Abstract
Although both broth microdilution (BMD) and disk diffusion (DD) are listed by NCCLS as acceptable methods for testing Acinetobacter spp. for antimicrobial susceptibility, few studies have compared the results generated by the two methods. We tested 196 isolates of Acinetobacter spp. from nine U.S. hospitals and from the Centers for Disease Control culture collection by using BMD and DD and clinically appropriate antimicrobial agents. Categorical results for amikacin, ciprofloxacin, gatifloxacin, gentamicin, imipenem, levofloxacin, meropenem, tobramycin, and trimethoprim-sulfamethoxazole were comparable for the two methods: there was only one very major (VM) error, with tobramycin, and only one major (M) error, with meropenem, when DD results were compared with BMD results. However, VM errors were frequent with the β-lactams and β-lactam-β-lactam inhibitor combinations, while M errors were often observed with tetracyclines. For BMD, tests frequently exhibited subtle growth patterns that were difficult to interpret, especially for β-lactams. If subtle growth (i.e., granular, small button, or “starry” growth) was considered positive, error rates between BMD and DD were unacceptably high for ampicillin-sulbactam (VM error, 9.8%; minor [m] error, 16.1%), piperacillin (VM error, 5.7%; m error, 13.5%), piperacillin-tazobactam (VM error, 9.3%; m error, 12.9%), ceftazidime (VM error, 6.2%; m error, 11.4%), cefepime (VM error, 6.2%; m error, 13.0%), cefotaxime (m error, 21.2%), ceftriaxone (m error, 23.3%), tetracycline (M error, 11.4%; m error, 32.1%), and doxycycline (M error, 2.6%). When subtle growth patterns were ignored, the agreement still did not achieve acceptable levels. To determine if the problems with BMD testing occurred in other laboratories, we sent frozen BMD panels containing β-lactam drugs and nine isolates to six labs with experience in performing BMD and DD. Among these laboratories, cefepime MICs ranged from ≤8 to ≥32 μg/ml for four of the nine strains, confirming the problem in interpreting BMD results. Discrepancies between the categorical interpretations of BMD and DD tests were noted primarily with cefepime and piperacillin, for which the BMD results were typically more resistant. Clinical laboratories should be aware of these discrepancies. At present, there are no data to indicate which method provides more clinically relevant information.
Acinetobacter species are ubiquitous in nature and are the most common gram-negative organisms found on the skin of hospital personnel (1). Because of their ability to develop resistance to a variety of antimicrobial agents and to cause infection in debilitated hosts, isolates that are clinically significant must often be tested for antimicrobial susceptibility in order to guide anti-infective therapy (16). Although this group of organisms is included with Pseudomonas aeruginosa in NCCLS disk diffusion (DD) interpretive tables (Table 2B in NCCLS document M100-S14, M2) (15a), no published reports document the performance of the NCCLS reference methods, broth microdilution (BMD) and DD, for Acinetobacter spp. Therefore, we compared DD to BMD for this organism group.
TABLE 2.
Activities of eight β-lactam agents against 195a randomly selected isolates of Acinetobacter spp., using both conservative and liberal MIC readings
| Antimicrobial agent | Readingc | MIC (μg/ml)
|
% Susceptible isolatesb | ||
|---|---|---|---|---|---|
| 50% | 90% | Range | |||
| Ampicillin-sulbactam | Cons | 8 | >64 | 0.12->64 | 63.6 |
| Lib | 4 | >64 | 0.12->64 | 68.7 | |
| Piperacillin | Cons | 128 | >128 | ≤1.0->128 | 30.8 |
| Lib | 32 | >128 | ≤1.0->128 | 38.5 | |
| Piperacillin-tazobactam | Cons | 64 | >128 | ≤1.0->128 | 43.1 |
| Lib | 16 | >128 | ≤1.0->128 | 55.9 | |
| Ticarcillin-clavulanate | Cons | 32 | >128 | ≤1.0->128 | 46.7 |
| Lib | 16 | >128 | ≤1.0->128 | 54.4 | |
| Ceftazidime | Cons | 16 | >64 | ≤0.5->64 | 44.6 |
| Lib | 8 | >64 | ≤0.5->64 | 50.8 | |
| Cefotaxime | Cons | 32 | >64 | ≤0.5->64 | 15.9 |
| Lib | 32 | >64 | ≤0.5->64 | 20.5 | |
| Ceftriaxone | Cons | 32 | >64 | ≤0.5->64 | 19.0 |
| Lib | 32 | >64 | ≤0.5->64 | 22.1 | |
| Cefepime | Cons | 16 | >64 | ≤0.5->64 | 48.5 |
| Lib | 8 | >64 | ≤0.5->64 | 54.1 | |
One unidentified isolate did not grow in the MIC plate.
According to NCCLS criteria.
Cons, conservative reading, i.e., not ignoring any growth; Lib, liberal reading, i.e., ignoring colonies or subtle growth patterns.
(This work was presented in part at the 103rd General Meeting of the American Society for Microbiology, Washington, D.C., 18 to 22 May, 2003.)
MATERIALS AND METHODS
Bacterial strains.
A total of 196 isolates of Acinetobacter spp. were tested. Of this total, 117 isolates were obtained from 11 hospital laboratories in nine different states (California, Georgia, Illinois, Massachusetts, New York, New Jersey, North Carolina, Texas, and Washington), 15 isolates were obtained from the Project ICARE (Intensive Care Antimicrobial Resistance Epidemiology) collection (3), and 64 were obtained from the Centers for Disease Control and Prevention (CDC) collection. All of the isolates from the hospital laboratories were selected randomly, i.e., they were not chosen because of any particular resistance characteristic or mechanism. Isolates from the ICARE and CDC collections were selected to include isolates representing all resistance patterns and species available. Appropriate quality control organisms were used for all testing. All isolates were frozen upon receipt and, when removed from the freezer, were subcultured twice prior to testing.
Identification.
All strains were identified at the CDC by restriction fragment analysis of their ribosomal DNAs amplified as described by Vaneechoutte et al. (17, 19), except that a different reverse primer was used (TCA CAA AGT GGT AAG CGC CCT C). The PCR assay was validated by the use of genetically characterized strains from the CDC prior to use. Some of the strains were also identified by traditional biochemical methods (16).
Susceptibility test methods.
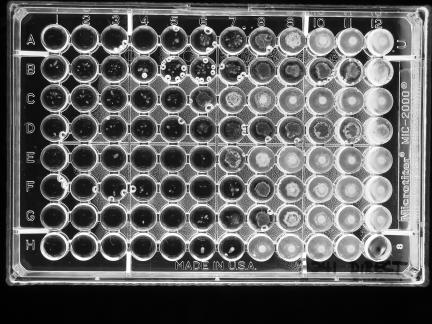
All strains were tested by NCCLS BMD and DD methods (10), using cation-adjusted Mueller-Hinton broth (Difco, Sparks, Md.) and Mueller-Hinton agar (BBL MH II; Becton Dickinson Microbiology Systems, Cockeysville, Md.) (11, 13). For BMD, when trailing or subtle growth patterns occurred above an obvious end point, two MIC readings were made, a conservative one at the highest concentration at which no growth occurred and a liberal one at a concentration that ignored any subtle growth above an obvious end point (Fig. 1).
FIG. 1.
Example of subtle growth patterns for an isolate of an Acinetobacter sp. Conservative MICs read were A4, >B1, >C1, >D1, E (unreadable), F2, G3, and H5. Liberal MICs read were A7, B7, C5, D6, E6, F7, G6, and H7. The bubbles in several wells can be ignored.
Antimicrobial agents.
The antimicrobial agents included in this study were as follows (concentrations tested, in micrograms per milliliter; source): amikacin (1 to 128; Sigma-Aldrich, St. Louis, Mo.), ampicillin-sulbactam (2:1) (0.03-0.015 to 64-32; Sigma and United States Pharmacopoeia, Rockville, Md.), cefepime (0.5 to 64; Bristol-Myers Squibb Co., Wallingford, Conn.), cefotaxime (0.5 to 64; Sigma), ceftazidime (0.5 to 64; Lilly Research Laboratories, Indianapolis, Ind.), ceftriaxone (0.5 to 64; Sigma), ciprofloxacin (0.12 to 16; Bayer Corporation, West Haven, Conn.), doxycycline (0.12 to 16; Sigma), gatifloxacin (0.25 to 32; Bristol-Myers Squibb), imipenem (0.12 to 16; Merck & Co., Rahway, N.J.), levofloxacin (0.25 to 32; Johnson & Johnson, Spring House, Pa.), meropenem (0.25 to 32; AstraZeneca Pharmaceuticals LP, Wilmington, Del.), piperacillin (1 to 128; Sigma), piperacillin-tazobactam (1-4 to 128-4; Sigma and Wyeth-Ayerst Pharmaceuticals, Pearl River, N.Y.), polymyxin B (0.5 to 16; Sigma), tetracycline (0.25 to 32; Sigma), ticarcillin-clavulanic acid (1-2 to 128-2; GlaxoSmithKline, Collegeville, Pa.), tobramycin (0.25 to 32; Sigma), and trimethoprim-sulfamethoxazole (1:19) (0.25-4.8 to 8-152; Sigma).
Inoculum effect.
Twelve strains that showed colonies beyond an obvious end point in the broth microdilution plates were retested at 0.2, 1, and 2 times the NCCLS recommended inoculum concentration of 5 × 105 CFU/ml.
Fixed concentration versus fixed ratio of β-lactamase inhibitor.
The concentrations of β-lactam and β-lactamase inhibitor drugs approved for use in laboratory testing by the NCCLS are a 2:1 ratio for ampicillin-sulbactam and fixed concentrations of inhibitors for piperacillin-tazobactam (4 μg/ml) and ticarcillin-clavulanate (2 μg/ml). In order to determine if the number of discrepancies between test results would decrease with a different configuration of β-lactam agents and β-lactamase inhibitors, we retested all strains by using both a fixed ratio (2:1) of β-lactam agent to β-lactamase inhibitor and a fixed concentration of β-lactamase inhibitor with the β-lactam agent for ampicillin-sulbactam (fixed concentration, 8 μg/ml), piperacillin-tazobactam (4 μg/ml), and ticarcillin-clavulanic acid (2 μg/ml). In addition, tazobactam and sulbactam were tested alone at concentrations of 0.12 to 128 μg/ml.
Interlaboratory testing.
Frozen BMD panels prepared at the CDC containing ampicillin-sulbactam, piperacillin-tazobactam, piperacillin, ceftazidime, and cefepime were sent along with nine selected Acinetobacter isolates to five laboratories with experience in performing NCCLS reference methods. Each of the five laboratories and the CDC tested the nine strains by BMD and DD with the BMD plates and disks supplied to them, but they used their own lot of Mueller-Hinton agar.
RESULTS
Identification.
The identifications of the 196 isolates used for this study (to the species or genomospecies level) are shown in Table 1, along with the frequencies of very major errors (for one or more antimicrobial agents) in DD results when compared with the results from BMD. The Acinetobacter calcoaceticus-baumannii complex (genomospecies 1, 2, 3, and 13) comprised 149 (76%) of the 196 isolates and gave 79.5% (35 of 44) of the very major errors. Three of the isolates did not yield adequate growth by one of the methods; therefore, the total number of isolates with BMD results was 195 and the number with both BMD and DD results was 193.
TABLE 1.
Species identification of 196 Acinetobacter sp. isolates and frequency of isolates that express very major errors by disk diffusion
| Species (genomospecies) | Frequency (%) | No. (%)a with very major errors by DD |
|---|---|---|
| A. baumannii (GS 2)b | 96 (49.0) | 17 (38.6) |
| A. calcoaceticus (GS 1)b | 5 (2.6) | 1 (2.3) |
| A. haemolyticus (GS 4) | 2 (1.0) | |
| A. johnsonii (GS 7) | 5 (2.6) | 1 (2.3) |
| A. junii (GS 5) | 2 (1.0) | |
| A. lwoffii (GS 8/9) | 6 (3.1) | 3 (6.8) |
| GS 3b | 24 (12.2) | 5 (11.4) |
| GS 10 | 2 (1.0) | |
| GS 11 | 3 (1.6) | |
| GS 10/11 | 1 (0.5) | |
| GS 12 | 4 (2.0) | |
| GS 13b | 24 (12.2) | 12 (27.3) |
| GS 14 | 3 (1.6) | 1 (2.3) |
| GS 15 | 1 (0.5) | |
| GS 16 | 1 (0.5) | 1 (2.3) |
| Unable to identify to species level | 17 (8.7) | 3 (6.8) |
| Total | 196 (100) | 44 (100) |
All errors were with β-lactam agents, except for one with tobramycin.
Genomospecies 1, 2, 3, and 13 make up the A. calcoaceticus-baumannii complex.
Broth microdilution MICs.
In the BMD tests, very small colonies or a star-like growth type (unique from the trailing observed with trimethoprim-sulfamethoxazole) was observed in wells containing high concentrations of several drugs, i.e., concentrations above which an obvious reduction in growth had occurred. This was noted for 64 (32.8%) of the 195 organisms and almost exclusively with the β-lactam agents tested (Fig. 1). When such growth was noted, two end points were recorded, with one taking into account any growth in the well, even small colonies or subtle growth patterns after the large reduction in growth (called the conservative end point), and one that ignored colonies or subtle growth and called the MIC at the obvious reduction in growth (called the liberal end point). The MICs at which 50% of the isolates were inhibited (MIC50 values), the MIC90 values, and the ranges of MICs for 19 antimicrobial agents tested are shown in Tables 2 and 3. The percentages of strains that were categorized as susceptible by the use of NCCLS interpretive criteria (except for polymyxin B) are also presented. For polymyxin B, a susceptible breakpoint of ≤2 μg/ml and a resistant breakpoint of ≥4 μg/ml were used, as suggested by Gales et al. (6). For the β-lactam agents, with which the presence of subtle growth above an obvious end point was most likely to occur, activities are given for both the conservative and liberal MIC readings (Table 2). When conservative MIC end points were used for the β-lactam agents (Table 2), the percentages of isolates that were susceptible were always lower than those when liberal MIC readings were used. The differences in percentages of susceptible isolates for the two readings were all significant (P < 0.01, except for ceftriaxone [P = 0.03], by McNemar's chi-square test). Since patterns of subtle growth were not observed for the non-β-lactam agents, only one MIC result is given for each in Table 3.
TABLE 3.
Activity of 11 non-β-lactam agents against 195 randomly selected isolates of Acinetobacter spp.
| Antimicrobial agent | MIC (μg/ml)
|
% Susceptible isolates | ||
|---|---|---|---|---|
| 50% | 90% | Range | ||
| Ciprofloxacin | 1.0 | >16 | ≤0.12->16 | 56.4 |
| Levofloxacin | ≤0.25 | 32 | ≤0.25->32 | 60.5 |
| Gatifloxacin | ≤0.25 | 32 | ≤0.25->32 | 60.0 |
| Gentamicin | 2 | >32 | ≤0.25->32 | 58.0 |
| Tobramycin | 1.0 | >32 | ≤0.25->32 | 69.7 |
| Amikacin | 4 | 64 | ≤1.0->128 | 80.0 |
| Tetracycline | 2 | >32 | ≤0.25->32 | 61.5 |
| Doxycycline | 0.25 | >16 | ≤0.12->16 | 74.9 |
| Imipenem | 0.25 | 8 | ≤0.12->16 | 89.2 |
| Meropenem | 1.0 | 16 | ≤0.25->32 | 80.5 |
| Polymyxin B | ≤0.5 | 2 | ≤0.5-16 | 91.3a |
| Trimethoprim- sulfamethoxazole | 0.5 | >8 | ≤0.25->8 | 58.5 |
| Sulbactam | 8 | 128 | 0.5->128 | —b |
| Tazobactam | 16 | 128 | 0.5->128 | — |
Using a susceptible category of ≤2 μg/ml (see reference 6).
—, no interpretive criteria are available.
Ampicillin-sulbactam was the most active β-lactam agent tested (63.6 to 68.7% of isolates were susceptible); the least active agent was cefotaxime (15.9 to 20.5% of isolates were susceptible) (Table 2). As expected, the activity increased when the liberal MIC end point was used. Of the nonpenicillin and noncephalosporin agents tested (Table 3), the most active were polymyxin B (91.3% of isolates were susceptible) and imipenem (89.2% of isolates were susceptible). Of the two β-lactamase inhibitors tested, sulbactam exhibited a slightly higher activity than tazobactam, as judged by the MIC50 values.
Inoculum effect.
Twelve strains were tested with inocula at 0.2, 1, and 2 times the recommended concentration of 5 × 105 CFU/ml. The presence of subtle growth or small colonies beyond the obvious end point increased as the inoculum increased; however, even using a 0.2× inoculum (i.e., 105 CFU/ml) did not eliminate the growth of colonies beyond the obvious end point.
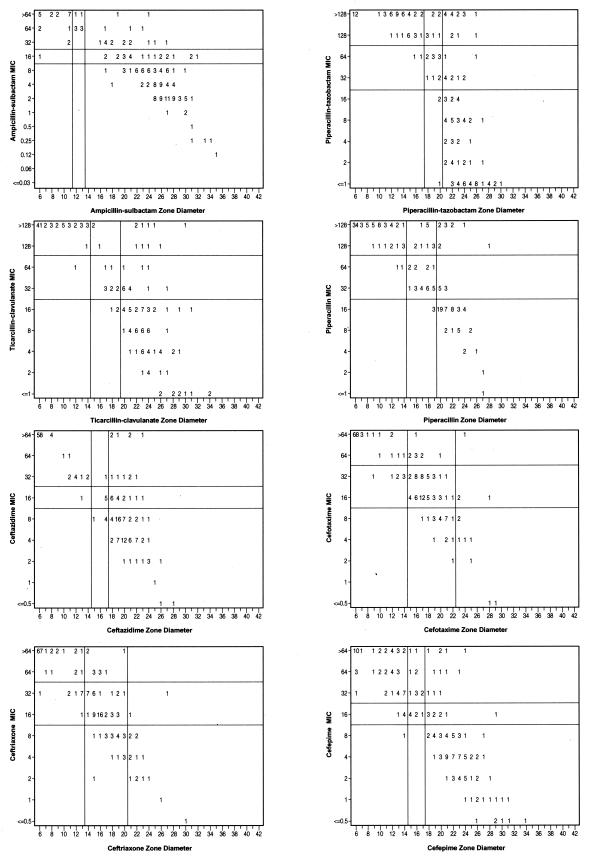
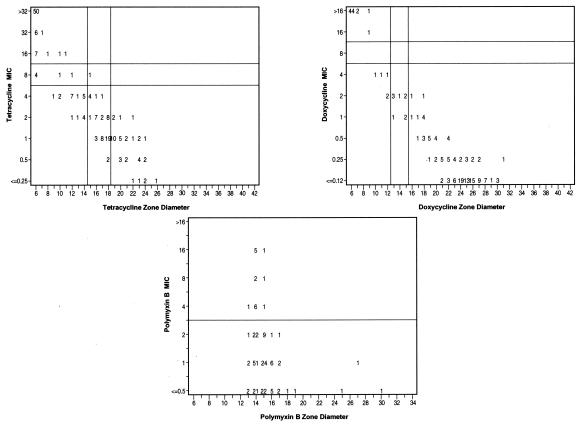
Correlation of MICs and zone diameters.
Discrepancy rates for BMD versus DD are presented in Table 4, with corresponding scatter plots for the β-lactam agents (Fig. 2) and for tetracycline, doxycycline, and polymyxin B (Fig. 3). Unacceptable error rates were noted for all of the β-lactam agents tested and for tetracycline. Very major errors (susceptible according to DD but resistant according to BMD) occurred with ampicillin-sulbactam, piperacillin, piperacillin-tazobactam, ticarcillin-clavulanate, ceftazidime, and cefepime. Although using the liberal readings for the β-lactam agents (Table 5) reduced the error rates slightly, they remained at an unacceptable level for all of the agents tested.
TABLE 4.
MIC and zone diameter discrepancy rates for 19 antimicrobial agents and 193a Acinetobacter spp.
| Antimicrobial agent | No. (%)b of discrepancies
|
||
|---|---|---|---|
| Very major | Major | Minor | |
| Ampicillin-sulbactam | 19 (9.8) | 0 | 31 (16.1) |
| Piperacillin | 11 (5.7) | 0 | 26 (13.5) |
| Piperacillin-tazobactam | 18 (9.3) | 0 | 25 (12.9) |
| Ticarcillin-clavulanate | 10 (5.2) | 0 | 22 (11.4) |
| Ceftazidime | 12 (6.2) | 0 | 22 (11.4) |
| Cefepime | 12 (6.2) | 1 (0.5) | 25 (13.0) |
| Cefotaxime | 1 (0.5) | 0 | 41 (21.2) |
| Ceftriaxone | 0 | 0 | 45 (23.3) |
| Ciprofloxacin | 0 | 0 | 9 (4.7) |
| Levofloxacin | 0 | 0 | 2 (1.0) |
| Gatifloxacin | 0 | 0 | 8 (4.1) |
| Gentamicin | 0 | 0 | 12 (6.2) |
| Tobramycin | 1 (0.5) | 0 | 9 (4.7) |
| Amikacin | 0 | 1 (0.5) | 16 (8.3) |
| Tetracycline | 0 | 22 (11.4) | 62 (32.1) |
| Doxycycline | 0 | 5 (2.6) | 9 (4.7) |
| Imipenem | 0 | 0 | 4 (2.1) |
| Meropenem | 0 | 1 (0.5) | 13 (6.7) |
| Trimethoprim-sulfamethoxazole | 0 | 0 | 6 (3.1) |
Three isolates did not grow when tested by DD.
The total population was used as the denominator. Unacceptable levels are shown in bold. They are ≥1.5% for very major errors and ≥3% for major errors, as recommended in NCCLS document M23 (12). Minor errors of ≥10% are also shown in bold.
FIG. 2.
Scatter plots comparing BMD MICs and DD zone diameters for eight β-lactam antimicrobial agents against 193 isolates of Acinetobacter spp.
FIG. 3.
Scatter plots comparing BMD MICs and DD zone diameters for tetracycline, doxycycline, and polymyxin B against 193 isolates of Acinetobacter spp.
TABLE 5.
BMD and DD discrepancy rates for eight antimicrobial agents, with comparisons of conservative readings and liberal readings
| Antimicrobial agent | Discrepancy ratea (%)
|
|||||
|---|---|---|---|---|---|---|
| Very major
|
Major
|
Minor
|
||||
| Cons | Lib | Cons | Lib | Cons | Lib | |
| Ampicillin-sulbactam | 9.8 | 7.8 | 0 | 0 | 16.1 | 13.0 |
| Piperacillin | 5.7 | 2.6 | 0 | 0 | 13.5 | 13.0 |
| Piperacillin-tazobactam | 9.3 | 2.6 | 0 | 0 | 12.9 | 10.4 |
| Ticarcillin-clavulanate | 5.2 | 1.6 | 0 | 0 | 11.4 | 8.8 |
| Ceftazidime | 6.2 | 2.1 | 0 | 0 | 11.4 | 9.8 |
| Cefepime | 6.2 | 1.6 | 0.5 | 0.5 | 13.0 | 9.3 |
| Cefotaxime | 0.5 | 0.5 | 0 | 0 | 21.2 | 23.8 |
| Ceftriaxone | 0 | 0 | 0 | 0 | 23.3 | 22.3 |
The total population was used as the denominator. Unacceptable levels are shown in bold. Cons, conservative reading, i.e., not ignoring colonies or subtle growth; Lib, liberal reading, i.e., ignoring growth beyond an obvious end point.
NCCLS has not established interpretive breakpoints for the DD method for polymyxin B. However, in this study and in a study by Gales and coworkers, DD did not differentiate the presumed resistant population (MIC ≥ 4 μg/ml) from the susceptible population (MIC ≤ 2 μg/ml) (6).
Using a fixed concentration versus a fixed ratio of β-lactamase inhibitor for BMD.
To determine if the problems with the β-lactam-β-lactamase inhibitor combinations could be resolved by using a fixed ratio of inhibitor to β-lactam agent instead of a fixed concentration of inhibitor, we used BMD to test both fixed ratios and fixed concentrations of β-lactamase inhibitors. A comparison of the discrepancy rates is presented in Table 6. For all three agents, there were fewer very major errors for the 2:1 fixed ratio of β-lactam to β-lactamase inhibitor than for the fixed concentration tested, but the error rates (very major or minor) remained at an unacceptable level for both formulations.
TABLE 6.
BMD and DD discrepancy rates for three antimicrobial agents when testing a fixed ratio versus a fixed concentration of β-lactamase inhibitor
| Antimicrobial agent or expt conditionb | n | No. (%) of discrepancies
|
% Susceptible isolatesa | ||
|---|---|---|---|---|---|
| Very major | Major | Minor | |||
| Ampicillin-sulbactam | |||||
| Fixed ratio (2:1) | 191 | 25 (13.1) | 0 | 28 (14.7) | 52.9 |
| Fixed concentration (8 μg/ml) | 186 | 63 (33.9) | 0 | 15 (8.1) | 46.0 |
| Piperacillin-tazobactam | |||||
| Fixed ratio (2:1) | 193 | 6 (3.1) | 0 | 42 (21.9) | 48.2 |
| Fixed concentration (4 μg/ml) | 192 | 25 (13.0) | 0 | 48 (24.9) | 37.6 |
| Ticarcillin-clavulanate | |||||
| Fixed ratio (2:1) | 192 | 0 | 0 | 36 (18.8) | 51.3 |
| Fixed concentration (2 μg/ml) | 192 | 5 (2.6) | 0 | 21 (10.9) | 51.8 |
According to the NCCLS breakpoint for the β-lactam agent.
NCCLS conditions are shown in bold.
Interlaboratory testing.
The testing of nine selected strains in six different laboratories confirmed that the testing problems could be replicated in other laboratories, even in those with considerable experience in performing NCCLS reference testing methods. Variations in both the MIC results and the categorical interpretations were observed for all of the strains for one or more of the β-lactam agents tested (Table 7). For example, categorical interpretations for cefepime results varied from susceptible to resistant, and cefepime MIC results ranged from ≤8 to ≥32 μg/ml for four of the nine isolates. The occurrence of very major errors for BMD versus DD was also highest for cefepime; however, very major errors occurred with five of the nine strains for at least one of the β-lactam agents tested (data not shown).
TABLE 7.
Variation in MIC and zone category from testing nine problem Acinetobacter spp. in six laboratories
| Strain | Method | No. of isolates in categorya
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ampicillin- sulbactam
|
Piperacillin- tazobactam
|
Pipera- cillin
|
Cefta- zidime
|
Cefepime
|
||||||||||||
| Sb | Ib | Rb | S | I | R | S | I | R | S | I | R | S | I | R | ||
| 1235 | MIC | 5 | 1 | 5 | 1 | 2 | 4 | 2 | 4 | 2 | 1 | 2 | ||||
| Disk | 6 | 4 | 1 | 5 | 1 | 6 | 6 | |||||||||
| 1266 | MIC | 6 | 2 | 4 | 5 | 1 | 4 | 2 | 2 | 1 | 2 | |||||
| Disk | 6 | 6 | 2 | 4 | 6 | 6 | ||||||||||
| 1974 | MIC | 6 | 6 | 3 | 3 | 5 | 1 | 5 | 1 | |||||||
| Disk | 6 | 6 | 6 | 6 | 6 | |||||||||||
| 1975 | MIC | 3 | 3 | 6 | 6 | 6 | 6 | |||||||||
| Disk | 6 | 6 | 6 | 6 | 6 | |||||||||||
| 5065 | MIC | 1 | 5 | 5 | 5 | 1 | 4 | 5 | ||||||||
| Disk | 5 | 1 | 2 | 3 | 1 | 2 | 4 | 6 | 6 | |||||||
| 8213 | MIC | 1 | 5 | 5 | 5 | 3 | 3 | 1 | 3 | 2 | ||||||
| Disk | 4 | 2 | 4 | 2 | 4 | 2 | 5 | 1 | 6 | |||||||
| 24318 | MIC | 6 | 6 | 2 | 4 | 6 | 6 | |||||||||
| Disk | 6 | 6 | 6 | 6 | 6 | |||||||||||
| 24325 | MIC | 6 | 4 | 1 | 3 | 3 | 6 | 6 | ||||||||
| Disk | 6 | 6 | 4 | 2 | 6 | 6 | ||||||||||
| 2241 | MIC | 5 | 1 | 5 | 1 | 4 | 1 | 3 | 2 | 1 | 2 | 2 | ||||
| Disk | 6 | 3 | 3 | 5 | 1 | 6 | 6 | |||||||||
When the total number of results is <6, a laboratory reported that MICs were uninterpretable.
S, susceptible; I, intermediate; R, resistant.
DISCUSSION
The increasing resistance of Acinetobacter spp. to many antimicrobial agents has been well documented (4, 5, 7, 18, 20, 21). However, the optimal method for determining the in vitro susceptibility of Acinetobacter spp. to β-lactams and other antimicrobial agents in the clinical laboratory has yet to be determined. Although the testing of Acinetobacter spp. by DD is recommended in NCCLS document M2, Performance Standards for Antimicrobial Disk Susceptibility Tests: Approved Standard, 8th ed. (15), the breakpoints given in this document were established primarily with large numbers of Enterobacteriaceae and P. aeruginosa and relatively few isolates of Acinetobacter. Few studies have directly compared the results of BMD and DD for this genus. Because all commercial methods for antimicrobial susceptibility testing are verified by comparison to NCCLS reference methods, it is important that the accuracy of reference testing methods be validated.
In this study, the error rates for all of the non-β-lactam antimicrobial agents tested, with the exception of tetracycline, were within the acceptable ranges established by the NCCLS (12). Although an unacceptable level of major errors occurred with tetracycline, the number of errors with DD for tetracycline could be reduced if the breakpoints for the disk diffusion test were adjusted.
Several problems with testing β-lactam agents were encountered in this study. For many of the isolates tested, the presence of sporadic or subtle growth beyond an obvious end point made determining an MIC end point difficult. Others have also seen trailing end points with some β-lactams (8). If, as the NCCLS recommends in document M7 (14), the end point is read as the lowest concentration “that completely inhibits growth of the organism…as detected by the unaided eye,” then the presence of subtle growth and small colonies in the well should not be ignored. Because the high number of very major errors may have been due to reading the MIC end points too conservatively as a result of these subtle growth patterns, a second MIC determination, which ignored the individual colonies and star-like growth patterns, was included. Despite the manner in which the end points were determined, there remained an unacceptable level of very major errors for all of the β-lactam agents, with the exceptions of cefotaxime and ceftriaxone (which showed unacceptable minor error rates). Unfortunately, there are neither human nor animal model data to indicate which end point (a DD or liberally or conservatively read BMD end point) is more clinically relevant.
Because polymyxin B has excellent activity against this group of organisms, for which there are limited therapeutic options, the need for interpretive criteria, particularly for BMD, is critical. Unfortunately, it does not appear from our data and from those of Gales and colleagues that a DD assay for polymyxin B is likely to yield accurate results for this organism group. As Gales et al. discuss, this may be due to the fact that the polymyxin molecule diffuses poorly in agar, perhaps due to its size (6).
The therapeutic potential of using sulbactam alone has also been discussed (2). Since the activity of the combination of ampicillin and sulbactam against Acinetobacter spp. comes almost exclusively from the sulbactam component (2, 8, 9), the use of a combination disk may be able to predict the activity of sulbactam alone. However, further studies are needed to determine if breakpoints for sulbactam alone can be developed.
In summary, the results of BMD and DD are concordant for most non-β-lactam agents. Thus, DD can be used with confidence for Acinetobacter spp. and these agents. While an unacceptably high rate of major errors was observed with tetracycline, this problem may be resolved by readjusting the DD breakpoints to smaller zone diameters. The BMD tests for the β-lactam agents, which were difficult to read because of subtle growth beyond an obvious end point, continue to pose a problem of interpretation. Further studies are needed to determine appropriate methods for testing β-lactam antimicrobial agents and for testing sulbactam and polymyxin B.
Acknowledgments
We thank the following individuals and institutions for donating clinical strains: Tamara L. Underwood, Duke University Medical Center, Durham, N.C.; Jean Spargo, Massachusetts General Hospital, Boston, Mass.; Stephen Jenkins, Mt. Sinai Hospital, New York, N.Y.; Donna Hacek, Northwestern Memorial Hospital, Chicago, Ill.; Doug Prince, Piedmont Hospital, Atlanta, Ga.; Susan Munro, Stanford Health Services, Palo Alto, Calif.; Janet Hindler, UCLA Medical Center, Los Angeles; Lettie McElmeel and Sharon Crawford, UTHSC, San Antonio; Judy Rothberg, UMDNJ-Robert Wood Johnson Medical School, New Brunswick, N.J.; and Thomas Fritsche and Sue Swanzy, University of Washington and Harborview Medical Center, Seattle, Wash. We also thank the following for their help during the multilaboratory phase of the study: Mary Jane Ferraro and Jean Spargo, Massachusetts General Hospital, Boston, Mass.; Janet Hindler, UCLA, Los Angeles; Ronald Jones and Douglas Biedenbach, JMI Labs, North Liberty, Iowa; James Jorgensen and Lettie McElmeel, UTHSC, San Antonio; and Ellen Jo Baron and Susan Munro, Stanford Health Services, Palo Alto, Calif.
The use of trade names is for identification purposes only and does not constitute endorsement by the Public Health Service or the U.S. Department of Health and Human Services.
REFERENCES
- 1.Allen, D. M., and B. J. Hartman. 2000. Acinetobacter species, p. 2339-2344. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practices of infectious diseases. Churchill Livingstone, Inc., Philadelphia, Pa.
- 2.Corbella, X., J. Ariza, C. Ardanuy, M. Vuelta, F. Tubau, M. Sora, M. Pujol, and F. Gudiol. 1998. Efficacy of sulbactam alone and in combination with ampicillin in nosocomial infections caused by multiresistant Acinetobacter baumannii. J. Antimicrob. Chemother. 42:793-802. [DOI] [PubMed] [Google Scholar]
- 3.Fridkin, S. K., C. D. Steward, J. R. Edwards, E. R. Pryor, J. E. McGowan, Jr., L. Archibald, R. P. Gaynes, F. C. Tenover, and Project Intensive Care Antimicrobial Resistance Epidemiology Hospitals. 1999. Surveillance of antimicrobial use and antimicrobial resistance in U.S. hospitals: project ICARE phase 2. Clin. Infect. Dis. 29:252. [DOI] [PubMed] [Google Scholar]
- 4.Friedland, I., L. Stinson, M. Ikaiddi, S. Harm, and G. L. Woods. 2003. Phenotypic antimicrobial resistance patterns in Pseudomonas aeruginosa and Acinetobacter: results of a multicenter intensive care unit surveillance study, 1995-2000. Diagn. Microbiol. Infect. Dis. 45:245-250. [DOI] [PubMed] [Google Scholar]
- 5.Gales, A.C., R. N. Jones, K. R. Forward, J. Liñares, H. S. Sader, and J. Verhoef. 2001. Emerging importance of multidrug-resistant Acinetobacter species and Stenotrophomonas maltophilia as pathogens in seriously ill patients: geographic patterns, epidemiological features, and trends in the SENTRY antimicrobial surveillance program (1997-1999). Clin. Infect. Dis. 32(Suppl. 2):104-113. [DOI] [PubMed] [Google Scholar]
- 6.Gales, A. C., A. O. Reis, and R. N. Jones. 2001. Contemporary assessment of antimicrobial susceptibility testing methods for polymyxin B and colistin: review of available interpretative criteria and quality control guidelines. J. Clin. Microbiol. 39:183-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Héritier, C., L. Poirel, D. Aubert, and P. Nordmann. 2003. Genetic and functional analysis of the chromosome-encoded carbapenem-hydrolyzing oxacillinase OXA-40 of Acinetobacter baumannii. Antimicrob. Agents Chemother. 47:268-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Higgins, P. G., H. Wisplinghoff, D. Stefanik, and H. Seifert. 2004. In vitro activities of the β-lactamase inhibitors clavulanic acid, sulbactam, and tazobactam alone or in combination with β-lactams against epidemiologically characterized multidrug-resistant Acinetobacter baumannii strains. Antimicrob. Agents Chemother. 48:1586-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levin, A. S. 2002. Multiresistant Acinetobacter infections: a role for sulbactam combinations in overcoming an emerging worldwide problem. Clin. Microbiol. Infect. 8:144-153. [DOI] [PubMed] [Google Scholar]
- 10.NCCLS. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard, 5th ed. NCCLS document M7-A5. NCCLS, Wayne, Pa.
- 11.NCCLS. 2000. Performance standards for antimicrobial disk susceptibility tests. Approved standard, 7th ed. NCCLS document M2-A7. NCCLS, Wayne, Pa.
- 12.NCCLS. 2001. Development of in vitro susceptibility testing and quality control parameters. Approved guideline, 2nd ed. NCCLS document M23-A2. NCCLS, Wayne, Pa.
- 13.NCCLS. 2002. Performance standards for antimicrobial susceptibility testing. Twelfth informational supplement. NCCLS document M100-S12. NCCLS, Wayne, Pa.
- 14.NCCLS. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard, 6th ed. NCCLS document M7-A6. NCCLS, Wayne, Pa.
- 15.NCCLS. 2003. Performance standards for antimicrobial disk susceptibility tests. Approved standard, 8th ed. NCCLS document M2-A8. NCCLS, Wayne, Pa.
- 15a.NCCLS. 2004. Performance standards for antimicrobial disk susceptibility testing. Fourteenth informational supplement. NCCLS document M100-514. NCCLS, Wayne, Pa.
- 16.Schreckenberger, P. C., M. I. Daneshvar, R. S. Weyant, and D. G. Hollis. 2003. Acinetobacter, Achromobacter, Chryseobacterium, Moraxella, and other nonfermentative gram-negative rods, p. 749-779. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. A. Pfaller, and R. H. Yolken (ed.), Manual of clinical microbiology. ASM Press, Washington, D.C.
- 17.Seifert, H., L. Dijkshoorn, P. Gerner-Smidt, N. Pelzer, I. Tjernberg, and M. Vaneechoutte. 1997. Distribution of Acinetobacter species on human skin: comparison of phenotypic and genotypic methods. J. Clin. Microbiol. 35:2819-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Urban, C., S. Segal-Maurer, and J. J. Rahal. 2003. Considerations in control and treatment of nosocomial infections due to multidrug-resistant Acinetobacter baumannii. Clin. Infect. Dis. 36:1268-1274. [DOI] [PubMed] [Google Scholar]
- 19.Vaneechoutte, M., L. Dijkshoorn, I. Tjernberg, A. Elaichouni, P. De Vos, G. Claeys, and G. Verschraegen. 1995. Identification of Acinetobacter genomic species by amplified ribosomal DNA restriction analysis. J. Clin. Microbiol. 33:11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vila, J., J. Ruiz, M. Navia, B. Becerril, I. Garcia, S. Perea, I. Lopez-Hernandez, I. Alamo, F. Ballester, A. M. Planes, J. Martinez-Beltran, and T. J. De Anta. 1999. Spread of amikacin resistance in Acinetobacter baumannii strains isolated in Spain due to an epidemic strain. J. Clin. Microbiol. 37:758-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yum, J. H., K. Yi, H. Lee, D. Yong, K. Lee, J. M. Kim, G. M. Rossolini, and Y. Chong. 2002. Molecular characterization of metallo-β-lactamase-producing Acinetobacter baumannii and Acinetobacter genomospecies 3 from Korea: identification of two new integrons carrying the blaVIM-2 gene cassette. J. Antimicrob. Chemother. 49:837-840. [DOI] [PubMed] [Google Scholar]