Abstract
The transmission dynamics of Rocky Mountain spotted fever in Montana appears to be regulated by Rickettsia peacockii, a tick symbiotic rickettsia that interferes with transmission of virulent Rickettsia rickettsii. To elucidate the molecular relationships between the two rickettsiae and glean information on how to possibly exploit this interference phenomenon, we studied a major rickettsial outer membrane protein gene, ompA, presumed to be involved in infection and pathogenesis of spotted fever group rickettsiae (SFGR) but which is not expressed in the symbiont. Based on PCR amplification and DNA sequence analysis of the SFGR ompA gene, we demonstrate that R. peacockii is the most closely related of all known SFGR to R. rickettsii. We show that R. peacockii, originally described as East Side agent in Dermacentor andersoni ticks from the east side of the Bitterroot Valley in Montana, is still present in that tick population as well as in D. andersoni ticks collected at two widely separated locations in Colorado. The ompA genes of R. peacockii from these locations share three identical premature stop codons and a weakened ribosome binding site consensus sequence relative to ompA of R. rickettsii. The R. peacockii ompA promoter closely resembles that of R. rickettsii and is functional based on reverse transcription-PCR results. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting showed that OmpA translation products were not detected in cultured tick cells infected with R. peacockii. Double immunolabeling studies revealed actin tail structures in tick cells infected with R. rickettsii strain Hlp#2 but not in cells infected with R. peacockii.
Ticks are major vectors of emerging and reemerging disease agents in North America, Europe, and elsewhere in temperate zones. Because their complex life cycle alternates between arthropods and vertebrates and commonly involves a wildlife reservoir host, vector-borne pathogens are difficult to eradicate. In addition, growing concerns over toxicity of acaricides have renewed interest in environmentally compatible strategies for control of ticks and tick-borne pathogens. In addition to bacterial pathogens, obligate intracellular proteobacteria are sometimes found in ticks as apparently nonpathogenic endosymbionts that are transmitted transovarially from generation to generation (29). Closely related microorganisms are more likely to interfere with each other in arthropod vectors than are distant relatives (4, 10, 23), and endosymbionts could possibly be exploited to ultimately render ticks incapable of transmitting closely related pathogens.
Rickettsia peacockii, an endosymbiont of Dermacentor andersoni, the Rocky Mountain wood tick (28), is of particular interest because it may have played a role in the declining prevalence in the North American Rockies of Rickettsia rickettsii, the etiologic agent of Rocky Mountain spotted fever (RMSF) (9). It has long been known that in the Bitterroot Valley of Montana the absence of RMSF outbreaks correlates with the presence of nonpathogenic spotted fever group rickettsiae (SFGR) (6, 7, 18). R. peacockii, also known as the East Side agent, was first detected in ticks collected from the east side of Montana's Bitterroot Valley with a prevalence of up to 80% (7, 28). In contrast, R. rickettsii is nearly absent in ticks on the east side of the valley (34), and most cases of RMSF occur on the west side of the valley, where prevalence of R. rickettsii in ticks is considerably lower (less than 1% [16]) than that of R. peacockii on the valley's east side. While a low prevalence of R. rickettsii may be due to its pathogenic effects on ticks (27), a high prevalence of R. peacockii in tick ovaries might further interfere with maintenance and transmission of R. rickettsii (7).
Although the genomes of R. rickettsii, Rickettsia sibirica, and Rickettsia conorii (31) have been sequenced, very few DNA sequence data are available for nonpathogenic SFGR that could help define their symbiotic nature on a molecular basis. Several genes have been used to establish rickettsial relationships, principally the 16S rRNA gene, citrate synthase gene (gltA), rickettsial outer membrane protein genes A and B (ompA and ompB, respectively), and gene D (15, 40-42, 44). The latter four genes encode proteins and undergo stabilizing (neutral) selection (14), indicating that they are suitable for taxonomic purposes. The molecular and biological traits that differentiate R. peacockii from R. rickettsii (28, 47) include an inability of the former to make a functional OmpA, which could underlie its endosymbiotic nature and apparent confinement to D. andersoni. Pathogenic R. rickettsii expresses OmpA and polymerizes host cell actin, facilitating its adhesion to host cells, cell-to-cell spread, and replication within mammalian and tick hosts (19, 22, 24). Unlike surface protein genes that rapidly evolve in response to host antibodies (52), Fournier et al. (14, 15) found by calculating the ratio of synonymous and nonsynonymous amino acid substitution rates that the ompA and -B genes undergo neutral selection. Because SFGR are predominantly maintained in nature by transovarial transmission through successive tick generations, their evolution is probably more influenced by tick factors than by those of the mammalian host. In order to gain a better understanding of the biological and evolutionary relationship between R. rickettsii and R. peacockii, we expanded analysis of the R. peacockii ompA gene to include a 3.5-kb region downstream of its tandem repeats (Fig. 1). We also sequenced the promoter region and examined ompA transcription using reverse transcription-PCR (RT-PCR). Our analysis included PCR-amplified ompA sequences from genomic DNA of R. peacockii-infected ticks collected in Colorado and from ticks on the east and west sides of the Bitterroot Valley. We anticipate that the new data presented here will aid in developing methods for the genetic manipulation and transformation of tick symbiotic rickettsiae with the aim of improving their potential for the control of tick-borne pathogens.
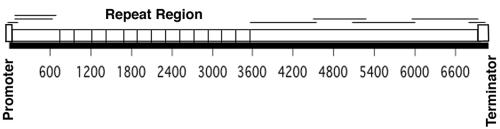
FIG. 1.
PCR amplification of the rickettsial ompA gene. The 7,088-bp R. rickettsii R strain ompA sequence (GenBank accession no. M31277) (2) is represented by the solid line, with a numerical nucleotide scale. The protein-encoding portion of the gene is represented by the open rectangle, with the tandem repeat region indicated by vertical bars. Overlapping PCR fragments (primer positions in Table 1) are indicated by lines above the open rectangle. The left- and right-most fragments contain the promoter and transcription terminator regions, respectively. Amplified sequences were assembled into a 4,032-bp composite sequence excluding the primer binding sites at the termini.
MATERIALS AND METHODS
Rickettsiae and DNA extraction.
R. peacockii strain DAE100R (isolate from Rustic, Colo.) was grown in the chronically infected D. andersoni cell line DAE100 (46). In addition, for routine maintenance R. peacockii was grown in Ixodes scapularis, the black-legged tick, cell line ISE6 (25). Initially, rickettsiae were released from infected DAE100 cells by forcing cell suspensions through a 27-gauge needle and removing large debris by low-speed centrifugation (275 × g for 10 min). The supernatant was filtered through 0.8-μm syringe filters, and the host cell-free rickettsial suspension was added to a culture of ISE6 cells. Subsequently, R. peacockii was transferred every 2 weeks by introducing an aliquot (0.1 to 0.5 ml) of an infected ISE6 culture to an uninfected ISE6 culture (5 ml of ISE6 cells at 5 × 106 cells/ml in a 25-cm2 flask). All cultures were maintained in antibiotic-free L15B300 medium supplemented with fetal bovine serum (5%), tryptose phosphate broth (5%), and bovine lipoprotein concentrate (0.1%), pH 7.2 to 7.5 (25). Two nonpathogenic rickettsial isolates, R. rickettsii Hlp#2, originally cultured from rabbit ticks (Haemaphysalis leporis-palustris) (33), and MOAa, an isolate from the lone star tick (Amblyomma americanum), were grown in I. scapularis cell lines ISE6, IDE2, and IDE8 (45, 52).
To extract genomic DNA, rickettsiae were recovered (38) and lysed in 300 μl of buffer containing 10 mM Tris-HCl (pH 7.8), 0.5 mM EDTA, 0.5% sodium dodecyl sulfate (SDS), 40 μg of RNase A (Promega, Madison, Wis.)/ml, and 250 μg of proteinase K (Fisher, Pittsburgh, Pa.)/ml at 55°C for 2 to 16 h. Lysates were phenol-chloroform extracted, and DNA was ethanol precipitated and resuspended in nuclease-free H2O (Life Technologies, Rockville, Md.).
Ticks and DNA extraction.
We collected adult female D. andersoni ticks on the north fork of Crestone Creek 4 miles upstream of Crestone, Colo. (200 miles south of Rustic, Colo.) in May 2002. We also obtained adult female D. andersoni ticks collected on the east side (Skalkaho Mountain and Burnt Fork) of the Bitterroot Valley, Ravalli County, Mont., or on the west side (near Como Lake) in May 2003 (we thank Tom Schwan, Rocky Mountain Laboratories, Hamilton, Mont., for providing us with these ticks).
To harvest DNA, ticks were surface disinfected by 5 min of agitation in 0.5% bleach followed by 5 min in 70% ethanol and two rinses in sterile H2O. Ticks were dissected individually in sterile Hanks balanced salt solution, and internal tissues were separated from the integument and transferred to 300 μl of lysis buffer (Puregene; Gentra Systems, Minneapolis, Minn.) with 0.2 μg of proteinase K (Sigma, St. Louis, Mo.)/μl and incubated overnight at 55°C. Recovered DNA was resuspended in nuclease-free H2O.
Strain designations.
The R. peacockii strains that we analyzed, whether they were in culture (i.e., DAE100R isolated from ticks originally collected near Rustic, Colo. [47]) or from field-collected ticks (i.e., Skalkaho Mountain, Mont., or Crestone, Colo.) are designated according to the site or locale where the ticks were originally collected.
Primers and PCR amplification.
The primers that we used are listed in Table 1. Gene positions and primer designations are based on those given for R. rickettsii (2, 15, 36, 39, 41, 42) unless stated otherwise. Lyophilized primers were purchased from Invitrogen (Carlsbad, Calif.) or Integrated DNA Technologies (Coralville, Iowa) and dissolved in TE buffer (10 mM Tris-HCl, 1 mM EDTA; pH 8.0). All PCRs were performed in a RoboCycler thermocycler (Stratagene, La Jolla, Calif.), with 0.5 μg, or less, of template DNA and 2.5 U of Taq polymerase (Promega), unless stated otherwise, in a 50-μl reaction volume. Standard buffer, MgCl2, and deoxynucleoside triphosphate concentrations were as recommended by the enzyme manufacturers.
TABLE 1.
Primers used for PCR amplification and sequencing
| Gene and primer | Nucleotide sequence (5′-3′) | Gene positionc | Reference |
|---|---|---|---|
| ompA | |||
| 190-(−110)a | GCAACAAGGCCAACACCGC | (−110)-(−92)d | 36 |
| 190-70pa | ATGGCGAATATTTCTCCAAAA | 70-90 | 39 |
| 190-271a | CTGCAGCCGTTATCTCATTCC | 271-251 | This report |
| 190-485b | GCAAAAGCTTAACTTTAAA | 485-503 | This report |
| 190-602nb | AGTGCAGCATTCGCTCCCCCT | 602-582 | 39 |
| 190-701a | GTTCCGTTAATGGCAGCATCT | 701-681 | 15 |
| 190-3588a | AACAGTGAATGTAGGAGCAG | 3588-3607 | 15 |
| 190-3817b | CTGCAACTGTTCCTATAG | 3817-3800 | 15 |
| 190-3968b | TAGCAGCTGATTTAGTAGCT | 3968-3986 | 15 |
| 190-4388a | TTCAGGAAACGACCGTACG | 4338-4356 | 15 |
| 190-4406a | ACTATACCCTCATCGTCATT | 4406-4387 | 15 |
| 190-4710b | AACATTTACATTGTTATTTA | 4710-4690 | 15 |
| 190-4859b | GCGAAATCCAAGGTACAGG | 4859-4877 | 15 |
| 190-5125a | GCGGTTACTTTAGCCAAAGG | 5125-5144 | 15 |
| 190-5238a | ACTATTAAAGGCTAGGCTATT | 5238-5218 | 15 |
| 190-5561b | CGGTTGTCATTATTAATAG | 5561-5543 | 15 |
| 190-5768b | CACCGCTACAGGAAGCAGAT | 5768-5787 | 15 |
| 190-5917a | TCAGGGAATAAAGGTCCTG | 5917-5935 | 15 |
| 190-6013a | TCTTCTGCGTTGCATTACCG | 6013-5994 | 15 |
| 190-6427b | ATCTAAGCCCAGCTAGCGGT | 6427-6408 | 15 |
| 190-6585b | CGCAATGGTCGATTATGC | 6585-6602 | 15 |
| 190-6808a | CACGAACTTTCACACTACC | 6808-6790 | 15 |
| 190-6984a | TGCAAGGAAATTACGAAGTAAGTG | 6984-6961 | This report |
| 17-kDa antigen | |||
| Rr17kDa1a | GCTCTTGCAACTTCTATGTT | 31-51 | 3 |
| Rr17kDa2a | CATTGTTCGTCAGGTTGGCG | 463-444 | 3 |
| Citrate synthase | |||
| RpCS.877pa | GGGGACCTGCTCACGGCGG | 877-895 | 42 |
| RpCS.1273ra | CATAACCAGTGTAAAGCTG | 1273-1255 | 42 |
| 16S rRNA | |||
| Rs16S354a | CAGCAATACCGAGTGAGTGATGAAG | 357-381 | This report |
| Rs16S647a | AGCGTCAGTTGTAGCCCAGATG | 697-676 | This report |
| E45a | GCTTAACACATGCAAG | 45-60 | 32 |
| E1242a | CCATTGTAGCACGTGT | 1242-1227 | 32 |
| F5a | CCTTTTTGAGTTTCGCTCC | 1290-1272 | 13 |
| F11a | TACCAGTTGGAAACGACTGT | 149-168 | 13 |
Primers used for PCR amplification and sequencing of amplicons.
Primers used only for sequencing.
Gene positions are relative to those reported for R. rickettsii (see text).
Sequence within putative promoter region.
RFLP analysis of the 5′ end of rickettsial ompA.
The SFGR-specific primer pair 190-70p and 190-602n was used to amplify a 532-bp fragment at the 5′ end of ompA (cycling parameters of 95°C for 5 min; 35 cycles of 95°C for 20 s, 48°C for 30 s, and 60°C for 2 min, followed by a final 60°C 5-min elongation step) (39) for restriction fragment length polymorphism (RFLP) analysis. Fifty to 250 ng of the gel-purified (see below) ompA PCR fragment was digested for 2 h at 37°C with restriction enzyme PstI (Promega) or RsaI (Life Technologies) in the manufacturer's buffer in 10- to 30-μl volumes. Digests were electrophoresed through 4% agarose gels (3% Gene Pure LE agarose, 1% NuSieve GTG agarose; ISC BioExpress, Kaysville, Utah) and stained with ethidium bromide. RFLP patterns were compared to those described for SFGR (39) and R. peacockii (28).
PCR amplification and sequencing of rickettsial ompA.
We used primers designed by Fournier et al. (15) to amplify the 5′ and 3′ ends of the ompA gene (Table 1). Figure 1 provides a map of the ompA gene, indicating the regions targeted by the primers. We amplified the PCR products for sequencing using Pfu Turbo Hotstart DNA polymerase or cloned Pfu DNA polymerase (Stratagene) at 5 U per reaction mixture of 50 μl. Cycling parameters for the cloned polymerase were 94°C for 45 s followed by 35 cycles of 94°C for 45 s, X°C (X = annealing temperature specified below for each primer pair) for 2 min, and 72°C for 1 min followed by a final 10-min extension step at 72°C. For Turbo Hotstart polymerase, denaturation temperatures were increased to 95°C and lengthened by 15 s, and total cycles were reduced to 30. The 5′ end of the ompA gene, including the promoter, was amplified as a 381-bp fragment with primer pair 190-(−110) and 190-271 (X = 50°C). A 631-bp fragment overlapping the 532-bp product described above was amplified with primer pair 190-70p and 190-701 (X = 48°C). The 3′-end region of the gene was amplified with primer pairs that produced five overlapping fragments as follows: 818 bp with 190-3588 and 190-4406 (X = 42°C); 850 bp with 190-4388 and 190-5238 (X = 46°C); 888 bp with 190-5125 and 190-6013 (X = 46°C); 891 bp with 190-5917 and 190-6808 (X = 42°C); 399 bp with 190-6585 and 190-6984 (X = 46°C). PCR products were purified by electrophoresis (1% agarose), recovered with QIAquick gel extraction spin columns (QIAGEN, Valencia, Calif.), and directly sequenced in forward and reverse directions two to four times with an ABI 377 automated sequencer (Advanced Genetic Analysis Center, University of Minnesota). Consensus sequences were obtained by alignment using the ClustalX multiple-sequence alignment program (20).
Phylogenetic analysis.
Nucleotide sequences of R. peacockii and R. rickettsii Hlp#2 corresponding to R. rickettsii (R strain) ompA positions 91 to 680 and 3635 to 6789 (2) were joined (the tandem repeat region was excluded) so that 3,764 nucleotides were included in the analysis (15). However, because of a premature stop codon in the 5′ prerepeat region of the R. peacockii ompA gene, translated amino acid sequences were not used for phylogenetic analysis (28, 47). The ompA sequences of R. peacockii, R. rickettsii Hlp#2, and other Rickettsia species (Table 2) were manually edited in a word processing file with ClustalX. Alignments were imported into PAUP* 4.0b10 (51) for construction of phylogenetic trees, and distance matrices were determined using Kimura's two-parameter option (21). Relationships were analyzed with neighbor-joining (43) and maximum parsimony (48) methods. Node stability of dendrograms was estimated using bootstrap analysis (12) of values obtained from 1,000 trees generated randomly.
TABLE 2.
GenBank accession numbers of Rickettsia ompA sequences used in the phylogenetic analysis
| Species | Strain | GenBank accession no.
|
|
|---|---|---|---|
| Prerepeat (5′) | Postrepeat (3′) | ||
| R. rickettsii | RT | M31227 | M31227 |
| R. rickettsii | HIp2 | AY319293 | AY319290 |
| R. peacockii | Skalkaho | AY357765 | AY357764 |
| R. peacockii | Crestone | AY357766 | AY357763 |
| R. peacockii | Rustic | AY319292 | AY319291 |
| R. japonica | YM | U43795 | U83442 |
| R. massiliae | MtulT | U43799 | U83445 |
| R. montanensis | ATCC VR-611 (M5/6) | U43801 | U83447 |
| R. rhipicephali | 3-7-6 | U43803 | U83450 |
| Bar29 | Bar-29 | U43792 | U83438 |
| R. aeschlimanii | MC-16T | U43800 | U83446 |
| “R. slovaca” | 13-B | U43808 | U83454 |
| R. honei | TT-118 | U43809 | U83456 |
| “R. mongolotimonae” | HA-91 | U43796 | U83439 |
| R. parkeri | Maculatum 20 | U43802 | U83449 |
| R. africae | ESF-5 (2500-1) | U43790 | U83436 |
| Strain S | S | U43805 | U83452 |
| Israeli tick typhus | ISTT CDC1 | U43797 | U83441 |
| Astrakhan fever rickettsia | A-167 | U43791 | U83437 |
| R. conorii | Malish ATCC VR-613T | U43806 | U83453 |
| R. heilongjiangii | HLJ-054 | AF179362 | AF179363 |
| R. hulinii | HL-93 | AF179364 | AF179366 |
| R. sibirica | 246, ATCC VR-151T | U43807 | U83455 |
| R. felis | URRWXCal2T | AF210694 | AF231136 |
| R. australis | PHS | AF149108 | AF149108 |
| Ixodes ricinus rickettsia | IRS3 | AF141909 | AF141910 |
| Ixodes ricinus rickettsia | IRS4 | AF14911 | AF141912 |
PCR and sequence analysis of 16S rRNA, 17-kDa antigen, and gltA (citrate synthase) genes.
16S rRNA gene targets were amplified with the general bacterial primers E45 and E1242 (32) (1,197 bp; cycling parameters of 95°C for 2 min, 30 cycles of 95°C for 45 s, 42°C for 45 s, and 72°C for 1 min, and a final 72°C 10-min extension), Francisella-specific primers F5 and F11 (13) (1,141 bp; cycling parameters of 95°C for 2 min, 30 cycles of 95°C for 45 s, 48°C for 45 s, and 72°C for 90 s and a final 72°C 10-min step), and rickettsial primers Rs16S354 and Rs16S647 (this report) (249 bp; cycling conditions of 95°C for 1 min, then 30 cycles of 95°C for 1 min, 54°C for 1 min, and 72°C for 1 min, and a final 10 min at 72°C). We used primer pair RpCS.877p and RpCS.1273r (42) to amplify a 381-bp citrate synthase gene fragment with cycling parameters of 95°C for 5 min, 35 cycles of 95°C for 20 s, 48°C for 30 s, and 60°C for 2 min followed by a final 60°C 5-min step. A 432-bp fragment of the 17-kDa genus-common rickettsial antigen gene was amplified using primer pair Rr17kDA1 and Rr17kDA2 and the cycling parameters of Williams et al. (53).
RT-PCR assays.
RNA was purified from infected and uninfected tick DAE100 cells with the SV total RNA isolation system (Promega). DNase I-treated RNA samples were stored in H2O at −70°C. RT-PCR amplifications were performed using the Access RT-PCR system (Promega) and temperature profiles recommended by the manufacturer. Transcripts of the ompA, gltA, and 17-kDa antigen genes were amplified with the primers listed in Table 1, electrophoresed through 1.5% agarose gels, and stained with ethidium bromide for visualization by UV illumination.
SDS-PAGE and Western blot analyses.
Rickettsiae were gradient purified by centrifugation (20,000 × g for 40 min at 4°C) through 30% diatrizoate (Hypaque 76; Nycomed Inc., Princeton, N.J.). Rickettsiae were washed in Hank's balanced salt solution and resuspended in 0.5 M Tris-HCl (pH 6.8), and approximate protein concentrations were determined by UV spectrophotometry. Sixty-five micrograms of denatured proteins per well was separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) through 7.5% mini-gels and stained with Rapid Coomassie blue (Diversified Biotech, Boston, Mass.). For Western blot analyses, proteins were transferred to an Immobilon-P membrane (Millipore Corporation, Bedford, Mass.) (30) and reacted with mouse monoclonal antibody (MAb) 13-5 to rickettsial OmpA (1) diluted 500-fold in phosphate-buffered saline with 3% bovine serum albumin or hamster polyclonal anti-Rickettsia monacensis serum diluted 300-fold (45). Bound primary antibodies were detected using horseradish peroxidase-conjugated goat anti-mouse or anti-hamster immunoglobulin G diluted 1:1,000 and the 4CN membrane peroxidase system (Kirkegaard & Perry Laboratories, Gaithersburg, Md.).
Double fluorescence staining to assess actin tail formation.
IDE8 tick cells (ATCC CRL-11974) grown to confluency on glass coverslips in 24-well plates were inoculated with dilutions of host cell-free R. rickettsii Hlp#2 or DAE100 cells infected with R. peacockii and incubated in a candle jar at 34°C for 7 days (45). Coverslips were fixed, and rickettsiae were labeled with mouse MAb 13-2 to the rickettsial 120-kDa surface antigen OmpB (1), diluted 1:100, and anti-mouse immunoglobulin G conjugated to fluorescein isothiocyanate (Pierce, Rockford, Ill.), while tick cell F-actin was labeled with rhodamine-conjugated phalloidin (Molecular Probes, Eugene, Oreg.) as described elsewhere (19, 24). Coverslips were mounted on microscope slides in phosphate-buffered saline containing 3% bovine serum albumin, 10% glycerol, and 10% (wt/vol) 1,4-diamino-bicyclo(2,2,2)octane (Sigma) and viewed using a Nikon E400 microscope fitted with dual fluorescence illumination and a Nikon DX1200 digital camera.
Nucleotide sequence accession numbers.
GenBank accession numbers for the rickettsial ompA sequences reported in this paper are as follows: R. peacockii Skalkaho, AY357765 and AY357764; R. peacockii Crestone, AY357766 and AY357763; R. peacockii Rustic, AY319292 and AY319291; R. rickettsii Hlp#2, AY319293 and AY319290. Accession numbers for the partial sequences of the 16S rRNA gene for R. peacockii Crestone and Skalkaho strains are AY360093 and AY360094, respectively; for Hlp#2 the partial 16S rRNA sequence accession number is AY573599. The accession number for partial 17-kDa antigen gene sequences of R. rickettsii strain Hlp#2 is AY189818, and those for R. peacockii Crestone and Skalkaho are AY576905 and AY590153, respectively. The partial gltA sequence accession numbers for R. peacockii strains from Crestone and Skalkaho are AY576904 and AY590152, respectively, and for R. rickettsii strain Hlp#2 it is AY189819.
RESULTS
R. peacockii is present in Colorado and Montana tick populations.
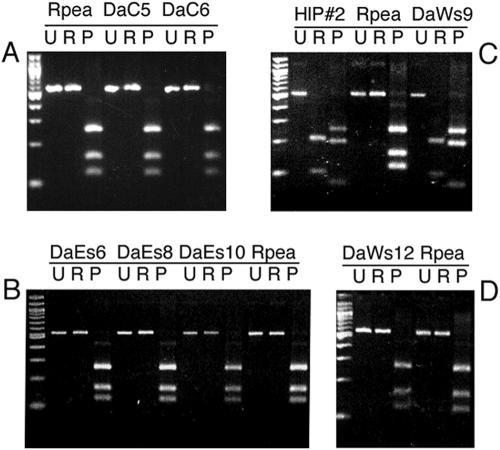
We detected R. peacockii in D. andersoni collected in Crestone, Colo., and the Bitterroot Valley of Montana by PCR and RFLP analysis (Table 3). Amplification of a 1,157-bp fragment with eubacterial 16S rRNA gene-specific primers indicated the presence of bacteria in all ticks, and all samples tested with Francisella 16S rRNA gene primers generated the expected 1,123-bp product (data not shown), suggesting they harbored the DAS endosymbiont (26). We sequenced 341 nucleotides of the 16S rRNA gene (nucleotides 357 to 697, using the numbering of Roux and Raoult for R. rickettsii [41]). Sequence alignment of the three R. peacockii strains (Skalkaho, Crestone, and DAE100R) with those of R. rickettsii strains Hlp#2 and R showed that all were identical. Seventy-eight percent of Crestone tick DNA samples (Table 3) yielded 532-bp ompA products with primers Rr190.70p and 190.602n (data not shown), indicating infection with SFGR. The RFLP analysis, as shown for ticks DaC 5 and 6 (Fig. 2A), produced the pattern (RsaI, uncut; PstI, 120, 160, and 250 bp) typical of the ompA fragment from R. peacockii strains Skalkaho (28) and DaE100R (46). Similarly, 69% of D. andersoni ticks collected on the east side of the Bitterroot Valley carried R. peacockii based on PCR and RFLP analyses, as shown for extracts DaEs 6, 8, and 10 (Fig. 2B), whereas only two D. andersoni ticks, DaWs 9 and 12, from the west side of the Bitterroot Valley tested PCR positive for ompA. DaWs9 had an RFLP pattern identical to that of R. rickettsii Hlp#2 (RsaI, 220, 220, and 106 bp; PstI, 266, 205, and 80 bp) (Fig. 2C) but an AluI digest that was indicative of an R. rickettsii R-like strain rather than Hlp#2 (data not shown) (16). The RFLP pattern of the DaWs12 and R. peacockii ompA PCR products were identical (Fig. 2D).
TABLE 3.
D. andersoni females PCR or RFLP positive for bacteria, Rickettsia, and Francisella species
| Site | % Positive (no. positive/no. tested)
|
||||
|---|---|---|---|---|---|
| PCR
|
RFLP
|
||||
| Bacteria (16S) | Rickettsia spp. (ompA) | Francisella sp. (16S) | R. peacockii | R. rickettsii | |
| Crestone | 100 (9/9) | 77.8 (7/9) | 100 (6/6) | 77.8 (7/9) | 0 (0/9) |
| East side | 100 (13/13) | 68.8 (11/16) | 100 (6/6) | 66.7 (10/15) | 0 (0/9) |
| West side | 100 (16/16) | 12.5 (2/16) | 100 (6/6) | 6.3 (1/16) | 6.3 (1/16) |
FIG. 2.
RFLP analysis of the rickettsial ompA gene 532-bp PCR product amplified from purified rickettsiae (DAE100R, Rustic) and D. andersoni tick extracts. PCR products were left uncut (U) or were digested with RsaI (R) or PstI (P). A 100-bp DNA size marker ladder is shown on the left of each panel. (A) R. peacockii strain DaE100R (Rpea) and Crestone tick 5 and 6 extracts (DaC5 and 6). (B) R. peacockii (Rpea) and Montana Bitterroot Valley east side tick extracts (DaEs 6, 8, and 10). (C) R. rickettsii Hlp#2 (HlP#2), R. peacockii (Rpea), and Bitterroot Valley west side tick 9 extract (DaWs9). (D) Bitterroot Valley west side tick 12 extract (DaWs12) and R. peacockii (Rpea).
We sequenced R. peacockii ompA PCR products, including the ompA promoter (bp 1 to 69), the protein coding region upstream of the tandem repeats (bp 70 to 680), and the coding region beginning just downstream of the repeats and continuing through the transcription terminator (bp 3608 to 6960) (Fig. 1). Sequences from R. peacockii Rustic (DaE100R) (46) and ticks collected in Montana (east side, Skalkaho ticks DaEs1, 3, and 4) and in Colorado (Crestone ticks DaC3 and 8) were identical except that one sample (DaEs1) had a G-to-A transition at bp 455. In contrast to R. rickettsii, R. peacockii Rustic (DaE100R) and tick-derived R. peacockii sequences all contained a deletion of G at bp 403 and insertions of GT and A following bp 4872 and 5828, respectively, resulting in premature stop codons.
DNA and translated sequence comparisons of R. peacockii and R. rickettsii ompA PCR products.
We compared the R. rickettsii R and Hlp#2 ompA sequences to those of R. peacockii DaE100R and tick extracts. Sequences of R. rickettsii Hlp#2 and R. peacockii (all strains) were 99.7 and 98.1% identical to R. rickettsii R and 98.3% identical to each other over the total of 4,032 bp. In order to compare the R. peacockii OmpA protein sequence to those of R. rickettsii R and Hlp#2, we reconstructed the open reading frame by restoring G at bp 403 and removing the insertions at bp 4872 and 5828. The reconstructed R. peacockii OmpA sequence was 95.9 and 96.1% identical to those of R. rickettsii R and Hlp#2, which were 99.5% identical.
Phylogeny of SFGR ompA sequences.
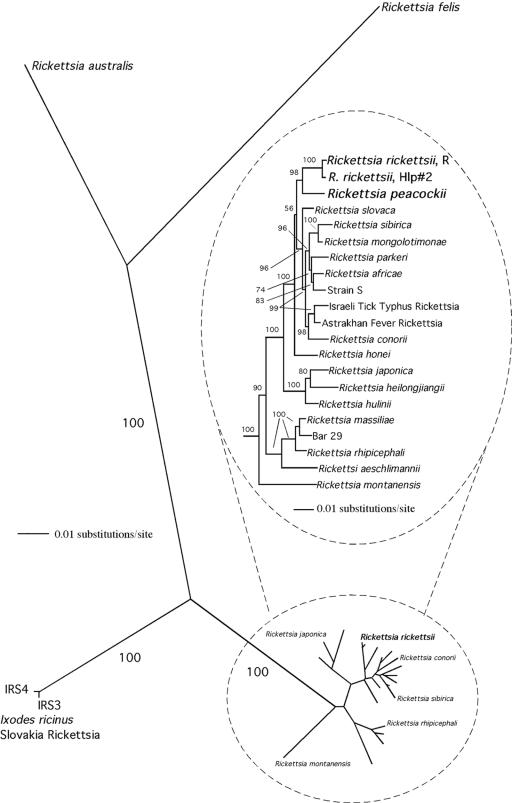
Both the neighbor-joining and maximum parsimony phylogenetic analyses revealed four major branches among the SFGR. Three of these branches, as shown in the unrooted phylogram (Fig. 3), were comprised of R. felis, R. australis, and the IRS3 and IRS4 rickettsiae, demonstrating how divergent their ompA sequences are. The remaining SFGR clustered together on the fourth branch, a direct consequence of their more-conserved ompA sequences (the inset phylogram in the figure is an expanded view of the fourth branch using R. felis as outgroup). Within this cluster, five distinct monophyletic groupings with bootstrap supports of 96% or better were obtained (Fig. 3, inset phylogram). One group contained R. rickettsii R and Hlp#2 and R. peacockii (98% bootstrap support), a second comprised R. rhipicephali, R. aeschlimannii, Bar 29, and R. massilae (100%), while R. japonica, R. hulinii, and R. heilongjiangii clustered together in a third group (100%). R. mongolotimoniae, R. sibirica strain S, R. africae, and R. parkeri formed another group (99%). A fifth group contained R. conorii, the Israeli tick typhus, and the Astrakhan fever rickettsiae (98%). R. montanensis, R. slovaca, and R. honei did not cluster with any other rickettsiae. Maximum parsimony and neighbor-joining analyses using the IRS as the sister outgroup and deleting R. felis and R. australis gave the same groupings with similar bootstrap values (data not shown).
FIG. 3.
Phylogenetic tree (neighbor-joining phylogram) of SFGR (Table 2) inferred from comparison of rickettsial ompA sequences. Numbers are the proportion of 1,000 bootstrap resamplings that supported the topology. Phylograms constructed using maximum parsimony tree-building analyses were similar. The inset phylogram in the figure is an expanded view of the fourth branch, using R. felis as an outgroup. A monophyletic group containing R. peacockii and R. rickettsii is shown in bold.
Analysis of gltA and 17-kDa antigen PCR products.
Analysis of the gltA and 17-kDa antigen gene sequences corroborated a close relationship of R. peacockii and R. rickettsii based on ompA and 16S rRNA gene sequences. Partial gltA gene sequences of all three R. peacockii strains were identical. All strains of R. peacockii had gltA sequences that differed from R. rickettsii strain Hlp#2 by 1 nucleotide (at position 998, based on the numbering of Roux et al. [42] for strain R) and from strain R by an insertion of 3 nucleotides (between nucleotides 1024 and 1025) and a point mutation (at nucleotide 1026). Partial 17-kDa antigen gene sequences of all R. peacockii strains were identical to each other and those for R. rickettsii strains Hlp#2 and Sheila Smith.
Transcriptional regulatory element sequence comparison and RT-PCR assays.
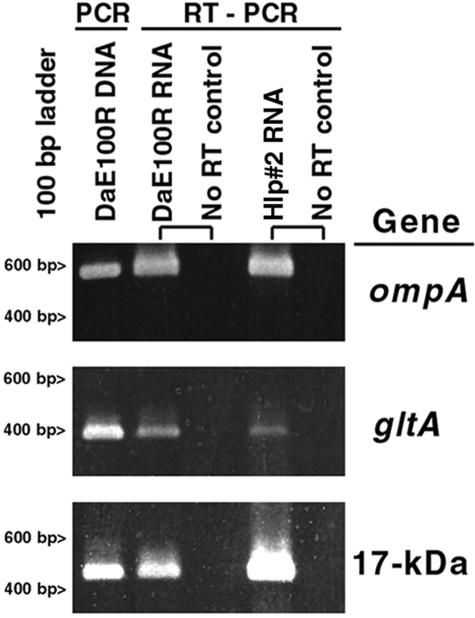
The R. rickettsii R and Hlp#2 promoter sequences were identical, but those from R. peacockii and ticks DaEs1 (Montana) and DaC3 and -8 (Colorado) contained a T-to-C transition at bp 12 and a G-to-T transversion at bp 54 (also present in the R. conorii sequence; GenBank accession no. AE006914). The R. peacockii and tick DNA sequences additionally contained a transversion at bp 61 that altered the putative ribosome binding site from AAGG (as in R. rickettsii ompA) to AAGC. The 140-bp transcription terminator region (bp 6820 to 6960) was identical among all sequences. The minor differences in the core promoter regions of ompA implied that the R. peacockii promoter was functional. Indeed, RT-PCR results obtained with the ompA primer set 190-70p and 190-701 indicated active transcription of ompA mRNA by R. peacockii Rustic (DAE100R). Control RT-PCR assays using RNA from R. rickettsii Hlp#2 and R. peacockii with primers for citrate synthase (gltA) or the 17-kDa antigen likewise yielded the predicted PCR products, whereas reactions lacking reverse transcriptase were negative (Fig. 4).
FIG. 4.
RT-PCR detection of R. peacockii DaE100R and R. rickettsii Hlp#2 ompA, citrate synthase (gltA), and 17-kDa surface antigen gene transcripts. Control reactions were performed on RNA extracts without reverse transcriptase, and PCR-only reactions were performed with DaE100R DNA for comparison of product sizes with those of RT-PCRs. The relative gel migration positions of 400- and 600-bp markers (Life Technologies) are indicated on the left.
SDS-PAGE and immunoblot assays of OmpA expression.
Despite an apparently functional promoter, the presence of at least three premature stop codons and a potentially weak ribosome binding site in the R. peacockii ompA gene mRNA suggested compromised translation. SDS-PAGE of R. peacockii Rustic (DAE100R) extracts did not resolve a protein of 190 kDa (the molecular mass of OmpA), but a prominent protein of that size was present in both Hlp#2 and MOAa extracts (Fig. 5A). Western blotting with MAb 13-5 against OmpA did not reveal an R. peacockii 190-kDa protein or possible truncated peptide bands, but results were positive for both Hlp#2 and MOAa (Fig. 5B). Likewise, a polyclonal hamster anti-R. monacensis serum detected a 190-kDa protein in Hlp#2 and MOAa, but not in R. peacockii (Fig. 5C), whereas a 120-kDa band corresponding to OmpB was present in all three extracts (35).
FIG. 5.
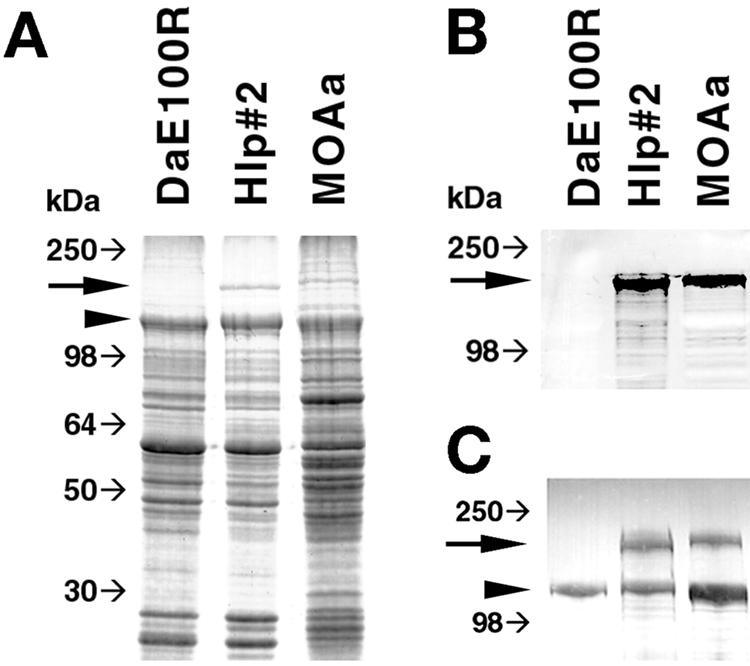
SDS-PAGE and immunoblot analyses of R. peacockii DaE100R, R. ricketsii Hlp#2, and Rickettsia sp. MOAa proteins. (A) Coomassie blue-stained SDS-PAGE gel. (B) Western blot probed with MAb 13-5 against rickettsial OmpA. (C) Western blot probed with polyclonal sera against R. monacensis to show absence of OmpA but presence of OmpB in R. peacockii DAE100R. Large arrows indicate the position of the 190-kDa OmpA, and arrowheads indicate the position of the 120-kDa OmpB. Small arrows indicate the relative gel migration positions of SeeBlue protein molecular mass markers (Invitrogen).
Double immunolabeling of actin associated with rickettsiae.
Because pathogenic SFGR facilitate their spread among host cells through actin tail formation, a process possibly mediated by OmpA (8, 24), we assessed the ability of R. peacockii to induce actin tails. There was no association of rhodamine-labeled host actin with fluorescein isothiocyanate-labeled R. peacockii, which was usually clumped or in chains (data not shown), while the host cell actin remained highly organized in filaments as in uninfected control cells. In cultures infected with R. rickettsii Hlp#2, actin tails often exceeding 20 μm in length were commonly associated with rickettsiae, and the host cells appeared to have disorganized cytoskeletal structure as indicated by diffuse actin staining.
DISCUSSION
Our data indicate that R. peacockii continues to be transovarially maintained in D. andersoni ticks collected in the Bitterroot Valley and that it also occurs in at least two widely separated D. andersoni populations in Colorado. Our investigators previously reported that the 16S rRNA gene sequence of R. peacockii DaE100R, isolated from ticks collected in Rustic, Colo. (47), differed by 2 nucleotides at positions 474 and 532 (incorrectly identified as nucleotides 475 and 533 [41, 47]) from that reported for the type strain, R. peacockii Skalkaho (28). This was unexpected in light of the 100% sequence homology between the ompA genes for these isolates. DNA of the original Skalkaho strain used to describe the R. peacockii type species is no longer available for comparison (T. Schwan, personal communication). This prompted us to reexamine 341 nucleotides spanning this region. We found that R. peacockii in four ticks collected in Skalkaho in 2003 had sequences identical to those for R. peacockii in ticks from Crestone, Colo., and to the culture isolate DaE100R (47), as well as R. rickettsii Hlp#2 (this study) and strain R (41). Analysis of the ompA gene showed that R. peacockii from all three locations possessed essentially identical sequences, including three premature stop codons. Although the R. peacockii ompA promoter closely resembled that of R. rickettsii and was functional, the putative ribosome binding site sequence within the mRNA contained a G-to-C transversion that would probably weaken translation efficiency (50). In support of this assumption, SDS-PAGE and Western blotting data indicated that neither full-length nor truncated OmpA products were expressed by R. peacockii. Compromised expression of OmpA and consequently its probable normal function as a host cell adhesion factor (22) may underlie the apparent restriction of R. peacockii to tick vectors and possibly to specific tissues involved in transmission of rickettsiae to progeny ticks.
We have shown that similar or identical R. peacockii strains are still prevalent in D. andersoni in the original Bitterroot Valley, Mont., location described for the East Side agent (7, 28) and that they also occur in both northern and southern Colorado D. andersoni populations. We propose that R. peacockii is likely to be more geographically widespread than previously believed even though, given its lack of infectivity for amplifying vertebrate hosts, transovarial transmission may alone be responsible for its maintenance in tick populations. The presence of R. peacockii in ticks is believed to influence the transmission dynamics of pathogenic R. rickettsii strains through a mechanism involving competitive exclusion from the tick host (7, 9), and further investigation of the prevalence of R. peacockii in tick populations is warranted given its potential role in the epidemiology of spotted fever.
Several earlier phylogenetic analyses of SFGR utilized sequences from only a portion of ompA, resulting in ambiguous trees that were not well supported by other data, such as biogeography (52). The new analysis presented here is based on the complete ompA gene except the repeats and demonstrates that among the SFGR, the nonvirulent R. peacockii is most closely related to R. rickettsii. This observation provides an opportunity to uncover mechanisms underlying the pathogenesis of R. rickettsii through molecular genetic approaches that would be facilitated by an effort to completely sequence the R. peacockii genome. The phylogenetic comparison further resolved a long-standing ambiguity (33) concerning the relationship of R. rickettsii Hlp#2 to other R. rickettsii strains. Despite the fact that virulent R. rickettsii is associated with Dermacentor ticks and that Hlp#2 was isolated from Haemaphysalis ticks collected from cottontail rabbits, they are clearly more closely related to each other than to any other member of the SFGR. Possible simultaneous circulation of attenuated Hlp#2-like (11, 33) and virulent Rickettsia strains with similar or identical major antigenic epitopes among vertebrates and ticks could be another significant variable in the epidemiology of RMSF.
DNA sequence comparison of the ompA promoter regions of R. peacockii, R. conorii, and R. rickettsii R and Hlp#2 strains demonstrated that they were nearly identical, with appropriately spaced (17 bp) −10 and −35 box consensus sequences, whose functional identity has been validated in the case of strain R by primer extension analysis (36). Curiously, this close identity did not extend to the ompA promoter regions of R. australis (49) and R. felis (5) (GenBank accession nos. AF149108 and AF191026), which have many differences including, respectively, a T-to-A transversion and an A-to-G transversion within the −10 box consensus. In addition, the R. felis sequence contains a 6-bp insertion immediately upstream of the −10 box consensus that results in a suboptimal 23-bp spacer between the −10 box and the −35 box consensus sequence (otherwise identical among all six of the above sequences).
In conclusion, the close relationship of R. peacockii to R. rickettsii was revealed by the high degree of sequence similarity among their ompA genes, supported by our analyses of the 16S rRNA, citrate synthase, and 17-kDa antigen gene sequences. The nearly identical R. peacockii ompA promoter region compared to that of R. rickettsii strain R suggested that it was functional, as confirmed by RT-PCR analysis. However, our sequence data showing a weakened ribosome binding site within the mRNA leader and multiple premature stop codons within the OmpA reading frame implied severely compromised translation. Not surprisingly, no OmpA translation products could be detected in R. peacockii protein extracts. R. peacockii is poorly infectious and exhibits slow growth in tick cells relative to that of Hlp#2 (46). Moreover, double immunolabeling studies failed to demonstrate actin tail structures that were present in the same cell line infected with R. rickettsii Hlp#2. These results support the hypothesis that OmpA, possibly in conjunction with RickA (17), accelerates cell-to-cell spread of rickettsiae. Genetic transformation of R. rickettsii and in vitro cell-free transcription-translation experiments would aid in testing this hypothesis, but no such expression system exists for the obligate intracellular rickettsiae, and rickettsial transformation techniques, including site-directed mutagenesis, are in their infancy (37).
Acknowledgments
This research was supported by National Institutes of Health grant RO1 AI49424 to U.G.M. This research was supported in part by a University of Minnesota graduate school doctoral dissertation fellowship awarded to J.A.S.
We are grateful to Michael Herron for critical review of the manuscript.
REFERENCES
- 1.Anacker, R. L., R. E. Mann, and C. Gonzales. 1987. Reactivity of monoclonal antibodies to Rickettsia rickettsii with spotted fever and typhus group rickettsiae. J. Clin. Microbiol. 25:167-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, B. E., G. A. McDonald, D. C. Jones, and R. L. Regnery. 1990. A protective protein antigen of Rickettsia rickettsii has tandemly repeated, near-identical sequences. Infect. Immun. 58:2760-2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson, B. E., and T. Tzianabos. 1989. Comparative sequence analysis of a genus-common rickettsial antigen gene. J. Bacteriol. 171:5199-5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azad, A. F., and C. B. Beard. 1998. Rickettsial pathogens and their arthropod vectors. Emerg. Infect. Dis. 4:179-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouyer, D. H., J. Stenos, P. Crocquet-Valdes, C. G. Moron, V. L. Popov, J. E. Zavala-Velazquez, L. D. Foil, D. R. Stothard, A. F. Azad, and D. H. Walker. 2001. Rickettsia felis: molecular characterization of a new member of the spotted fever group. Int. J. Syst. E vol. Microbiol. 51:339-347. [DOI] [PubMed] [Google Scholar]
- 6.Burgdorfer, W. 1988. Ecological and epidemiological considerations of Rocky Mountain spotted fever and scrub typhus, p. 33-50. In D. H. Walker (ed.), Biology of rickettsial diseases, vol. 1. CRC Press, Inc., Boca Raton, Fla.
- 7.Burgdorfer, W., S. F. Hayes, and A. J. Mavros. 1981. Nonpathogenic rickettsiae in Dermacentor andersoni: a limiting factor for the distribution of Rickettsia rickettsii, p. 585-594. In W. Burgdorfer and R. L. Anacker (ed.), Rickettsiae and rickettsial diseases. Academic Press, New York, N.Y.
- 8.Charles, M., J. Magdalena, J. A. Theriot, and M. B. Goldberg. 1999. Functional analysis of a rickettsial OmpA homology domain of Shigella flexneri IcsA. J. Bacteriol. 181:869-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Childs, J. E., and C. D. Paddock. 2002. Passive surveillance as an instrument to identify risk factors for fatal Rocky Mountain spotted fever: is there more to learn? Am. J. Trop. Med. Hyg. 66:450-457. [DOI] [PubMed] [Google Scholar]
- 10.de la Fuente, J., E. Blouin, and K. M. Kocan. 2003. Infection exclusion of the rickettsial pathogen Anaplasma marginale in the tick vector Dermacentor variabilis. Clin. Diagn. Lab. Immunol. 10:182-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eremeeva, M. E., G. Dasch, and D. J. Silverman. 2001. Quantitative analyses of variations in the injury of endothelial cells elicited by 11 isolates of Rickettsia rickettsii. Clin. Diagn. Lab. Immunol. 8:788-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Felsenstein, J. 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783-791. [DOI] [PubMed] [Google Scholar]
- 13.Forsman, M., G. Sandstrom, and A. Sjöstedt. 1994. Analysis of 16S ribosomal DNA sequences of Francisella strains and utilization for determination of the phylogeny of the genus and for identification of strains by PCR. Int. J. Syst. Bacteriol. 44:38-46. [DOI] [PubMed] [Google Scholar]
- 14.Fournier, P.-E., J. S. Dumler, G. Greub, J. Zhang, Y. Wu, and D. Raoult. 2003. Gene sequence-based criteria for identification of new Rickettsia isolates and description of Rickettsia heilongjiangensis sp. nov. J. Clin. Microbiol. 41:5456-5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fournier, P. E., V. Roux, and D. Raoult. 1998. Phylogenetic analysis of spotted fever group rickettsiae by study of the outer surface protein rOmpA. Int. J. Syst. Bacteriol. 48:839-849. [DOI] [PubMed] [Google Scholar]
- 16.Gage, K. L., M. E. Schrumpf, R. H. Karstens, W. Burgdorfer, and T. G. Schwan. 1994. DNA typing of rickettsiae in naturally infected ticks using a polymerase chain reaction/restriction fragment length polymorphism system. Am. J. Trop. Med. Hyg. 50:247-260. [DOI] [PubMed] [Google Scholar]
- 17.Gouin, E., C. Egile, P. Dehoux, V. Villiers, J. Adams, F. Gertler, R. Li, and P. Cossart. 2004. The RickA protein of Rickettsia conorii activates the Arp2/3 complex. Nature 427:457-461. [DOI] [PubMed] [Google Scholar]
- 18.Harden, V. A. 1990. Rocky Mountain spotted fever: history of a twentieth century disease. The Johns Hopkins University Press, Baltimore, Md.
- 19.Heinzen, R. A., S. F. Hayes, M. G. Peacock, and T. Hackstadt. 1993. Directional actin polymerization associated with spotted fever group rickettsia infection of Vero cells. Infect. Immun. 61:1926-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeanmougin, F., J. D. Thompson, M. Gouy, D. G. Higgins, and T. J. Gibson. 1998. Multiple sequence alignment with Clustal X. Trends Biochem. Sci. 23:403-405. [DOI] [PubMed] [Google Scholar]
- 21.Kimura, M. 1980. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 16:111-120. [DOI] [PubMed] [Google Scholar]
- 22.Li, H., and D. H. Walker. 1998. rOmpA is a critical protein for the adhesion of Rickettsia rickettsii to host cells. Microb. Pathog. 24:289-298. [DOI] [PubMed] [Google Scholar]
- 23.Macaluso, K. R., D. E. Sonenshine, S. M. Ceraul, and A. F. Azad. 2002. Rickettsial infection in Dermacentor variabilis (Acari: Ixodidae) inhibits transovarial transmission of a second Rickettsia. J. Med. Entomol. 39:809-813. [DOI] [PubMed] [Google Scholar]
- 24.Munderloh, U. G., S. F. Hayes, J. Cummings, and T. J. Kurtti. 1998. Microscopy of spotted fever rickettsia movement through tick cells. Microsc. Microanal. 4:115-121. [Google Scholar]
- 25.Munderloh, U. G., S. D. Jauron, V. Fingerle, L. Leitritz, S. F. Hayes, J. M. Hautman, C. M. Nelson, B. W. Huberty, T. J. Kurtti, G. G. Ahlstrand, B. Greig, M. A. Mellencamp, and J. L. Goodman. 1999. Invasion and intracellular development of the human granulocytic ehrlichiosis agent in tick cell culture. J. Clin. Microbiol. 37:2518-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niebylski, M. L., M. G. Peacock, E. R. Fischer, S. F. Porcella, and T. G. Schwan. 1997. Characterization of an endosymbiont infecting wood ticks, Dermacentor andersoni, as a member of the genus Francisella. Appl. Environ. Microbiol. 63:3933-3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niebylski, M. L., M. G. Peacock, and T. G. Schwan. 1999. Lethal effect of Rickettsia ricketsii on its tick vector (Dermacentor andersoni). Appl. Environ. Microbiol. 65:773-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niebylski, M. L., M. E. Schrumpf, W. Burgdorfer, E. R. Fischer, K. L. Gage, and T. G. Schwan. 1997. Rickettsia peacockii sp. nov., a new species infecting wood ticks, Dermacentor andersoni, in western Montana. Int. J. Syst. Bacteriol. 47:446-452. [DOI] [PubMed] [Google Scholar]
- 29.Noda, H., U. G. Munderloh, and T. J. Kurtti. 1997. Endosymbionts of ticks and their relationship to Wolbachia and tick-borne pathogens of man and animals. Appl. Environ. Microbiol. 63:3926-3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Obonyo, M., U. G. Munderloh, V. Fingerle, B. Wilske, and T. J. Kurtti. 1999. Borrelia burgdorferi in tick cell culture modulates expression of outer surface proteins A and C in response to temperature. J. Clin. Microbiol. 37:2137-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ogata, H., S. Audic, P. Renesto-Audiffren, P.-E. Fournier, V. Barbe, D. Samson, V. Roux, P. Cossart, J. Weissenbach, J.-M. Claverie, and D. Raoult. 2001. Mechanisms of evolution in Rickettsia conorii and R. prowazekii. Science 293:2093-2098. [DOI] [PubMed] [Google Scholar]
- 32.O'Neill, S. L., R. Giordano, A. M. E. Colbert, T. L. Karr, and H. M. Robertson. 1992. 16S rRNA phylogenetic analysis of the bacterial endosymbionts associated with cytoplasmic incompatibility in insects. Proc. Nat. Acad. Sci. USA 89:2699-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parker, R. R., E. G. Pickens, D. B. Lackman, E. J. Bell, and F. B. Thraikill. 1951. Isolation and characterization of Rocky Mountain spotted fever from the rabbit tick Haemaphysalis leporis-palustris Packard. Public Health Rep. 66:455-463. [PMC free article] [PubMed] [Google Scholar]
- 34.Philip, C. B. 1959. Some epidemiological considerations in Rocky Mountain spotted fever. Public Health Rep. 74:595-600. [PMC free article] [PubMed] [Google Scholar]
- 35.Policastro, P., U. G. Munderloh, E. R. Fischer, and T. Hackstadt. 1997. Rickettsia rickettsii growth and temperature-inducible protein expression in embryonic tick cell lines. J. Med. Microbiol. 46:839-845. [DOI] [PubMed] [Google Scholar]
- 36.Policastro, P. F., and T. Hackstadt. 1994. Differential activity of Rickettsia rickettsii ompA and ompB promoter regions in a heterologous reporter gene system. Microbiology 140:2941-2949. [DOI] [PubMed] [Google Scholar]
- 37.Qin, A., A. M. Tucker, A. Himes, and D. O. Wood. 2004. Transposon mutagenesis of the obligate intracellular pathogen Rickettsia prowazekii. Appl. Environ. Microbiol. 70:2816-2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rachek, L. I., A. M. Tucker, H. H. Winkler, and D. O. Wood. 1998. Transformation of Rickettsia prowazekii to rifampin resistance. J. Bacteriol. 180:2118-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Regnery, R. L., C. L. Spruill, and B. D. Plikaytis. 1991. Genotypic identification of rickettsiae and estimation of intraspecies divergence for portions of two rickettsial genes. J. Bacteriol. 173:1576-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roux, V., and D. Raoult. 2000. Phylogenetic analysis of members of the genus Rickettsia using the gene encoding the outer-membrane protein rOmpB (ompB). Int. J. Syst. E vol. Microbiol. 50:1449-1455. [DOI] [PubMed] [Google Scholar]
- 41.Roux, V., and D. Raoult. 1995. Phylogenetic analysis of the genus Rickettsia by 16S rDNA sequencing. Res. Microbiol. 146:385-396. [DOI] [PubMed] [Google Scholar]
- 42.Roux, V., E. Rydkina, M. Eremeeva, and D. Raoult. 1997. Citrate synthase gene comparison, a new tool for phylogenetic analysis, and its application for the rickettsiae. Int. J. Syst. Bacteriol. 47:252-261. [DOI] [PubMed] [Google Scholar]
- 43.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 44.Sekeyova, Z., V. Roux, and D. Raoult. 2001. Phylogeny of Rickettsia spp. inferred by comparing sequences of “gene D, ” which encodes an intracytoplasmic protein. Int. J. Syst. E vol. Microbiol. 51:1353-1360. [DOI] [PubMed] [Google Scholar]
- 45.Simser, J. A., A. T. Palmer, V. Fingerle, B. Wilske, T. J. Kurtti, and U. G. Munderloh. 2002. Rickettsia monacensis sp. nov., a spotted fever group rickettsia, from ticks (Ixodes ricinus) collected in a European city park. Appl. Environ. Microbiol. 68:4559-4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simser, J. A., A. T. Palmer, U. G. Munderloh, and T. J. Kurtti. 1999. Isolation and characterization of Rickettsia peacockii maintained in tick cell culture. Am. J. Trop. Med. Hyg. 61(Suppl.):359. [Google Scholar]
- 47.Simser, J. A., A. T. Palmer, U. G. Munderloh, and T. J. Kurtti. 2001. Isolation of a spotted fever group rickettsia, Rickettsia peacockii, in a Rocky Mountain wood tick, Dermacentor andersoni, cell line. Appl. Environ. Microbiol. 67:546-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sober, E. 1988. Reconstructing the past: parsimony, evolution and inference, p. 265. MIT Press, Cambridge, Mass.
- 49.Stenos, J., and D. H. Walker. 2000. The rickettsial outer-membrane protein A and B genes of Rickettsia australis, the most divergent rickettsia of the spotted fever group. Int. J. Syst. Evol. Microbiol. 50:1775-1779. [DOI] [PubMed] [Google Scholar]
- 50.Stromo, G. D., T. D. Schneider, and L. M. Gold. 1982. Characterization of translation initiation sites in E. coli. Nucleic Acids Res. 10:2971-2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Swofford, D. L. 2001. PAUP*. Phylogenetic analysis using parsimony (*and other methods), version 4.0b8 (PPC). Sinauer Associates, Sunderland, Mass.
- 52.Weller, S. J., G. D. Baldridge, U. G. Munderloh, H. Noda, J. Simser, and T. J. Kurtti. 1998. Phylogenetic placement of rickettsiae from the ticks Amblyomma americanum and Ixodes scapularis. J. Clin. Microbiol. 36:1305-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Williams, S. G., J. B. Sacci, Jr., M. E. Schriefer, E. M. Andersen, K. K. Fujioka, F. J. Sorvillo, A. R. Barr, and A. F. Azad. 1992. Typhus and typhuslike rickettsiae associated with opossums and their fleas in Los Angeles County, California. J. Clin. Microbiol. 30:1758-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]