Abstract
Periphyton (Cladophora sp.) samples from a suburban stream lacking detectable dissolved As were able to reduce added As(V) to As(III) when incubated under anoxic conditions and, conversely, oxidized added As(III) to As(V) with aerobic incubation. Both types of activity were abolished in autoclaved controls, thereby demonstrating its biological nature. The reduction of As(V) was inhibited by chloramphenicol, indicating that it required the synthesis of new protein. Nitrate also inhibited As(V) reduction, primarily because it served as a preferred electron acceptor to which the periphyton community was already adapted. However, part of the inhibition was also caused by microbial reoxidation of As(III) linked to nitrate. Addition of [14C]glucose to anoxic samples resulted in the production of 14CO2, suggesting that the observed As(V) reduction was a respiratory process coupled to the oxidation of organic matter. The population density of As(V)-reducing bacteria within the periphyton increased with time and with the amount of As(V) added, reaching values as high as ∼106 cells ml−1 at the end of the incubation. This indicated that dissimilatory As(V) reduction in these populations was linked to growth. However, As(V)-respiring bacteria were found to be present, albeit at lower numbers (∼102 ml−1), in freshly sampled periphyton. These results demonstrate the presence of a bacterial population within the periphyton communities that is capable of two key arsenic redox transformations that were previously studied in As-contaminated environments, which suggests that these processes are widely distributed in nature. This assumption was reinforced by experiments with estuarine samples of Cladophora sericea in which we detected a similar capacity for anaerobic As(V) reduction and aerobic As(III) oxidation.
Arsenic is a toxic trace element that is widely but unevenly distributed in the earth's atmosphere, hydrosphere, soils, sediments, and living organisms (6). Natural sources of environmental arsenic include volcanism, hydrothermal activity, and the weathering of sedimentary and igneous rocks that contain arsenic-bearing minerals. Anthropogenic activities such as ore smelting, fossil fuel combustion, and the use of agricultural chemicals also represent significant sources of arsenic in the environment (31). The contamination of drinking water aquifers by naturally occurring arsenic represents a significant environmental hazard that presently affects the health of millions of people worldwide (1, 20, 29, 45).
The mobility and toxicity of As in aqueous environments is controlled by oxidative and reductive (redox) microbial transformations of the element between its two most abundant inorganic oxyanions, arsenate [As(V)] and arsenite [As(III)]. Arsenate is the predominant species in aerobic surface waters. Arsenite predominates in reducing anaerobic environments but may also be present as a metastable species in aerobic settings due to its slow oxidation kinetics and biologically mediated reduction reactions (46). While both of these species are water soluble and toxic to a wide range of organisms, As(V) is highly sorptive to the surface of iron, manganese, and aluminum oxide minerals that are commonly present in sediments and soils. Arsenite adsorbs to most mineral surfaces less strongly than As(V) and is therefore generally the more mobile and bioavailable form.
Microbial redox transformations of arsenic between the As(V) and As(III) species can result from biochemical detoxification mechanisms that permit the organism to exclude or expel environmental arsenic from its cellular interior (38). Alternately, other redox transformations of these arsenic species include metabolic pathways linked to energy gain and growth for various heterotrophic and chemoautotrophic prokaryotes (25, 27, 31, 33, 43, and S. Silver and L. T. Phung, submitted for publication). While microbial reactions that utilize the reduction of As(V) or the oxidation of As(III) as strategies for arsenic detoxification and resistance have been identified in a wide range of organisms, detoxification processes alone cannot adequately account for the changes in arsenic speciation that occur upon experimental incubation of natural materials, such as sediments or anoxic waters (7, 28, 34). Most field-oriented studies of this kind have in turn focused on As-rich or As-contaminated settings (3, 14, 17, 18, 22, 34, 37, 46).
Over the past decade, a number of novel prokaryotes have been described that obtain energy for growth from metabolic reactions involving arsenic (31). These As-based metabolic pathways include dissimilatory (respiratory) As(V) reduction and chemoautotrophic As(III) oxidation. Aerobic, chemoautotrophic As(III)-oxidizing bacteria have been isolated from gold mines (16, 40, 41). Anaerobic As(III) oxidation coupled to nitrate was reported for a novel γ-Proteobacterium, strain MLHE-1, isolated from arsenic-rich Mono Lake, California (14, 32). Hydrochemical data suggests that biological As(III) oxidation coupled to nitrate occurs in other As-contaminated, anoxic environments, including a freshwater lake (42) and a groundwater aquifer in Bangladesh (12). Dissimilatory arsenate reduction (DAsR) has been described for about 20 strains of anaerobic prokaryotes from a wide variety of environments (27, 30, 31, 33), including recent isolations of chemolithoautotrophs that use sulfide (13) or H2 (21) as electron donors. Therefore, arsenic-based metabolism is widespread among phylogenetically diverse organisms isolated from arsenic-rich environments, and in turn these microbes are also adapted to a wide range of pHs, salinities, and temperatures.
Almost all As(V)-respiring organisms studied to date are also capable of using other electron acceptors in addition to As(V), most commonly nitrate (31, 43). The one exception to this general observation is a recently isolated chemoautotroph, strain MLMS-1 (13). This suggests that prokaryotes capable of DAsR need not be restricted to As-rich environments. Similar reasoning also extends to chemoautotrophic As(III) oxidizers, because they either can use other inorganic electron donors besides arsenic (e.g., sulfide or hydrogen) or can grow as conventional heterotrophs (32, 40, 41). However, we know little about the potential for microbial communities found in As-free environments to adapt to added As inputs, either by reducing As(V) or oxidizing As(III).
Here we report microbial As(III) oxidation and dissimilatory As(V) reduction by live samples of freshwater periphyton (Cladophora sp.) collected from San Francisquito Creek, a suburban freshwater drainage in Palo Alto, Calif., that is characterized by low (<13 nM) concentrations of ambient arsenic. In a much earlier investigation it was reported that these algal periphyton materials were copopulated by dense bacterial assemblages that were living off the cellular exudates generated by the algal component. We noted that freshly recovered periphyton samples had the constitutive ability to sustain high rates of denitrification when dark incubated in the absence of oxygen (44). The respiratory nitrate reductases, respiratory arsenate reductases, and arsenite oxidases of bacteria are all members of the broad dimethyl sulfoxide reductase family of Mo-containing enzymes (2, 24, 39), and many dissimilatory arsenate reducers can commonly respire nitrate as well (31, 43). Hence, we hypothesized that these communities would not be constrained by the availability of trace elements like Mo and would readily be able to respire arsenate by de novo synthesis of respiratory arsenate reductase if it was present in their genome. Therefore, we conducted more experiments with these periphyton materials to determine if they would respond quickly to perturbations made with arsenic oxyanions, as they had demonstrated previously for nitrate and denitrification activity (44). We chose a benthic periphyton system, because such a community would experience large diel variations in oxygen tension as a response to daytime net photosynthesis and nighttime net respiration, as was first noted in shallow marine mats (4, 36). Such an environment could provide niches for both As(V) respirers and As(III) oxidizers.
MATERIALS AND METHODS
Collection and preparation of samples.
Fresh periphyton and stream water for all experiments were collected from San Francisquito Creek on the day that the experiment was started. These freshwater Cladophora sp.-dominated periphyton mats contained abundant decomposing, as well as live, algal material. During periods of low stream flow the mats also entrapped fine stream sediments, therefore cobbles containing the periphyton were shaken in the stream prior to scraping so as to minimize the amount of entrained sediment in the scraped materials. Creek water was collected upstream of the periphyton in acid-washed nalgene bottles (2 liters). Periphyton was scraped from several streambed cobbles and was deposited into a single ziplock plastic bag. After returning to the laboratory, the algal material was transferred to a 250-ml glass beaker and mixed vigorously (5 to 10 min) by hand with a small stainless steel spatula to homogenize the sample. In an effort to demonstrate that this phenomenon was more widespread, we also conducted a limited sampling of Cladophora sericea material taken from Brisbane Lagoon, a saline (salinity, 34.5 g/liter) estuarine tidal slough located in San Francisco Bay, and processed them (along with the lagoon water) as outlined below.
Samples of creek water plus the homogenized periphyton as well as creek water without periphyton were incubated under aerobic or anaerobic conditions. For samples that received periphyton, wet algal material (0.25 g [wet weight], 0.04 g [dry weight]) was weighed into a 57-ml serum bottle prior to the addition of 30 ml of creek water. For incubations of creek water alone, the periphyton was omitted and 30 ml of creek water was dispensed into an empty serum bottle. Aerobic bottles were plugged with a permeable foam seal, allowing exchange with the atmosphere during incubation. Creek water for anaerobic samples was bubbled with O2-free N2 for 30 min prior to dispensing under a flow of N2. The anaerobic bottles were then crimp sealed with a butyl rubber stopper and flushed for 10 min with O2-free N2. Water and algae in heat-killed controls were autoclaved for 1 h at 121°C prior to any further additions.
Electron acceptors, electron donors, and inhibitors were added by syringe from anaerobic stock solutions. Experiments were conducted with As(V) added as Na2HAsO4 or with As(III) added as NaH2AsO3 at either low (10 μM) or high (1 mM) concentration. In samples which contained periphyton, the algal material itself served as the electron donor. Samples of As(V)-amended creek water that lacked algae were incubated with or without the addition of sodium lactate (1 mM) to serve as an electron donor. To determine if nitrate would either inhibit As(V) reduction or serve as an electron acceptor for anaerobic As(III) oxidation, a set of incubations was prepared with equimolar concentrations (1 mM) of nitrate and As(V) or As(III). Chloramphenicol (final concentration, 4.0 mg/ml) was added to a final set of As(V)-amended (10 μM and 1 mM) anaerobic incubations in order to block the synthesis of de novo enzymes. For the experiments with the estuarine C. sericea material, we only tested for reduction of 1 mM As(V) and oxidation of 1 mM As(III) with anaerobic and aerobic incubations, respectively.
All incubations were performed with triplicate bottles representing each experimental condition. Bottles were incubated in the dark to avoid photosynthetic activity and O2 production by the periphyton during the course of the experiment. The bottles were incubated at 20°C with constant rotary shaking (200 rpm), and the liquid phase was periodically subsampled (0.5 ml) by syringe. Subsamples were filter centrifuged (Spin-X centrifuge tube filters; pore size, 0.2 μm; Corning Inc., Corning, N.Y.) and diluted in deionized water prior to species-specific determination of As(III) and As(V) concentrations.
Determination of MPNs of DAsR bacteria.
The population density of DAsR bacteria within periphyton materials was determined by a three-tube most probable number (MPN) technique (34). Periphyton samples were prepared as described above at the following As(V) concentrations: 0, 1, and 5 mM. Each experimental condition was prepared in duplicate. Three 1-ml aliquots of the liquid phase were collected by syringe from each bottle at the beginning and at completion of As(V) reduction in the bottles. The length of the incubation period was determined by the time it took for the added As(V) to be completely reduced to As(III). For the 1 mM addition this was achieved in 10 days, but it required 58 days for the 5 mM addition. These 1-ml aliquots were then decimally diluted via serial transfers into triplicate sets of sealed, anaerobic culture tubes that contained 9 ml of freshwater media (35), with 5 mM lactate as electron donor and 5 mM As(V) as electron acceptor (highest decimal dilution, 10−10). The inoculated culture tubes, in turn, were incubated at ∼20°C in the dark for 27 days and then were subsampled for determination of As(III) and As(V) by high-performance liquid chromatography (HPLC). Following the incubation, the appearance of As(III) in a given tube was used to indicate the presence of As(V)-reducing microbes at that particular dilution level.
Radioisotope experiment.
Anaerobic incubations were prepared as described above, with the exception that an artificial creek water mixture was used in order to eliminate the presence of any ambient electron acceptors, such as nitrate. The artificial creek water consisted of phosphate-buffered deionized water (0.225 g of each K2HPO4 and KH2PO4 per liter) that was amended with NaCl (0.46 g/liter) and adjusted to pH 7.3 with 8.4% NaHCO3. The incubations were inoculated with fresh algae (0.25 g [wet weight]) that had been rinsed, centrifuged, and decanted three times with 25 ml of artificial creek water. The incubations were amended with As(V) (0 μM, 10 μM, 1 mM, or 5 mM) and uniformly labeled [14C]d-glucose (3.5 μCi; specific activity, 175 mCi/mmol; final added glucose concentration, ∼0.67 μM; ICN Pharmaceuticals, Irvine, Calif.). We monitored the As(III) and As(V) concentrations in a set of nonradioactive duplicates over the course of the incubation to ensure that adequate time had passed for complete reduction of As(V) at all concentration levels. After 15 days, the radioactive samples were injected with 3 N HCl (1 ml) to terminate the experiment and degas CO2 from the liquid phase. The headspace was then analyzed for 14C-labeled CO2 and CH4. Autoclaved controls and live samples incubated without As(V) were also prepared and analyzed.
Analytical techniques.
The total As concentrations of San Francisquito Creek and Brisbane Lagoon waters were measured by hydride generation-atomic absorption spectrophotometry (HG-AAS) using a Perkin Elmer 5000 atomic absorption spectrophotometer equipped with a Varian VGA-76 hydride generation accessory. Species-specific analysis of As(III) and As(V) concentrations in samples containing micromolar levels of arsenic were performed by HPLC-HG-AAS (23). This flowthrough analytical method permits nanomolar concentrations of As(III) and As(V) to be determined individually by separating the two species on an ion-exchange chromatography column that is directly interfaced with the spectrophotometer's hydride generation accessory. A Dionex Ion Pack AS-11 column was used with a 30 mM NaOH-1% methanol eluent mixture (flow rate, 1.0 ml/min). Retention times for As(III) and As(V) under these conditions were 2.3 and 3.9 min, respectively (detection limit for As, ∼70 nM). Arsenic speciation in samples containing millimolar levels of As was determined by HPLC with UV to visible wavelength detection (14). Nitrate was measured by ion chromatography with conductivity detection (33). The activity of 14C-labeled CO2 and CH4 in the gas phase of the radioisotope experiments was measured via gas chromatography in conjunction with gas proportional counting (5), using a Raytest Raga 93 model gas proportional counter attached to an HNU GC 301 gas chromatograph with a 3.04-m Porapack Q/N (80:20 mix) column.
RESULTS
Dissolved arsenic and nitrate in San Francisquito Creek.
The ambient arsenic in San Francisquito Creek water was below the detectable limit for HG-AAS analysis (<10 nM). The ambient nitrate concentration in the creek water was 90.9 μM (n = 3, σ = 0.5). The ambient inorganic arsenic concentration in the saline Brisbane Lagoon was 36 nM.
Anaerobic incubations.
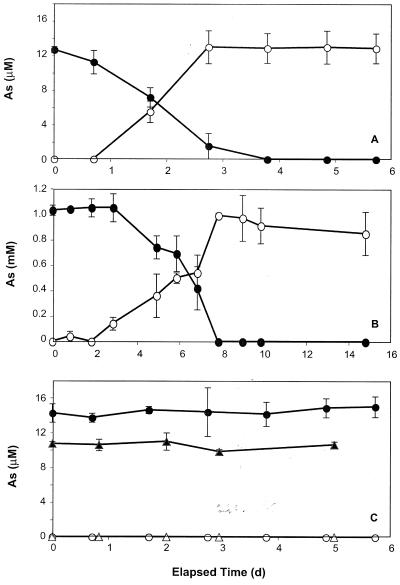
Freshwater periphyton samples showed noticeable As(V) reduction within 1 day of incubation and were able to completely reduce 10 μM As(V) to As(III) within 4 days (Fig. 1A). No As(V) reduction occurred in either heat-killed controls or in live samples incubated with chloramphenicol (Fig. 1C). Arsenate reduction to As(III) also occurred in samples with 1 mM As(V), although a lag of 2 days was evident before this was first noticeable (Fig. 1B). A complete reduction of As(V) to As(III) in these samples occurred within 8 days. As was the case at the lower concentrations, no arsenate reduction occurred in heat-killed controls or in live samples that were amended with chloramphenicol (data not shown). Furthermore, we did not note any loss of added (1 mM) As(V) or production of As(III) in aerobically incubated periphyton (data not shown). For the experiments with the estuarine C. sericea materials, As(V) reduction was evident by 3.8 days incubation (Table 1). No activity occurred in autoclaved controls (data not shown).
FIG. 1.
Reduction of As(V) (closed symbols) to As(III) (open symbols) by creek water inoculated with periphyton in anaerobic incubations amended with 10 μM (A) or 1 mM (B) As(V). (C) Controls consisting of samples that were heat sterilized (○ and •) or incubated with chloramphenicol (Δ and ▴). Symbols represent the means of three samples, and bars indicate ±1 standard deviation. The absence of bars indicates that the error was smaller than the symbols.
TABLE 1.
Changes in As redox chemistry made with anaerobic or aerobic incubation of estuarine C. sericea materialsa
| Oxyanion and incubation | Change (mM) at:
|
|
|---|---|---|
| 3.8 days | 5.9 days | |
| Anaerobicb | ||
| Arsenate | 0.36 ± 0.17 | 0.19 ± 0.16 |
| Arsenite | 0.43 ± 0.23 | 0.61 ± 0.26 |
| Aerobicc | ||
| Arsenate | 0.06 ± 0.01 | 0.27 ± 0.14 |
| Arsenite | 0.79 ± 0.02 | 0.59 ± 0.15 |
Mean of three samples ± 1 standard deviation.
Initial As(V) added, 0.87 ± 0.03 mM.
Initial As(III) added, 0.79 ± 0.06 mM.
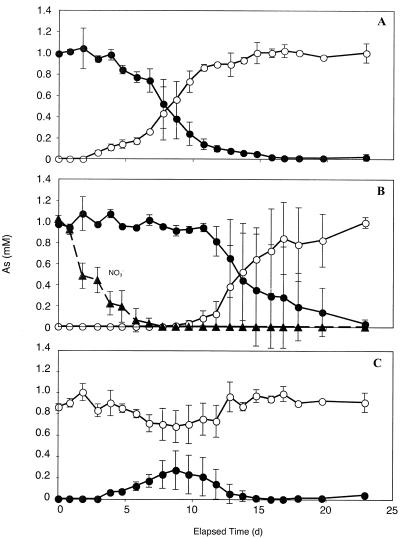
Addition of nitrate to periphyton samples inhibited the reduction of As(V) to As(III) (Fig. 2). Whereas in samples without nitrate As(V) reduction was notable after a 2-day lag (Fig. 2A), the lag was nearly 10 days long in samples containing nitrate (Fig. 2B). Only after the complete consumption of nitrate was arsenate reduction detected. Periphyton samples incubated with 1 mM As(III) plus 1 mM nitrate had significant As(III) removal and As(V) production notable by 4 days of incubation (Fig. 2C). However, this was a transient phenomenon, peaking by 9 days and then reversing itself completely by 15 days of incubation.
FIG. 2.
Anaerobic incubations of periphyton showing the effect of nitrate (▴) on the anaerobic reduction of 1 mM As(V) (•) and oxidation of 1 mM As(III) (○). Incubation conditions were with 1 mM As(V) but without nitrate additions (A), with 1 mM As(V) plus 1 mM nitrate (B), and with 1 mM As(III) plus 1 mM nitrate (C). Symbols represent the means of three samples, and bars indicate ±1 standard deviation. The absence of bars indicates that the error was smaller than the symbols.
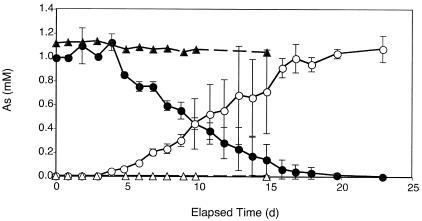
Anaerobic samples of creek water incubated without periphyton did not reduce As(V) (Fig. 3). However, when creek water was amended with 1 mM lactate, a complete reduction of 1 mM As(V) to As(III) occurred within 23 days. No As(V) reduction occurred in autoclaved creek water controls incubated with 1 mM lactate (data not shown).
FIG. 3.
Reduction of As(V) to As(III) in anaerobic creek water lacking periphyton. Shown are As(V) (▴) and As(III) (Δ) in live samples without added electron donor and As(V) (•) and As(III) (○) in live samples with lactate. Symbols represent the means of three samples, and bars indicate ±1 standard deviation. The absence of bars indicates that the error was smaller than the symbols.
Aerobic incubations.
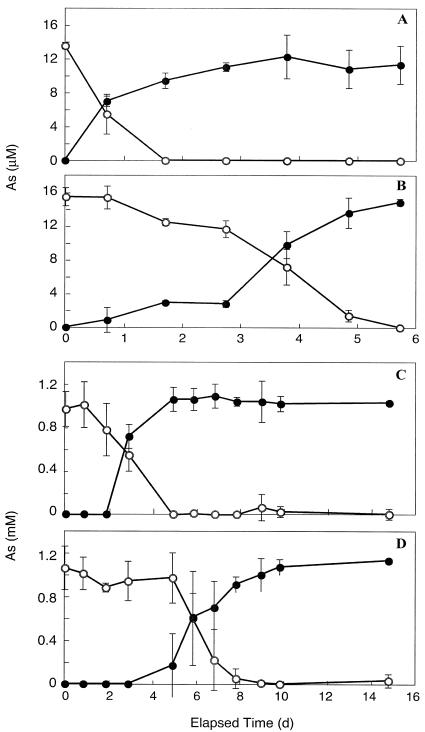
The oxidation of As(III) was observed in live samples of creek water that were incubated either with or without the periphyton component. Creek water alone completely oxidized 10 μM As(III) to As(V) within 6 days, while the presence of periphyton essentially doubled the rate of oxidation (Fig. 4A). Increasing the As(III) concentration to 1 mM merely lengthened the lag time by 1 day before oxidation was noticeable, but the results were essentially the same as those observed at the lower concentration (Fig. 4B). No As(III) oxidation occurred in heat-killed periphyton or water-only controls at either concentration (data not shown). For the experiments with estuarine C. sericea materials, a small amount of oxidation of ∼1 mM As(III) (7.5%) was evident at 3.8 days, which increased to 34.1% by 5.9 days (Table 1). No such activity was observed in autoclaved controls (data not shown).
FIG. 4.
Oxidation of As(III) (○) to As(V) (•) during aerobic incubation of creek water with periphyton (A and C) or without periphyton (B and D). As(III) was added at either 10 μM (A and B) or 1 mM (C and D). Symbols represent the means of three samples, and bars indicate ±1 standard deviation. The absence of bars indicates that the error was smaller than the symbols.
MPN and radioisotope experiments.
In order to estimate the population density of arsenate respirers in the periphyton samples before and after incubation with As(V), we carried out the MPN growth experiment as summarized in Table 2. There was a small (102 to 103 cells ml−1) but significant population of prokaryotes capable of DAsR present in the originally freshly scraped periphyton samples at the start of the experiment. The population of DAsR prokaryotes increased by the end of the incubations for both the As(V)-free and As(V)-added conditions, with the highest increase (∼3 orders of magnitude) occurring in the samples incubated with 5 mM As(V). However, a 2-order-of-magnitude increase in cell numbers was also noted in the samples incubated without added As(V) by the end of the incubation.
TABLE 2.
MPN estimates of bacterial cell density before and after incubation of periphyton samples amended with 0, 1, or 5 mM As(V)
| AS(V) added (mM) | MPN (cells/ml)
|
|||
|---|---|---|---|---|
| Beginning time point
|
Final time pointa
|
|||
| Avg | Range | Avg | Range | |
| 0 | 1.9 × 102 | 1.5 × 102-2.4 × 102 | 1.3 × 104 | 2.3 × 103-2.4 × 104 |
| 1.0 | 2.2 × 103 | 1.4 × 102-4.3 × 103 | 5.1 × 104 | 9.3 × 103-9.3 × 104 |
| 5.0 | 2.5 × 102 | 0.7 × 102-4.3 × 102 | 9.5 × 105 | 4.3 × 105-1.5 × 106 |
The incubation times were 10 days for the 1 mM addition and 58 days for the 0 and 5 mM additions. The range of values determined for two periphyton incubations of each condition are presented along with the average for each time point.
The experiments with [14C]glucose were conducted to determine if a larger amount of mineralization of this tracer to 14CO2 would occur upon exposure of the samples to higher As(V) concentrations (Table 3). Periphyton samples incubated with [14C]glucose but without As(V) formed both 14CO2 and 14CH4. These results were not significantly different for samples containing 10 μM As(V). Additions of 1 and 5 mM As(V) completely inhibited the formation of 14CH4. Samples amended with 1 mM As(V) formed similar amounts of 14CO2, but the largest proportion of glucose oxidized to 14CO2 was in samples amended with 5 mM As(V). No 14CO2 or 14CH4 was produced in heat-killed controls (data not shown).
TABLE 3.
Percentage of [14C]glucose metabolized recovered as 14CO2 and 14CH4 after incubation of periphyton samples amended with As(V)
| As(V) added (mM) | % Glucose recovered asa:
|
|
|---|---|---|
| 14CO2 produced | 14CH4 produced | |
| 0.00 | 26.1 (1.6) | 8.9 (0.5) |
| 0.01 | 24.7 (2.0) | 7.7 (1.1) |
| 1.00 | 26.8 (1.4) | 0 |
| 5.00 | 40.4 (2.6) | 0 |
Incubation time, 15 days; [14C] glucose concentration, 0.67 μM. Values in parentheses represent standard deviations of three replicate samples.
DISCUSSION
Studies of microbially mediated As redox chemistry employing naturally occurring materials have generally focused on As-contaminated systems. Hence, the response of these communities to experimental additions of As(III) or As(V) was fast, taking as little as a few minutes (10, 19, 46) to as long as 1 or 2 days (3, 11, 18, 37). In addition, these were unidirectional investigations, following either As(III) oxidation or As(V) reduction. Freeman et al. (8) established enrichment cultures from As-rich Lake Ohakuri that could carry out either oxidative or reductive transformations of arsenic oxyanions, thereby showing that both types of microbes were present in the original sediment. However, no work has been reported that studied the response time of unadapted, natural populations to perturbations made with As(V) under anoxic conditions and As(III) under oxic conditions. Considering that microbial degradation of some xenobiotics can require long adaptation periods (i.e., months or years) before activity can be detected (for examples see reference 15), it was not known just how fast a microbial community from a pristine environment would respond to perturbation with toxic arsenic oxyanions.
As(V) reduction.
In the case of the freshwater Cladophora sp. mat, researchers had previously noted rapid (within 1 h), constitutive (i.e., chloramphenicol-insensitive) denitrification activity as soon as these materials were dark incubated (44). In our present experiments, the response time for As(V) reduction was considerably longer, ranging from within 1 day at 10 μM As(V) (Fig. 1A) to between 3 to 5 days at 1 mM As(V) (Fig. 1C). The fact that As(V) reduction at each of these concentrations was inhibited by chloramphenicol (Fig. 1B) proves that a de novo synthesis of As(V)-reducing enzymes was required, and As(V) reductase was not constitutive in this population as it was for nitrate reductase and N2O reductase. Nonetheless, the response time for observable As(V) reduction was comparable to that which had been found previously in As(V)-amended estuarine sediments (7), and the faster response at the lower concentration was probably just a matter of the greater sensitivity of the analytical methods we employed at the different concentrations (e.g., atomic absorption versus ion chromatography). Clearly, the periphyton microbial community quickly adapts to the presence of As(V) by synthesizing enzymes for its reduction. We also noted that the presence of nitrate effectively blocked the reduction of As(V) (Fig. 2B), suggesting that it acted as a preferred electron acceptor for anaerobic respiration over As(V). This finding is in agreement with results reported for salt marsh sediments by Dowdle et al. (7), who noted that the oxidation of electron donors by nitrate is thermodynamically preferable to that with As(V). However, this interpretation is complicated by the possibility of a rapid co-occurrence of nitrate-linked As(III) oxidation (14, 32) masking the reduction of As(V) (see below).
We were also able to elicit As(V) reduction upon anoxic incubation of just the stream water itself, incubated without periphyton (Fig. 3). This suggests that prokaryotes capable of As(V) reduction are readily transported by fluvial processes downstream, where they can colonize other habitats. This also holds true for the microbes that oxidize As(III) (see below). The fact that promotion of this As(V) reduction required addition of lactate to the water while no such additions were needed for the periphyton incubations proves that the natural algal exudate provided an abundant supply of electron donor. Indeed, the increase in population sizes of DAsR prokaryotes with time in both the As(V)-amended and As(V)-free incubation conditions (Table 1) suggests that they were heterotrophs growing at the expense of an algal exudate electron donor. Because most DAsR prokaryotes are opportunists capable of respiratory growth on a wide diversity of electron acceptors (31), such as the ∼91 μM nitrate available in the stream water, it is not surprising that their numbers would increase with incubation time under these conditions, even in the absence of As(V).
The question arises as to whether the As(V) reductase activity was entirely attributable to dissimilatory reduction or to microbes that expressed their As resistance enzymes (e.g., ArsC). The fact that we detected a clear presence of DAsR prokaryotes in the MPN cultures (Table 2) and that increasing the As(V) concentration in the periphyton experiments enhanced the production of 14CO2 as the flora shifted from fermentation and methanogenesis to DAsR (Table 3) argues that at least As(V) respirers were a significant component of the microbial flora. If resistance-based As(V) reduction was also an important facet of the As redox reactions we observed, As(III) accumulation should have occurred both under aerobic as well as anaerobic incubation of the periphyton materials (8). The fact that it happened only under the latter condition argues that DAsR was the dominant, but perhaps not the exclusive, process.
As(III) oxidation.
The rate of aerobic As(III) oxidation in samples of periphyton (∼0.35 μmol liter−1 h−1) was approximately threefold faster than that observed with creek water incubated without periphyton (Fig. 4). This implies that the bacterial community responsible for As(III) oxidation was much more abundant in the periphyton mats than in the stream water, and it probably acted as the ultimate seeding source for its fluvial transport downstream. Oxidation of As(III) proceeded without a lag at the micromolar level, a situation similar to that seen for As(V) reduction (Fig. 1A), although the oxidation rate was approximately threefold faster than that observed for As(V) reduction. The rate of oxidation increased nearly 40-fold by increasing the amount of As(III) added to periphyton from ∼10 μM to 1 mM (Fig. 3C). Presumably, the 1-day lag we observed before oxidation commenced at this concentration was associated with an increase in the population size of the As(III) oxidizers.
It is not entirely clear whether the As(III) oxidation we observed was due to chemoautotrophy or heterotrophy. However, in support of the former process, the fact that we could elicit As(III) oxidation in the stream water without addition of exogenous electron donors suggests that the As(III) was itself acting as an electron donor to fuel a chemoautotrophic type of oxidation. Hence, this observation was exactly the opposite of what we found for anaerobic As(V) reduction with stream water, which required the addition of an exogenous organic electron donor (i.e., lactate) in order to fuel the observed activity.
Freshwater periphyton samples incubated under anaerobic conditions when supplied with nitrate were able to oxidize As(III) to As(V). Approximately 30% of the added As(III) was converted to As(V) before the nitrate was depleted, and subsequently all the As(V) that had been produced was reduced back to As(III) (Fig. 2C). However, there was an initial lag of about 4 days before As(V) accumulation was evident, and nitrate consumption occurred steadily over this initial interval. Therefore, most of the nitrate consumption was linked to the activity of heterotrophic denitrifying bacteria within this periphyton community (44). Because As(III) oxidation occurred only after a substantial lag (Fig. 2C), the initial inhibition of As(V) reduction caused by nitrate (Fig. 2B) was primarily due to its use as a preferred electron acceptor to which it was already adapted, unlike the case with As(V). However, these results present direct evidence for the presence of As(III)-oxidizing, nitrate-reducing bacteria within this freshwater microbial community, a chemoautotrophic microflora that previously has been observed only in Mono Lake, and these bacteria are typified by the facultative chemoautotroph, strain MLHE-1 (14, 32).
These results demonstrate that prokaryotes with the potential to carry out As(V) reduction and both aerobic and anaerobic As(III) oxidation are likely to coexist in periphyton communities and are probably ubiquitous in freshwater aquatic systems that are not originally exposed to arsenic contamination. The positive results with the estuarine C. sericea materials also suggest that this phenomenon is widespread. Because As(V) reduction and As(III) oxidation can be readily elicited in naturally occurring stream periphyton communities that are routinely exposed to nitrate, it is implied that these microbial ecosystems will quickly adapt [e.g., by expressing As-metabolizing enzymes or by increasing the population densities of As(V)-respiring and/or As(III)-oxidizing bacteria] to any anthropogenic inputs of arsenic into the stream arising from point sources upstream, such as mine tailing wastes. We have demonstrated that these activities will be induced rapidly at environmentally realistic concentrations (e.g., micromolar) of polluting As oxyanions. Some previously identified factors that affect arsenic speciation and mobility in streams include reductive processes in the hyporheic zone (26) as well as changes in streambed sorptive affinities caused by photosynthesis-induced pH excursions (9). In addition, we have now shown that periphyton communities themselves should also be examined in any future studies of arsenic speciation and mobility in streams.
Acknowledgments
This work was conducted while T.R.K. was an NRC postdoctoral associate at the USGS. We acknowledge financial support from the USGS and from an Exobiology grant from NASA.
We are grateful to J. F. Stolz and J. Santini for their constructive comments on an earlier draft of the manuscript.
REFERENCES
- 1.Acharyya, S. K., P. Chakraborty, S. Lahiri, B. C. Raymahashay, S. Guha, and A. Bhowmik. 1999. Arsenic poisoning in the Ganges delta. Nature 401:545. [DOI] [PubMed] [Google Scholar]
- 2.Afkar, E., J. Lisak, C. Saltikov, P. Basu, R. S. Oremland, and J. F. Stolz. 2003. The respiratory arsenate reductase from Bacillus selenitireducens strain MLS10. FEMS Microbiol. Lett. 226:107-112. [DOI] [PubMed] [Google Scholar]
- 3.Ahmann, D., L. R. Krumholz, H. F. Hemond, D. R. Lovley, and F. M. M. Morel. 1997. Microbial mineralization of arsenic from sediments of the Aberjona watershed. Environ. Sci. Technol. 31:2923-2930. [Google Scholar]
- 4.Bebout, B. M., H. W. Paerl, K. M. Krocker, and L. E. Prufert. 1987. Diel interactions of oxygenic photosynthesis and N2 fixation (acetylene reduction) in a marine mat microbial community. Appl. Environ. Microbiol. 53:2353-2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Culbertson, C. W., A. J. B. Zehnder, and R. S. Oremland. 1981. Anaerobic oxidation of acetylene by estuarine sediments and enrichment cultures. Appl. Environ. Microbiol. 41:396-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cullen, W. R., and K. J. Reimer. 1989. Arsenic speciation in the environment. Chem. Rev. 89:713-764. [Google Scholar]
- 7.Dowdle, P. R., A. M. Laverman, and R. S. Oremland. 1996. Bacterial dissimilatory reduction of arsenic(V) to arsenic(III) in anoxic sediments. Appl. Environ. Microbiol. 62:1664-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freeman, M. C., J. Aggett, and G. O'Brien. 1986. Microbial transformations of arsenic in Lake Ohakuri, New Zealand. Water Res. 20:283-294. [Google Scholar]
- 9.Fuller, C. C., and J. A. Davis. 1989. Influence of sorption and photosynthetic processes on trace element cycles in natural waters. Nature 340:52-54. [Google Scholar]
- 10.Gihring, T. M., G. K. Druschel, R. B. McCleskey, R. J. Hamers, and J. F. Banfield. 2001. Rapid arsenite oxidation by Thermus aquaticus and Thermus thermophilus: field and laboratory investigations. Environ. Sci. Technol. 35:3857-3862. [DOI] [PubMed] [Google Scholar]
- 11.Harrington, J. M., S. E. Fendorf, and R. F. Rosenzweig. 1998. Biotic generation of arsenic (III) in metal(oid)-contaminated freshwater lake sediments. Environ. Sci. Technol. 32:2425-2430. [Google Scholar]
- 12.Harvey, C. F., C. H. Swartz, A. B. M. Badruzzaman, N. Keon-Blute, W. Yu, M. A. Ali, J. Jay, R. Beckie, V. Niedan, D. Brabander, P. M. Oates, K. N. Ashfaque, S. Islam, H. F. Hemond, and M. F. Ahmed. 2002. Arsenic mobility and groundwater extraction in Bangladesh. Science 298:1602-1606. [DOI] [PubMed] [Google Scholar]
- 13.Hoeft, S. E., T. R. Kulp, J. F. Stolz, J. T. Hollibaugh, and R. S. Oremland. 2004. Dissimilatory arsenate reduction with sulfide as the electron donor: experiments with Mono Lake water and isolation of strain MLMS-1, a chemoautotrophic arsenate-respirer. Appl. Environ. Microbiol. 70:2741-2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoeft, S. E., F. Lucas, J. T. Hollibaugh, and R. S. Oremland. 2002. Characterization of microbial arsenate reduction in the anoxic bottom waters of Mono Lake, California. Geomicrobiol. J. 19:23-40. [Google Scholar]
- 15.Horowitz, A., J. M. Suflita, and J. M. Tiedje. 1983. Reductive dehalogenations of halobenzenes by anaerobic lake sediment microorganisms. Appl. Environ. Microbiol. 45:1459-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iletdinov, A. N., and S. A. Abdrashitova. 1981. Autotrophic oxidation of arsenic by a culture of Pseudomonas arsenitoxidans. Mikrobiologiya 50:51-57. [PubMed] [Google Scholar]
- 17.Jackson, C. R., E. F. Jackson, S. L. Dugas, K. Gamble, and S. E. Williams. 2003. Microbial transformations of arsenite and arsenate in natural environments. Recent Res. Dev. Microbiol. 7:103-118. [Google Scholar]
- 18.Jones, C. A., H. W. Langner, K. Anderson, T. R. McDermott, and W. P. Inskeep. 2000. Rates of microbially mediated arsenate reduction and solublilization. Soil Sci. Soc. Am. J. 64:600-608. [Google Scholar]
- 19.Langner, H. W., C. R. Jackson, T. R. McDermott, and W. P. Inskeep. 2001. Rapid oxidation of arsenite in a hot spring ecosystem, Yellowstone National Park. Environ. Sci. Technol. 35:3302-3309. [DOI] [PubMed] [Google Scholar]
- 20.Lianfang, W., and H. Jianzhong. 1994. Chronic arsenicism from drinking water in some areas of Xinjiang, China, p. 159-172. In J. O. Nriagu (ed.), Arsenic in the environment. Part II: human health and ecosystem effects. John Wiley, Inc., New York, N.Y.
- 21.Liu, A., E. Garcia-Dominguez, E. D. Rhine, and L. Y. Young. 2004. A novel arsenate respiring isolate that can utilize aromatic substrates. FEMS Microbiol. Ecol. 48:323-332. [DOI] [PubMed] [Google Scholar]
- 22.Macur, R. E., J. T. Wheeler, T. R. McDermott, and W. P. Inskeep. 2001. Microbial populations associated with the reduction and enhanced mobilization of arsenic in mine tailings. Environ. Sci. Technol. 35:3676-3682. [DOI] [PubMed] [Google Scholar]
- 23.Manning, B. A., and D. A. Martens. 1997. Speciation of arsenic(III) and arsenic(V) in sediment extracts by high-performance liquid chromatography-hydride generation atomic absorption spectrophotometry. Environ. Sci. Technol. 31:171-177. [Google Scholar]
- 24.McEwan, A. G., J. P. Ridge, C. A. McDevitt, and P. Hugenholtz. 2002. The DMSO reductase family of microbial molybdenum enzymes: molecular properties and role in the dissimilatory reduction of toxic elements. Geomicrobiol. J. 19:3-22. [Google Scholar]
- 25.Mukhopadhyay, B., P. Rosen, L. T. Phung, and S. Silver. 2002. Microbial arsenic: from geocycles to genes and enzymes. FEMS Microbiol. Rev. 26:311-325. [DOI] [PubMed] [Google Scholar]
- 26.Nagorski, S. A., and J. A. Moore. 1999. Arsenic mobilization in the hyporheic zone of a contaminated stream. Water Res. 35:3441-3450. [Google Scholar]
- 27.Newman, D. K., D. Ahmann, and F. M. M. Morel. 1998. A brief review of microbial arsenate reduction. Geomicrobiol. J. 15:255-268. [Google Scholar]
- 28.Nicholas, D. R., S. Ramamoorthy, V. Palace, S. Springs, J. M. Moore, and F. Rosenzweig. 2003. Biogeochemical transformations of arsenic in circumneutral freshwater sediment. Biodegradation 14:123-137. [DOI] [PubMed] [Google Scholar]
- 29.Nickson, R., J. McArthur, W. Burgess, K. M. Ahmed, P. Ravenscroft, and M. Rahman. 1998. Arsenic poisoning of Bangladesh groundwater. Nature 395:338. [DOI] [PubMed] [Google Scholar]
- 30.Oremland, R. S., J. F. Stolz, and J. T. Hollibaugh. 2004. The microbial arsenic cycle in Mono Lake, California. FEMS Microbiol. Ecol. 48:15-27. [DOI] [PubMed] [Google Scholar]
- 31.Oremland, R. S., and J. F. Stolz. 2003. The ecology of arsenic. Science 300:939-944. [DOI] [PubMed] [Google Scholar]
- 32.Oremland, R. S., S. E. Hoeft, J. M. Santini, N. Bano, R. A. Hollibaugh, and J. T. Hollibaugh. 2002. Anaerobic oxidation of arsenite in Mono Lake water and by a facultative, arsenite-oxidizing chemoautotroph, strain MLHE-1. Appl. Environ. Microbiol. 68:4795-4802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oremland, R. S., D. K. Newman, B. W. Kail, and J. F. Stolz. 2001. Bacterial respiration of arsenate and its significance in the environment, p. 273-296. In W. T. Frankenberger, Jr. (ed.), The environmental chemistry of arsenic. Marcel Dekker, New York, N.Y.
- 34.Oremland, R. S., P. R. Dowdle, S. Hoeft, J. O. Sharp, J. K. Schaefer, L. G. Miller, J. S. Blum, R. L. Smith, N. S. Bloom, and D. Wallschlaeger. 2000. Bacterial dissimilatory reduction of arsenate and sulfate in meromictic Mono Lake, California. Geochim. Cosmochim. Acta 64:3073-3084. [Google Scholar]
- 35.Oremland, R. S., J. Switzer Blum, C. W. Culbertson, P. T. Visscher, L. G. Miller, P. Dowdle, and F. E. Strohmaier. 1994. Isolation, growth and metabolism of an obligately anaerobic, selenate-respiring bacterium, strain SES-3. Appl. Environ. Microbiol. 60:3011-3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Revsbech, N. P., and B. B. Jørgensen. 1981. Primary production of microalgae in sediments measured by oxygen microprofile, H14CO3− fixation, and oxygen exchange method. Limnol. Oceanogr. 26:717-730. [Google Scholar]
- 37.Rittle, K. A., J. I. Drever, and P. S. Colberg. 1995. Precipitation of arsenic during bacterial sulfate reduction. Geomicrobiol. J. 13:1-12. [Google Scholar]
- 38.Rosen, B. P. 2002. Biochemistry of arsenic detoxification. FEBS Lett. 529:86-92. [DOI] [PubMed] [Google Scholar]
- 39.Saltikov, C. W., and D. K. Newman. 2003. Genetic identification of a respiratory arsenate reductase. Proc. Natl. Acad. Sci. USA 100:10983-10988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santini, J. M., L. I. Sly, A. Wen, D. Comrie, P. de Wulf-Durand, and J. M. Macy. 2002. New arsenite-oxidizing bacteria isolated from Australian gold mining environments-phylogenetic relationships. Geomicrobiol. J. 19:67-76. [Google Scholar]
- 41.Santini, J. M., L. I. Sly, R. D. Schnagl, and J. M. Macy. 2000. A new chemolithoautotrophic arsenite-oxidizing bacterium isolated from a gold mine: phylogenetic, physiological, and preliminary biochemical studies. Appl. Environ. Microbiol. 66:92-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Senn, D. B., and H. F. Hemond. 2002. Nitrate controls on iron and arsenic in an urban lake. Science 296:2373-2376. [DOI] [PubMed] [Google Scholar]
- 43.Stolz, J. F., and R. S. Oremland. 1999. Bacterial respiration of arsenic and selenium. FEMS Microbiol. Rev. 23:615-627. [DOI] [PubMed] [Google Scholar]
- 44.Triska, F. J., and R. S. Oremland. 1981. Denitrification associated with periphyton communities. Appl. Environ. Microbiol. 42:745-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Welch, A. H., D. B. Westjohn, D. R. Helsel, and R. B. Wanty. 2000. Arsenic in ground water of the United States: occurrence and geochemistry. Ground Water 38:589-604. [Google Scholar]
- 46.Wilkie, J. A., and J. G. Hering. 1998. Rapid oxidation of geothermal arsenic(III) in streamwaters of the Eastern Sierra Nevada. Environ. Sci. Technol. 32:657-662. [Google Scholar]