biosynthesize PHB in a bacterial biofilm. The batch mode cell attachment experiment showed recovery efficiencies as high as 90%, obtained by manipulating the ionic strength of the attachment medium. It is a good indication that small volumes of highly concentrated NaCl solution can be used as a very cheap and effective method to recover A. eutrophus biofilm from a microcarrier. It is usually a difficult task to recover biomass from packed-bed biofilm (5). Additional evidence for this efficient detachment method is demonstrated in Fig. 7c and d. From the column used, 0.064 g of dried cells was recovered, which corresponds to 5.71 g of cells per liter in the void volume or to 0.0032 g of cells per g of wet microcarrier. Figure 7 shows the entire process of biofilm development and detachment on the DEAE-Sephadex A-25. The microscopic and quantitative evaluations supported each other.
The saturation curve of A. eutrophus cell attachment to DEAE-Sephadex A-25 showed that 0.0068 g of biomass per g of microcarrier is the maximal attachment capacity obtained with the batch mode in 0.01 M phosphate buffer (Fig. 4). In the packed-bed biofilm reactor, the yield based on the microcarrier weight was only 0.0032 g of cells per g of microcarrier, so cell growth and/or binding to the microcarrier in the packed bed were limited. One of the possible reasons could be oxygen limitation in packed-bed reactor culture. It has been observed that a thick biofilm requires a sufficient amount of soluble oxygen (2, 10). A. eutrophus, as an aerobic strain, is always cultured with dissolved oxygen concentrations 20% above the concentration in air (13). In the packed-bed reactor culture system used in this study, oxygen limitation could also occur along the column height, although the medium was saturated continuously with oxygen. Therefore, cells might not grow enough to get the maximal attachment capacity obtained in batch mode. Without aeration of the medium, we observed very little biofilm (data not shown).
One important property associated with surface-attached bacterial biofilm stability is the ability to synthesize an extracellular polysaccharide (EPS) (7). A Vibrio cholerae strain that is defective in EPS synthesis is also defective in biofilm development (17). To our knowledge, there is no report available about EPS production by A. eutrophus. A possible reason is that A. eutrophus belongs to such an EPS-defective strain and thus there can be no thick biofilm formation. With regard to this obstacle to forming a thick biofilm on solid surfaces, microcarriers with appropriately sized pores able to hold large bacterial colonies could be considered in future research.
Although biofilm formation in packed-bed reactors is limited, the yield of 5.714 g of cells per liter in the void volume is comparable to the batch culture yield (Table 3). Therefore, this is a promising and advantageous process for industrial purposes. It does not require any great effort to harvest the cells, such as centrifugation of large volumes; the carrier can be reused many times; and finally, the culture conditions do not require large volumes for fermentation.
Since extraction and recovery of PHB from the biomass is another important factor of PHB producing, further work will be done to try an economical and environmentally beneficial method, e.g., supercritical fluid of carbon dioxide extraction, to extract PHB from the biomass. For analytical purposes, the solubility of PHB in supercritical carbon dioxide has already been studied by other authors (12).
Acknowledgments
This work was supported by Kemira Kemi AB and the C. F. Lundströms Foundation.
We are grateful to Simon Gough for critical reading of the manuscript.
REFERENCES
- 1.Amersham Biosciences. 2004. Microcarrier cell culture: principles and methods, p. 10-13. Amersham Biosciences, Uppsala, Sweden.
- 2.Casey, E., B. Glennon, and G. Hamer. 1999. Oxygen mass transfer characteristics in a membrane-aerated biofilm reactor. Biotechnol. Bioeng. 62:183-192. [DOI] [PubMed] [Google Scholar]
- 3.Choi, J., and S. Y. Lee. 1999. Factors affecting the economics of polyhydroxyalkanoate production by bacterial fermentation. Appl. Microbiol. Biotechnol. 51:13-21. [Google Scholar]
- 4.DeFlaun, M. F., and C. W. Condee. 1997. Electrokinetic transport of bacteria. J. Hazard. Mater. 55:263-277. [Google Scholar]
- 5.Fassnacht, D., and R. Pörtner. 1999. Experimental and theoretical considerations on oxygen supply for animal cell growth in fixed-bed reactors. J. Biotechnol. 72:169-184. [DOI] [PubMed] [Google Scholar]
- 6.Griffiths, B., and D. Looby. 1991. Fixed immobilized beds for the cultivation of animal cells, p. 165-189. In S. H. Chester and D. I. C. Wang (ed.), Animal cell bioreactors. Butterworth-Heinemann, Reading, Mass. [DOI] [PubMed]
- 7.Hall-Stoodley, L., and P. Stoodley. 2002. Developmental regulation of microbial biofilms. Curr. Opin. Biotechnol. 13:228-233. [DOI] [PubMed] [Google Scholar]
- 8.Haywood, G. W., A. J. Anderson, L. Chu, and E. A. Dawes. 1988. Characterization of two 3-ketothiolases in the polyhydroxyalkanoate synthesizing organism Alcaligenes eutrophus. FEMS Microbiol. Lett. 52:91-96. [Google Scholar]
- 9.Haywood, G. W., A. J. Anderson, L. Chu., and E. A. Dawes. 1988. The role of NADH- and NADPH-linked acetoacetyl-CoA reductases in the poly-3-hydroxyalkanoate synthesizing organism Alcaligenes eutrophus. FEMS Microbiol. Lett. 52:259-264. [Google Scholar]
- 10.Jang. A., P. L. Bishop, S. Okabe, S. G. Lee, and I. S. Kim. 2003. Effect of dissolved oxygen concentration on the biofilm and in situ analysis by fluorescence in situ hybridization (FISH) and microelectrodes. Water Sci. Technol. 47:49-57. [PubMed] [Google Scholar]
- 11.Kersters, K., and J. D. Ley. 1984. Genus Alcaligenes Castellani and Chalmers 1919, 936 AL, p. 361-373. In J. G. Holt (ed.), Bergey's manual of systematic bacteriology, vol. 4. Williams & Wilkins, Baltimore, Md.
- 12.Khosravi-Darani, K., E. Vasheghani-Farahani, Y. Yamini, and N. Bahramifar. 2003. Solubility of poly (β-hydroxybutyrate) in supercritical carbon dioxide. J. Chem. Eng. Data 48:860-863. [Google Scholar]
- 13.Kim, B. S., S. C. Lee, S. Y. Lee, H. N. Chang, Y. K. Chang, and S. I. Woo. 1994. Production of poly(3-hydroxybutyric acid) by fed-batch culture of Alcaligenes eutrophus with glucose concentration control. Biotechnol. Bioeng. 43:892-898. [DOI] [PubMed] [Google Scholar]
- 14.Korotkova, N. A., V. V. Ashin, N. V. Doronina, and Y. A. Trotsenko. 1997. A new method for quantitative determination of poly-3-hydroxybutyrate and 3-hydroxybutyrate-3-hydroxyvelarate copolymer in microbial biomass by reversed-phase high performance liquid chromatography. Appl. Biochem. Microbiol. 33:302-305. [Google Scholar]
- 15.Lee, S. Y. 1996. Bacterial polyhydroxyalkanoates. Biotechnol. Bioeng. 49:1-14. [DOI] [PubMed] [Google Scholar]
- 16.Noda, Y., and Y. Kanemasa. 1984. Determination of surface charge of some bacteria by colloid titration. Physiolog. Chem. Phys. Med. NMR 16:263-274. [PubMed] [Google Scholar]
- 17.O'Toole, G., H. B. Kaplan, and R. Kolter. 2000. Biofilm formation as microbial development. Annu. Rev. Microbiol. 54:49-79. [DOI] [PubMed] [Google Scholar]
- 18.O'Toole, G. A., L. A. Pratt., P. I. Watnick., D. K. Newman, and R. Kolter. 1999. Genetic approaches to study of biofilms. Methods Enzymol. 310:93. [DOI] [PubMed] [Google Scholar]
- 19.Reddy, C. S. K., R. Ghai, Rashmi, and V. C. Kalia. 2003. Polyhydroxyalkanoates: an overview. Bioresour. Technol. 87:137-146. [DOI] [PubMed] [Google Scholar]
- 20.Ryu, H. W., S. K. Hahn, Y. K. Chang, and H. N. Chang. 1997. Production of poly(3-hydroxybutyrate) by high cell density fed-batch culture of Alcaligenes eutrophus with phosphate limitation. Biotechnol. Bioeng. 55:28-32. [DOI] [PubMed] [Google Scholar]
- 21.Shimizu, H., Y. Kozaki, H. Kodama, and S. Shioya. 1999. Maximum production strategy for biodegradable copolymer P(HB-co-HV) in fed-batch culture of Alcaligenes eutrophus. Biotechnol. Bioeng. 62:518-525. [PubMed] [Google Scholar]
- 22.Varani, J., M. Dame, T. F. Beals, and J. A. Wass. 1983. Growth of three established cell lines on glass microcarriers. Biotechnol. Bioeng. 25:1359-1372. [DOI] [PubMed] [Google Scholar]
- 23.Wang, F., and S. Y. Lee. 1997. Poly(3-hydroxybutyrate) production with high polymer content by fed-batch culture of Alcaligenes latus under nitrogen limitation. Appl. Environ. Microbiol. 63:3703-3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Widell, A., B. Hanson, and E. Nordenfelt. 1984. A microcarrier cell culture system for large scale production of hepatitis A virus. J. Virol. Methods 8:63-71. [DOI] [PubMed] [Google Scholar]
- 25.Worthington Diagnostic Systems. 1982. Enzyme and related biochemicals, p. 83-84. Worthington Diagnostic Systems, Inc., Freehold, N.J.
- 26.Zhang, S. P., and Y. Sun. 2001. Further studies on the contribution of electrostatic and hydrophobic interactions to protein adsorption on dye-ligand adsorbents. Biotechnol. Bioeng. 75:710-717. [DOI] [PubMed] [Google Scholar]