Abstract
The bacterial and temperature factors leading to yellow blotch/band disease (YBD), which affects the major reef-building Caribbean corals Montastrea spp., have been investigated. Groups of bacteria isolated from affected corals and inoculated onto healthy corals caused disease signs similar to those of YBD. The 16S rRNA genes from these bacteria were sequenced and found to correspond to four Vibrio spp. Elevating the water temperature notably increased the rate of spread of YBD on inoculated corals and induced greater coral mortality. YBD-infected corals held at elevated water temperatures had 50% lower zooxanthella densities, 80% lower division rates, and a 75% decrease in chlorophyll a and c2 pigments compared with controls. Histological sections indicated that the algal pyrenoid was fragmented into separate segments, along with a reconfiguration and swelling of the zooxanthellae, as well as vacuolization. YBD does not appear to produce the same physiological response formerly observed in corals undergoing temperature-related bleaching. Evidence indicates that YBD affects primarily the symbiotic algae rather than coral tissue.
The increasing prevalence of coral diseases (6, 8, 28, 30, 31, 32, 52, 58) and thermal stress (14, 15, 19, 21, 22, 23, 25, 26, 27, 67, 69) have severely impacted reef-building corals over the past 20 years. The decline of coral reefs is significant, because coral reefs maintain high levels of biodiversity, provide habitats for coastal fisheries (69), may contain potential pharmacological compounds, and protect shorelines from erosion (25).
There is a lack of knowledge about the causative agents of many diseases affecting corals (30, 32, 49, 52) and their symbiotic algae (zooxanthellae). However, microbial pathogens are being increasingly identified with coral diseases, as in black band disease (51). Microbial pathogens affecting corals include the fungi Aspergillus sydowii (47, 60) and Aurantimonas coralicida, which affect sea fans and other gorgonians and caused white plague type II in the Florida Keys in 1995 (12). At least 18 species of corals were reported to be susceptible to this disease, including massive branching and plating species. The bacterium Serratia marcescens was reported to be the pathogenic agent causing white-pox disease (48, 50). This disease reduced populations of a significant coastal reef-building coral, Acropora palmata. S. marcescens is a common human intestinal bacterium and is found in sewage and contaminated soils. Human sewage has been linked to the disease, marking the first time a gut bacterium has been found to harm a marine invertebrate (48). Bacteria associated with black band disease of corals also included common human gut microbiota, but these were not found in healthy coral tissue (18).
Members of the genus Vibrio are common marine bacteria. Various species have been isolated from almost all marine niches, including some associated with toxic-shellfish poisoning (11, 33). Vibrio communities associated with corals were shown to increase in bleached corals (A. Kushmaro, personal communication). Kushmaro et al. (43) suggested that bacterial bleaching is caused by a temperature-influenced infection by Vibrio shilonii (2). Vibrio coralliilyticus was also shown to cause bleaching and tissue lysis in the Indo-Pacific coral Pocillopora damicornis (3). Marine vibrios tend to favor temperatures between 17 and 35°C (56).
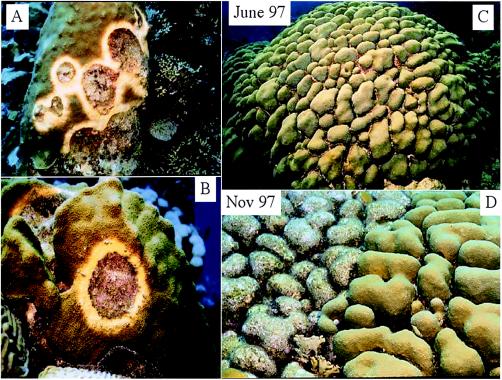
Any impairment of the host-symbiont relationship can affect coral growth (17, 19, 40, 62, 64). Yellow blotch/band disease (YBD), which affects the major reef-building corals Montastraea spp., has been documented throughout the Caribbean and can spread across a coral colony at ∼0.5 to 1.0 cm month−1 (5). During its earliest stages, YBD develops as a pale-yellow blotch on the coral tissue, which eventually expands in size into a band as the disease progresses (5, 59) (Fig. 1). This paleness represents a decrease in chlorophyll concentration and a lack of symbiotic zooxanthellae (5).
FIG. 1.
(A and B) Classic blotching yellow bands developing on the tissue of Montastraea spp. as described previously (8, 56). (C and D) Classic healthy Montastraea sp. during June 1997 and images of the same colony 5 months later with yellow lesions affecting half of the colony. This coral remains alive, with 50% of it overgrown with algae.
Corals harbor diverse bacterial communities and seem to exhibit some specificity within coral species (13, 55). Bacteria present on the coral surface utilize the rich mucopolysaccharide layer (13, 54). Rohwer at al. (55) recently described bacteria associated with Montastraea spp. and found a variety of gram-positive and gram-negative bacteria on the surfaces of these reef-building species. Zooxanthellae play a role in host mucus biosynthesis by synthesizing and relesing precursor biochemicals (1, 4, 65), thereby affecting the composition of mucus and the bacteria on it.
Thermal coral reef bleaching is the expulsion of whole symbiotic zooxanthellae from host endoderm tissue (7, 16, 19, 39, 46, 62, 64, 67, 70). Corals expel their zooxanthellae during the warmest months, when temperatures reach 1 to 2°C above their normal environmental threshold (26, 29). Corals can also bleach, or expel their symbiotic zooxanthellae, as a result of temperature stress coupled with high light levels, resulting in a loss of pigments and damage to photosystems (20, 34, 38, 40, 42, 63, 66, 67), or in response to changes in salinity (24), chemical exposure from cyanide fishing (9), or sedimentation (49).
Here, we show the effects of temperature and inoculation with exogenous Vibrio strains on the division rates of zooxanthella symbionts; we also show how this results in low symbiont densities and decreased chlorophyll concentrations in the corals.
MATERIALS AND METHODS
For microbial analysis, eight 10-ml sterile-syringe samples were drawn from the surface layers of YBD-infected and healthy Montastraea spp., two from the Florida Keys and six from Bonaire, Netherlands Antilles, by scuba diving. Syringe samples were immediately inoculated onto glycerol artificial seawater (GASW) plates (61) and thiosulfate-citrate-bile salt agar plates and then were incubated at 26°C. Colony morphology was examined using a standard stereomicroscope. Bacteria were isolated from the plates, with choices based on distinct morphological and growth characteristics, such as color and shape, and then were subcultured to pure cultures either on GASW or thiosulfate-citrate-bile salt agar.
In total, 143 pure cultures were isolated, 35 originating from control samples (the healthy samples) and 79 originating from diseased coral samples throughout the locations mentioned above. A 48-h agar culture of each isolate was then subjected to carbon source utilization pattern analysis using GN1 96-well microplates (BIOLOG Inc., Hayward, Calif.).
Bacteria associated with YBD were compared with those from healthy Montastraea annularis tissue, based on similarity of carbon source utilization patterns. Sixteen bacterial isolates were chosen, 6 isolates from healthy corals (controls) and 10 isolates that were present only in diseased corals. During preliminary testing, the 10 bacterial isolates from diseased corals were inoculated onto healthy coral tissue in different combinations, and all inoculations resulted in tissue paling. The six bacterial isolates from healthy corals were also inoculated onto healthy coral specimens and induced no yellow lesions.
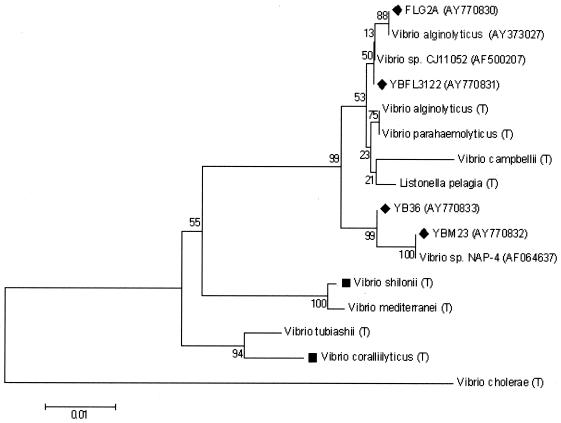
All 10 isolates that were suspected pathogens were then identified via sequence analysis of the PCR-amplified 16S rRNA gene. DNA isolation from the 10 bacterial isolates was followed by PCR amplification of the 16S rRNA gene, using universal bacterial 16S rRNA gene primers (27F [5′-AGAGTTTGATCCTGGCTAG-3′] and 1522R [5′ AAGGAGGTGATCCARCCGCA-3′]) (37), and sequencing. Sequencing was performed at the Georgia Tech core DNA facility using a BigDye Terminator version 3.1 cycle-sequencing kit on an automated capillary sequencer (model 3100 Gene Analyzer; Applied Biosystems). All 16S ribosomal DNA sequences were then determined by BLAST analysis from the National Center for Biotechnology Information nucleotide database. The dendrogram displaying phylogenetic positions of the four Vibrio spp. isolated and used in this study was based on alignment of a 511-bp region within the 16S rRNA gene. Sequence alignments were performed using DNA Star Lasergene MEGALIGN software (version 5.06) by the CLUSTAL W method. The tree was generated by the neighbor-joining method (57) using MEGA2 software (version 2.1). Sequence dissimilarity was determined following specific reference points (41). Bootstrap values were based on 500 replicates. Reference strain sequences were obtained from the Ribosomal Database Project (10).
Infection experiments.
Forty healthy coral specimens, each measuring 5 cm square, were used for inoculation experiments. Specimens were collected from Bonaire, Netherlands Antilles, and experiments were carried out at the University of South Carolina and the Mote Marine Laboratory, Summerland Key, Fla.
All corals were placed in outdoor aquarium tanks with fresh running seawater for testing and observation. Ten specimens were not inoculated with any bacteria (controls). Another 10 specimens were inoculated with Escherichia coli bacteria (K-12 strain MG1655; Carolina Biological). Another 10 specimens were inoculated with a mixture of the 10 bacterial strains isolated from the yellow lesion (YBFL3121, YBFL3122, YBM23, YBM22, YB36, YB2F2, YBFLG2A, YB15, YBFL362B, and YB23).
To create the mixture, one pure colony of each of the 10 YBD bacterial strains was cut from the culture plate media and smeared directly onto the surface of each coral. In total, 0.5 ml of mixed bacteria (50 μl from each) was smeared onto each of the 10 corals. The final 10 coral specimens were smeared with 0.5 ml of only 1 of the 10 YBD isolates each, to see if any of the isolates alone would induce the yellow band lesion. The experiment continued for 1 week. The first three groups of 10 were kept in separate tanks, all at 26 to 27.5°C. The corals in the fourth group were kept in 10 separate 1-liter beakers to avoid cross-contamination.
After a week, it became clear that the 10-strain mixture was the only bacterial inoculation causing yellow lesions similar to those found in the field. At that point, a swab from the yellow lesion was reisolated on GASW medium, incubated, and sequenced. Sequencing showed that four distinct strains of Vibrio bacteria were present in the yellow lesion that had been reisolated. (Most of the original 10 bacterial strains were actually duplicate strains of the same four bacteria.) The earlier experiment was then repeated. A combination of these four strains (125 μl each, totaling 500 μl) was smeared onto three different areas on the surface of a single healthy coral.
Cell densities, chlorophyll pigment analysis, and mitotic indices.
Samples were collected from healthy, stressed, and dying regions of Montastraea sp. corals. Total numbers of zooxanthellae and percent mitotic indices (MI) were recorded. The percent MI, which measures the number of dividing cells in a known population of algal cells over time, is calculated by determining the number of dividing algal cells divided by the total number of cells and multiplied by 100, as described by Wilkerson et al. (68).
Coral tissue was collected using a Water Pik (36) and was kept cold in ice-filled coolers. The slurry containing tissue and zooxanthellae was homogenized and centrifuged in a Beckman Accuspin at 5,000 rpm for 5 min to separate the host tissue from zooxanthellae. The liquid was discarded, and the pellet was resuspended in fresh filtered seawater-10% glutaraldehyde solution and recentrifuged for counting. The number of zooxanthellae and percent MI were determined by direct examination under a phase-contrast microscope at ×400 and ×1,000 magnifications, using a Neubauer ruling hemocytometer. Tissue was also extracted for pigment analysis of chlorophyll a and c2 (35).
Temperature experiments.
Fifteen samples of YBD-infected Montastraea sp. corals and 15 healthy samples from the same colony of Montastraea spp. were collected. The samples were placed in 100-ml polyethylene bottles with fresh filtered seawater and after 1 h were brought to the Mote Marine Laboratory facility. The corals were placed at 23°C and monitored for 48 h to make sure they remained alive for temperature experiments. For the experiments, the corals were placed in outdoor 10-gallon aquarium tanks at three different experimental temperatures: 20, 25, and 32°C. Controls (no YB lesions) were kept in separate tanks at the same temperatures in fresh running seawater. Five corals at 20°C, five at 25°C, and five at 32°C were placed in each experimental tank next to the tanks set up for the controls. This experiment was conducted to investigate the effect of temperature on healthy corals without YB lesions.
Ten 5-cm-square coral samples were collected from a colony with active YB lesions (0.741-cm-diameter average lesion/5-cm square) and placed in 20-gallon aquaria. An additional 10 5-cm-square samples with no yellow lesions on them (controls) were collected and placed in aquaria adjacent to the corals with yellow lesions. All 20 corals were exposed to the same temperatures over time: 20, 25, 30, and 33°C. Three 10-cm-square corals with active YB lesions on them were placed in aquaria and kept between 20 and 33°C for 120 h (the entire experiment). These time intervals were chosen to see how fast the YB lesions could increase during abrupt thermal-bleaching events. These corals were also used as experimental corals for isolation of mucus during bacterial-growth experiments and for monitoring because they had active YB lesions.
Histology and microscopy.
Samples of intact coral tissue displaying macroscopic features of YBD were placed in immersion, fixed in glutaraldehyde, and postfixed in 1.0% osmium tetroxide in phosphate buffer. These tissues were processed for examination by both optical and electron microscopy. After the coral tissues were decalcified, dehydrated in graded alcohols, and embedded in Spurr's epoxy resin, they were sectioned on an LKB Ultratome with a diamond knife. For light microscopy, thick sections were cut at 1.0 to 2.0 μm; for electron microscopy, thin sections were cut at 70 to 90 nm. All sections were prepared using a Dupont diamond knife. Thick sections (∼2.0 μm) were mounted on glass microscope slides and stained with aqueous toluidine blue 10B in sodium borate. Thin sections (∼90 nm) were mounted on Formvar-coated grids and stained with uranyl acetate and lead citrate. Optical photomicroscopy was conducted using a BandL Balplan microscope. Photographs were taken through the 100× objective lens with a film magnification of ×300. Fuji 200 ASA color print film was used for all photographic exposures. Electron photomicrographs were recorded on black-and-white film (Kodak 4489) at original magnifications of ×8,000 to 34,000.
RESULTS
Inoculation of corals with vibrios.
During preliminary testing, all corals inoculated with one YB bacterial isolate at a time showed a slight yellowing appearance similar to YBD. Bacterial samples were recovered from the experimentally infected corals during these testing trials. Pure cultures of the four bacteria unique to YBD, when inoculated (smeared) onto unaffected corals, resulted in signs resembling those of YBD. It is important to note that when all four bacteria together were smeared onto the coral, YB lesions appeared. Only slight paling was evident when bacterial species were inoculated onto the coral singly or in twos or threes. However, when all four of the Vibrio spp. were added together, classic YBD blotch lesions became evident. All corals remained at 20°C for 48 h with no evidence of advancing yellow lesions. Suspected YBD pathogens were inoculated onto healthy corals. These pathogens, which induced YB lesions in the healthy corals, were then reisolated once again and reinoculated onto healthy corals, causing YB lesions as seen in the field. These isolates were sequenced. Sequence analysis indicated that all 10 isolates could be narrowed down (after sequencing) to four isolates that are 99% similar to Vibrio spp.
Inoculation with other bacteria.
E. coli, as well as several other species of bacteria that had been isolated from the corals (including several gram-positive Bacillus spp.), showed no effect on the host tissue or symbiotic algae. No effect on the tissue or symbiotic algae was seen in any of the untreated corals.
Characterization of vibrios.
Two of the four Vibrio strains (YB36 and YBM23) formed cream-colored colonies, while two (YBFL3122 and YBFLG2A) formed yellow colonies. All were gram-negative motile curved rods that grew well at salt concentrations ranging from 1 to 10%. All strains grew on a wide variety of carbon sources. All four Vibrio spp. tested positive for utilization of the following carbon sources: d-mannose, d-galactose, d-lactose, l-fructose, d-fructose, d-mannitol, maltose, m-inositol, d-trehalose, dl-serine, l-leucine, d-gluconic acid, propionic acid, dl-lactic acid, glycerol, dl-[propto]-glycerol-phosphate, and glucose-6-phosphate.
Coupled effects of YBD-infected corals and variation in temperature.
Microscopic observations of all corals exposed to the four Vibrio spp. showed deleterious effects on zooxanthellae at higher temperatures within the gastroderm, leaving the coral host tissue intact at 32 to 33°C. Abnormalities associated with higher temperature and vibrios together were noted. For example, many zooxanthellae were degenerated and fragmented. There is no histological evidence of bacterial penetration into the zooxanthellae.
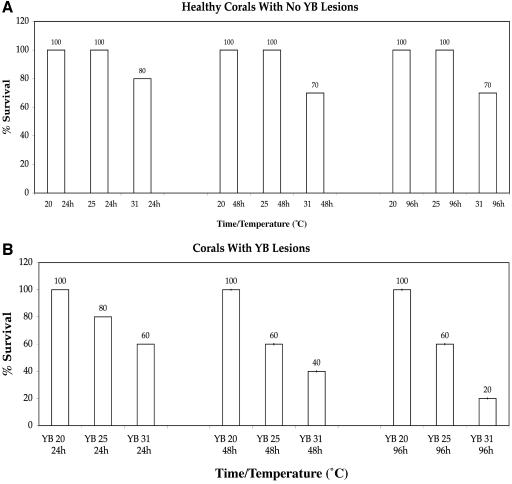
The mortality of healthy (control) corals exposed to temperatures of 20 to 32°C over time was evident (Fig. 3a). All healthy corals survived the experiments, except those exposed to 32 to 33°C, which experienced 20% mortality after 96 h. No mortality of the remaining controls was evident at any exposure durations. However, closer to the thermal-bleaching threshold, classic signs of temperature-induced expulsion of zooxanthellae were evident.
FIG. 3.
(a) Corals exposed to higher temperatures over time expel more of their symbiotic zooxanthellae from the gastroderm. (b) Corals exposed to bacterial pathogens coupled with increasing temperatures experience zooxanthella degradation within the gastroderm.
Mortality of YB-infected corals exposed to different temperatures could also be seen (Fig. 3b). The mortality of YBD-infected corals was highest (60 to 80%) with exposure to 31 to 32°C for 48 and 96 h. The corals experienced 40% mortality at 25°C and 0% mortality at 20°C. It is important to note that no expulsion of zooxanthellae from the yellow lesions was evident.
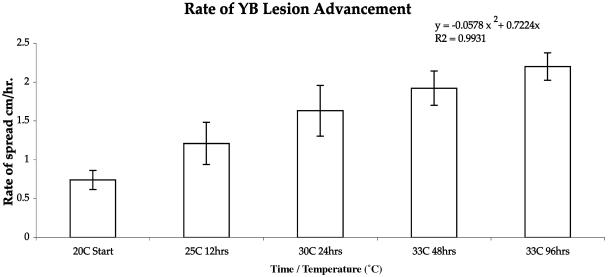
The rate-of-spread experiments also showed positive results when temperatures were increased. Temperatures were raised to 25°C at 12 h. At hour 24, the temperature was again raised to 30°C, and it was raised to 33°C at 48 and 96 h. The corals were spaced so that none of the specimens were in contact with each other for the duration of the experiment. None of the controls developed YB lesions. Corals with YB lesions that were placed in aquaria and kept at 20°C for 120 h showed no advancement of the yellow lesions at this temperature. The edge of the lesion spread with increasing temperature treatments showed an exponential increase in the yellow lesion (y = −0.0578x2 + 0.7224 × 2; R2 = 0.9931). At the starting point, the average yellow lesion diameter increased in 96 h from 0.74 cm with a temperature treatment of 20°C to 2.2 cm at 33°C toward the end of the experiment. None of the controls developed YB lesions (Fig. 4).
FIG. 4.
Lesion advancement after inoculation with YB pathogens together with increasing temperatures. The bar graph represents the area of YBD on experimental corals. At various times and temperatures, the zones of infection were estimated and the areas were calculated, and these values were plotted versus time. The error bars indicate standard deviations.
Microscopy, cell densities, mitotic index, and pigment analysis.
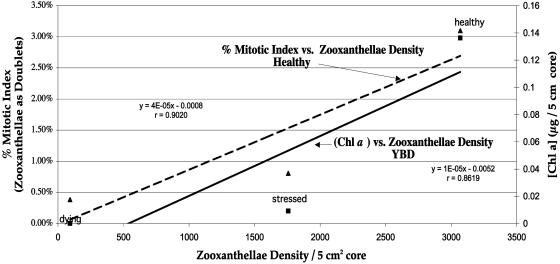
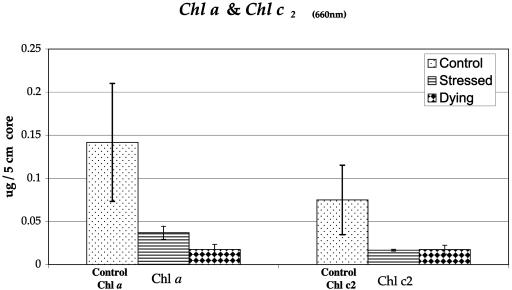
Microscopic images of experimentally infected host tissues were similar to those from field specimens of YBD. Corals exposed to YBD at 25°C for 72 h began to show cellular breakdown and necrosis. In YBD corals exposed to 20°C, this breakdown occurred more rapidly (after 48 h) (Fig. 5a and b) than in controls (Fig. 5d). Healthy zooxanthellae were seen surrounded by a bacterial mat 8 h after being inoculated with the four Vibrio spp. (Fig. 5c). No bacteria were evident in symbiotic zooxanthellae, as they were found only in host tissue surrounding the algal cell. Electron micrographs showed redistribution and degeneration of algal organelles (Fig. 6). Vacuolization, fragmentation, swelling, and displacement of cytological features were evident. A 50% decrease in algal density and an 80% decrease in the mitotic index occurred in YBD specimens. Compared with healthy tissue, diseased samples had a 75% decreased concentration of chlorophyll (Chl) a and c2 accessory pigments (Fig. 7 and 8).
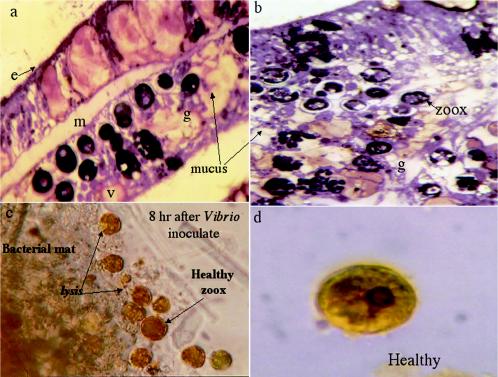
FIG. 5.
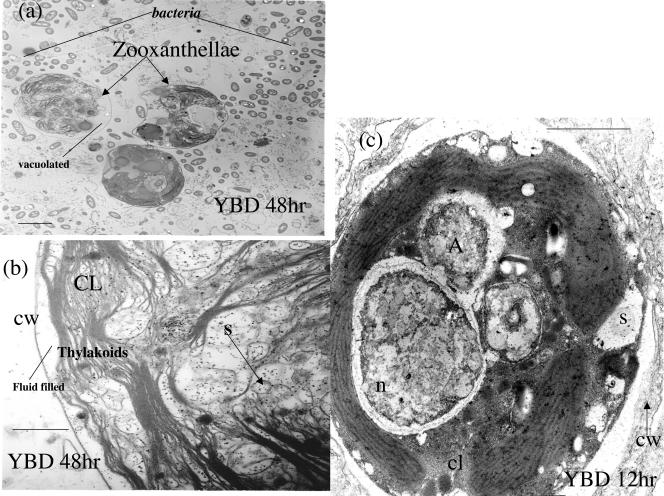
(a) Histology of Montastraea sp. exposed to YBD pathogens at 20°C. Vacuolation and slight fragmentation are evident. However, host structural features are also evident, indicating early breakdown. e, epidermis; m, mesogleoa; g, gastroderm; v, vacuole. Magnification, ×7,440. (b) Histological section of Montastraea sp. exposed to 25°C for 72 h. Symbiotic zooxanthella degeneration and vacuolation are extreme. The host tissue structural morphology has changed due to the virulence of YBD bacteria coupled with higher temperature. The mesogleoa is no longer evident. Magnification, ×7,440. zoox, symbiotic algae. (c) Light microscopic image of healthy zooxanthellae 8 h after inoculation with Vibrio. Evidence of algal lysis is seen surrounding the bacterial mat. Magnification, × 400. (d) Healthy zooxanthella isolated from controls. The average diameter of a cell in control tissues is ∼1.14 cm at a print magnification of ×1,250. This means that the unit diameter of the zooxanthella is ∼0.01 mm. Magnification, × 1,000.
FIG. 6.
Transmission electron micrographs (TEMs) of symbiotic zooxanthellae exposed to 25°C with symptoms of YBD. Degradation of the cell wall appears to be evident, along with vacuolation and cytoplasmic shrinkage. TEMs of zooxanthellae in situ indicate the same general morphology seen in light microscopy but reveal far greater detail. The chloroplasts are displaced to the outer margin of the cell; however, they appear intact. There are numerous non-membrane-bound vacuoles that appear within the nucleus and cytoplasm of the zooxanthellae. Some of these are filled with an electron-dense substance, but many are empty or filled with fluid. The cores of the algal cells are severely vacuolated. The normal morphology of the algal cell is disturbed beyond recognition, and the surface configuration of many organelles is unrecognizable. The pyrenoid body is apparent and normal in structure. However, in some cells, especially after being heated to 32°C, the pyrenoid is fragmented into separate segments. Algal swelling in the 32°C samples is primarily attributable to the expansion of the accumulation bodies and separation of the algal surface membranes from the peripheral cytoplasm containing chloroplasts. n, nucleus; CL, chloroplasts; S, starch vacuoles; cw, cell wall. Scale bars, 0.2 μm.
FIG. 7.
Linear regression of zooxanthella density, percent mitotic index, and Chl a analysis.
FIG. 8.
Graph comparing chlorophyll a adjacent to c2 in healthy tissues, stressed tissues (early advancing YB lesions), and dying tissue regions. The error bars indicate standard deviations.
DISCUSSION
Yellow band disease appears to affect zooxanthellae rather than host tissue in Montastraea spp. This hypothesis is based on the fact that corals exposed to 20°C for 96 h all survived while still showing signs of YBD. Corals exposed to 31°C showed an 80% mortality rate, and the 20% that survived had YB lesions covering the entire surface (host tissue remained alive). When the four Vibrio spp. were inoculated together, the YBD signs developed more rapidly and closely matched the disease progression and cell impairment features found in field samples of YBD (8). This suggests that these four species may act as a consortium. Field specimens of corals with YBD remain alive for years, depending upon the stress conditions placed upon the coral. As the lesion advances, the tissue remains alive, albeit without its symbionts. Over time, if stress conditions remain and the lesion advances quickly, the tissue starts to die and the skeleton is exposed. The symbionts are either degenerate, vacuolated, or absent. YBD has an appearance similar to that of thermal coral bleaching and can sometimes be confused with it. It is important to take careful photographs and analyze samples using microbiological probes to confirm the differences between YBD and thermal bleaching.
While Koch's postulates have ostensibly been fulfilled for each of the four Vibrio sp. isolates tested during this study, the postulates lack the flexibility to be applied directly to disease symptoms that may be induced by more than one bacterium. The poorly understood and complex nature of the microbial community associated with the coral makes it very difficult to ascertain the stress conditions needed to initiate the disease. The isolates may be acting as destabilizing agents within the microbial community, allowing some normally innocuous member of the coral-associated microbial community to opportunistically cause infection. It is also possible that each of the isolates directly induces the disease symptoms, either by some common pathogenic mechanism or by disparate means. Variations in the proportions of different bacteria in the consortium could affect the color, width, and seasonal progress rate of the lesions. These questions merit further research, as the answers could determine whether YBD continues to be treated as a single disease or as a collection of symptoms common to several diseases.
The temperatures that induce symbiotic algal stress are between 26 and 30°C, depending on the geographic region. In YBD-infected corals, neither a complete expulsion of dead and damaged algae nor a release of mucus from the coral gastrodermis occurs, as in bleaching. The symbiotic zooxanthellae appear to be degraded inside the gastroderm instead of being expelled during YBD. The corals that are in a weakened state due to higher temperatures are likely to be more susceptible to disease. Past studies have shown that many Vibrio spp., including Vibrio cholerae, are present on the surfaces of marine species but are not toxic until triggered by various stress factors (11).
Temperature may be causing the YB Vibrio spp. to reach their toxic state, but direct demonstration of toxin production remains to be established. Future work investigating the specific virulence factors, including biochemical characterization of the isolates, is warranted (53). Due to the close sequence homology between the Vibrio spp. isolated in this study and V. alginolyticus and Vibrio parahaemolyticus, such studies should include the examination of possible roles of known toxins produced by these species (i.e., various hemolysins) and determining if reference strains of V. parahaemolyticus and V. alginolyticus are capable of inducing disease signs in Montastraea spp.
The symbiotic zooxanthellae appear to be lysed. Similar cell wall damage is evident in harmful algal-bloom species when free-floating dinoflagellates are attacked by Pseudoalteromonas (45). If the mechanism of Pseudoalteromonas can be identified, it may provide insights into how a similar phenomenon occurs in YBD.
Our data suggested that the Vibrio spp. had a stronger effect on the symbiotic dinoflagellate when the temperature was increased. Histological examinations of whole tissues with symbiotic zooxanthellae harbored in the gastrodermal tissue and subjected to various temperatures, namely, 24.5, 29, and 33°C, showed the effects of temperature and pathogens together. These changes are not present when a coral undergoes thermal bleaching without pathogens, as they are expelled through the gastroderm as wholly intact cells. When pathogens are present, they do not trigger the coral to expel zooxanthellae, or to bleach; the increased sea surface temperatures cause the pathogens to be more virulent, resulting in zooxanthella cells dying in situ (Fig. 4).
The degeneration of YB-infected zooxanthella cells is consistent with a decrease in Chl a and c2 pigments, thereby possibly disrupting biochemical reactions normally taking place on the thylakoid membranes during photosynthesis, as seen in thermal bleaching (40). Symbiotic zooxanthellae, chlorophyll a and c2, and densities begin to substantially decrease from the border of the healthy tissue toward the yellow band lesion. The loss of photosynthetic capabilities of the reef builder Montastraea slowly diminishes due to YB exposure (Fig. 8).
According to electron micrographs, disfigured chloroplasts are evident in the zooxanthellae. Redistribution and degeneration of algal organelles accompanied by vacuolization, fragmentation, swelling, and displacement of cytological features are evident (Fig. 6). These symbiotic zooxanthellae are broken down during bacterially induced stress, hindering normal growth of the coral. We further clarified the characteristics of this breakdown due to the decrease in cell density and mitotic indices of YBD-exposed corals. The measurement of zooxanthella loss as a bioassay for assessing stress in coral has been used before (39, 46, 63, 68) (Fig. 7). Future research focusing on longer exposure times of the pathogenic bacteria and its mechanism of action are needed.
This research provides evidence for the correlation between increased temperature and the infectious natures of four marine Vibrio spp. in a major reef-building coral in the Caribbean. YBD can therefore be best interpreted as a primary disease of symbiotic algae that only secondarily affects host coral tissues of the reef builder Montastraea spp. Links of global warming and temperature stress to coral bleaching have been hypothesized (19, 23, 26, 27). High temperatures associated with global warming may exacerbate YBD (8) and other coral diseases (29, 31, 33).
Previous documentation indicated that V. shilonii (44) and V. coralliilyticus (3) caused coral-bleaching-like effects with increased temperature (43). We provide new evidence that indicates that YBD is a symbiont infection that results in in situ zooxanthella degradation rather than expulsion from host tissue on Montastraea spp,. as in thermal bleaching. When Chl a and c2 pigment concentrations decrease due to stress, photosynthesis becomes impaired, decreasing energy and slowing growth (15, 40, 66). It has been found that under thermal-bleaching conditions, Montastrea spp. and other corals have lower zooxanthella chlorophyll a and c2 concentrations than normal pigmented colonies (14, 40, 42). Any future research should make use of various molecular techniques, such as denaturing gradient gel electrophoresis, which could provide further insight into the dynamics of the total microbial community during the disease process. Further experiments using fluorescence in situ hybridization probes of the four bacterial species, exposing the symbiotic algae to YBD-associated Vibrio spp., and comparing the results to normal thermal bleaching would be interesting.
FIG. 2.
Dendrogram displaying phylogenetic positions of the four Vibrio spp. Scale bar = 1% dissimilarity in nucleotide sequences. (T), type strain; ⧫, strain used in this study; ▪, published coral pathogen. GenBank accession numbers for nontype strains are in parentheses.
Acknowledgments
We thank the Leslie Jones Foundation and the Global Coral Reef Alliance (GCRA) for partially funding this research.
We also thank the Mote Marine Laboratory (Summerland Key facility) and Patricia Sobecky at the Georgia Institute of Technology for laboratory space needed to carry out important laboratory experiments during this project. We thank Derek Kelly of the New York City Fire Department for field assistance. For scientific discussions and feedback, we thank Robert Trench, Forest Rohwer, Les Kaufman, John Willams, Andrew Baker, Norm Wainwright, and Esther Peters. Special thanks for editorial comments are due to Kathryn Winiarski of the New York Academy of Medicine, Jeff Houdret of the GCRA, Joseph Claro of the science research program at St. Francis Prep Science Research High School in New York City, and an anonymous reviewer who improved the clarity of the manuscript.
REFERENCES
- 1.Battey, J. F., and J. S. Patton. 1984. A reevaluation of the role of glycerol in carbon translocation in zooxanthellae-coelenterate symbiosis. Mar. Biol. 79:27-38. [Google Scholar]
- 2.Ben-Haim Y., and E. Rosenberg. 2002. A novel Vibrio sp. pathogen of the coral Pocillopora damicornis. Mar. Biol. 141:47-55. [Google Scholar]
- 3.Ben-Haim, Y., K. M. Zicherman, and E. Rosenberg. 2003. Temperature-regulated bleaching and lysis of the coral Pocillopora damicornis by the novel pathogen Vibrio coralliilyticus. Appl. Environ. Microbiol. 69:4236-4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benson A. A., and L. Muscatine. 1974. Wax in coral mucus: energy transfer from corals to reef fishes. Limnol. Oceanogr. 19:810-814. [Google Scholar]
- 5.Brown, B. E., M. D. A. Le Tissier, and J. C. Bythell. 1995. Mechanisms of bleaching deduced from histological studies of reef corals sampled during a natural bleaching event. Mar. Biol. 122:655-663. [Google Scholar]
- 6.Bruckner, A. W., and R. J. Bruckner. 1997. Spread of a black-band disease epizootic through the coral reef system in St. Ann's Bay, Jamaica. Bull. Mar. Sci. 61:919-928. [Google Scholar]
- 7.Cervino, J. M. 1996. Classes of coral reef mortality inducing processes and their possible interactions. Masters thesis. Boston University Marine Program, Woods Hole, Mass.
- 8.Cervino J. M., T. J. Goreau, I. Nagelkerken, G. W. Smith, and R. L. Hayes. 2001. Yellow band and dark spot syndromes in Caribbean corals: distribution, rate of spread, cytology and effects on abundance and division rate of zooxanthellae. Hydrobiologia 460:53-63. [Google Scholar]
- 9.Cervino, J. M., R. L. Hayes, M. Honovich, T. J. Goreau, S. Jones, and P. J. Rubec. 2003. Changes in zooxanthellae density, morphology, and mitotic index in hermatypic corals and anemones exposed to cyanide. Mar. Pollut. Bull. 46:573-586. [DOI] [PubMed] [Google Scholar]
- 10.Cole, J. R., B. Chai, T. L. Marsh, R. J. Farris, Q. Wang, S. A. Kulam, S. Chandra, D. M. McGarrell, T. M. Schmidt, G. M. Garrity, and J. M. Tiedje. 2003. The Ribosomal Database Project (RDP-II): previewing a new autoaligner that allows regular updates and the new prokaryotic taxonomy. Nucleic Acids Res. 31:442-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colwell, R. 1996. Global climate and infectious disease: the cholera paradigm. Science 274:2025-2031. [DOI] [PubMed] [Google Scholar]
- 12.Denner E. B. M., G. W. Smith, H. J. Busse1, P. Schumann, T. Narzt, S. W. Polson, W. Lubitz, and L. L. Richardson. 2003. Aurantimonas coralicida gen. nov., sp. nov., the causative agent of white plague type II on Caribbean scleractinian corals. Int. J. Syst. Evol. Microbiol. 53:1115-1122. [DOI] [PubMed] [Google Scholar]
- 13.Ducklow, H. W., and R. Mitchell. 1979. Composition of mucus released by coral reef coelenterates. Limnol. Oceanogr. 24:706-714. [Google Scholar]
- 14.Fang, L. S., C. W. Liao, and M. Liu. 1995. Pigment composition in different-colored scleractinian corals before and during the bleaching process. Zool. Studies 34:10-17. [Google Scholar]
- 15.Fitt, W. K., H. J. Spero, J. Halas, M. W. White., and J. W. Porter. 1993. Recovery of the coral Montastraea annularis in the Florida Keys after the 1987 “bleaching event.” Coral Reefs 12:57-64. [Google Scholar]
- 16.Fitt, W. K., and M. E. Warner. 1995. Bleaching patterns of four species of Caribbean reef corals. Biol. Bull. 189:298-307. [DOI] [PubMed] [Google Scholar]
- 17.Fitt, W. K., B. E. Brown, M. E. Warner, and R. Dunn. 2001. Coral bleaching: interpretation of thermal tolerance limits and thermal thresholds in tropical corals. Coral Reefs 20:51-65. [Google Scholar]
- 18.Frias-Lopez, J. G., A.L. Zerkle, G. T. Bonheyo, and B. W. Fouke. 2002. Partitioning of bacterial communities between seawater and healthy, black band diseased and dead coral surfaces. Appl. Environ. Microbiol. 68:2214-2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gates, R. D., G. Baghdasarian, and L. Muscatine. 1992. Temperature stress causes host cell detachment in symbiotic cnidarians: implications for coral bleaching. Biol. Bull. Woods Hole 182:324-332. [DOI] [PubMed] [Google Scholar]
- 20.Gleason, D. F. 1993. Differential effects of ultraviolet radiation on green and brown morphs of the Caribbean coral Porites astreoides. Limnol. Oceanogr. 38:1452-1463. [Google Scholar]
- 21.Glynn, P. W., and L. D'Croz. 1990. Experimental evidence for high temperature stress as the cause of El Nino-coincident coral mortality. Coral Reefs 8:181-192. [Google Scholar]
- 22.Glynn, P. W. 1993. Coral-reef bleaching—ecological perspectives. Coral Reefs 12:1-17. [Google Scholar]
- 23.Glynn, P. W. 1994. Coral reef bleaching in the 1980s and possible connections with global warming. Trends Ecol. Evol. 6:175-179. [DOI] [PubMed] [Google Scholar]
- 24.Goreau, T. F. 1964. Mass expulsion of zooxanthellae from Jamaican reef communities after Hurricane Flora. Science 145:383-386. [DOI] [PubMed] [Google Scholar]
- 25.Goreau, T. F., N. I. Goreau, and T. J. Goreau. 1979. Corals and coral reefs. Sci. Am. 241:124-135. [Google Scholar]
- 26.Goreau, T. J., and R. L. Hayes. 1994. Coral bleaching and ocean “hot spots.” Ambio 23:176-180. [Google Scholar]
- 27.Goreau, T. J., R. L. Hayes, J. W. Clark, D. J. Basta, and C. N. Robertson. 1993. Elevated sea surface temperatures correlate with Caribbean coral reef bleaching, p. 225-255. In R. A. Geyer (ed.), A global warming forum: scientific, economic, and legal overview. CRC Press, Boca Raton, Fla.
- 28.Goreau, T. J., J. Cervino, M. Goreau, R. Hayes, M. Hayes, L. Richardson, G. Smith, K. DeMeyer, I. Nagelkerken, J. Garzon-Ferrera, D. Gil, G. Garrison, E. H. Williams, L. Bunkley-Williams, C. Quirolo, K. Patterson, J. Porter, and K. Porter. 1998. Rapid spread of Caribbean coral reef diseases. Rev. Biol. Trop. 46(Suppl. 5):157-171. [Google Scholar]
- 29.Goreau, T. J., T. McClanahan, R. Hayes, and A. Strong. 2000. Conservation of coral reefs after the 1998 global bleaching event. Conserv. Biol. 14:5-15. [Google Scholar]
- 30.Green, E., and A. W. Bruckner. 2000. The significance of coral disease epizootiology for coral reef conservation. Biol. Conserv. 96:347-361. [Google Scholar]
- 31.Harvell, C. D., K. Kim, J. M. Burkholder, R. R. Colwell, P. R. Epstein, D. J. Grimes, E. E. Hofmann, E. K. Lipp, A. D. M. E. Osterhaus, R. M. Overstreet, J. W. Porter, G. W. Smith, and G. R. Vasta. 1999. Emerging marine diseases—climate links and anthropogenic factors. Science 285:1505-1510. [DOI] [PubMed] [Google Scholar]
- 32.Hayes, R. L., and N. I. Goreau. 1998. The significance of emerging diseases in the tropical coral reef ecosystem. Rev. Biol. Trop. 46:(Suppl. 5): 73-185. [Google Scholar]
- 33.Huq, A., and R. R. Colwell. 1996. Vibrios in the marine and estuarine environment: tracking Vibrio cholerae. J. Ecosyst. Health 2:198-214. [Google Scholar]
- 34.Iglesias-Prieto, R., and R. K. Trench. 1997. Acclimation and adaptation to irradiance in symbiotic dinoflagellates. II. Response of chlorophyll 2-protein complexes to different photon-flux densities. Mar. Biol. 130:23-33. [Google Scholar]
- 35.Jeffery, S. W., and G. F. Humphrey. 1976. New spectrophotometric equations for determining CHl a,b,c and c2 in higher plants, algae and natural phytoplankton. Biochem. Physiol. Pflanz. 167:191-194. [Google Scholar]
- 36.Johannes, R. E., and W. J. Wiebe. 1970. A method for determination of coral tissue biomass and composition. Limnol. Oceanogr. 21:540-547. [Google Scholar]
- 37.Johnson, J. L. 1994. Similarity analysis of rRNAs, p. 683-700. In P. Gerhardt, R. G. E. Murray, W.A. Wood, and N. R. Krieg (ed.), Methods for general and molecular bacteriology. American Society for Microbiology, Washington, D.C.
- 38.Jokiel, P. L., and R. H. York. 1982. Solar ultraviolet photobiology of the reef coral Pocillopora damicornis and symbiotic zooxanthellae. Bull. Mar. Sci. 32:301-315. [Google Scholar]
- 39.Jones, R. J. 1997. Zooxanthellae loss as a bioassay for assessing stress in corals. Mar. Ecol. Prog. Ser. 149:163-171. [Google Scholar]
- 40.Jones, R. J. 1997. Changes in zooxanthellae densities and chlorophyll concentrations in corals during and after a bleaching event. Mar. Ecol. Prog. Ser. 158:51-59. [Google Scholar]
- 41.Jukes, T. H., and C. R. Cantor. 1969. Evolution of protein molecules, p. 21-132. In H. N. Munro (ed.), Mammalian protein metabolism. Academic Press, New York, N.Y.
- 42.Kleppel, G. S., R. E. Dodge, and C. J. Reese. 1989. Changes in pigmentation associated with the bleaching of stoney corals. Limnol. Oceanogr. 34:1331-1335. [Google Scholar]
- 43.Kushmaro, A., Y. Loya, M. Fine, and E. Rosenberg. 1996. Bacterial infection and coral bleaching. Nature 380:396. [Google Scholar]
- 44.Kushmaro, A., E. Banin, Y. Loya, E. Stackebrandt, and E. Rosenberg. 2001. Vibrio shiloi sp. nov., the causative agent of bleaching of the coral Oculina patagonica. Int. J. Syst., Evol. Microbiol. 51:1383-1388. [DOI] [PubMed] [Google Scholar]
- 45.Lovejoy, C., J. P. Bowman, and G. M. Hallegraef. 1998. Algicidal effects of a novel marine Pseudoalteromonas isolate (class Proteobacteria, gamma subdivision) on harmful algal bloom species of the genera Chattonella, Gymnodinium, and Heterosigma. Appl. Environ. Microbiol. 64:2806-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muscatine, L. 2000. Preferential expulsion of dividing algal cells as a mechanism for regulating algal-cnidarian symbiosis. Mar. Biol. Bull. 199:278-286. [DOI] [PubMed] [Google Scholar]
- 47.Nagelkerken, I., K. Buchan, G. W. Smith, K. Bonair, P. Bush, J. Garzon-Ferreira, I. Botero, P. Gayle, C. D. Harvell, C. Heberer, K. Kim, C. Petrovic, I. Pors, and P. Yoshioka. 1997. Widespread disease in Caribbean sea fans: II. Patterns of infection and tissue loss. Mar. Ecol. Prog. Ser. 160:255-263. [Google Scholar]
- 48.Patterson, K., J. W. Porter, K. B. Ritchie, S. W. Polson, E. C. Peters, D. L. Santavy, and G. W. Smith. 2002. The etiology of white pox, a lethal disease of the Caribbean elkhorn coral, Acropora palmata. Proc. Natl. Acad. Sci. USA 13:8725-8730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peters, E. 1984. A survey of cellular reactions to environmental stress and disease in Caribbean scleractinian corals. Helgol. Meeresunters. 37:113-137. [Google Scholar]
- 50.Porter, J. W., P. W. Dustan, W. Jaap, K. Patterson, V. Kosmynin, M. Patterson, and M. Parsons. 2001. Patterns of spread of coral disease in the Florida Keys. Hydrobiology 460:1-24. [Google Scholar]
- 51.Richardson, L. 1996. Motility patterns of Phormidium corallyticum and Beggiatoa spp. associated with black band disease of corals. Microb. Ecol. 32:323-335. [DOI] [PubMed] [Google Scholar]
- 52.Richardson, L. 1998. Coral diseases: what is really known? Trends Ecol. Evol. 13:438-443. [DOI] [PubMed] [Google Scholar]
- 53.Richardson, L. L., G. W. Smith, K. B. Ritchie, and R. G. Carlton. 2001. Integrating microbiological, microsensor, molecular, and physiologic techniques for the study of coral disease pathogenesis. Hydrobiology 460:71-89. [Google Scholar]
- 54.Ritchie, K. B., S. W. Polson and G. W. Smith. 2001. Microbial disease causation in marine invertebrates: problems, practices and future prospects. Hydrobiology 460:131-139. [Google Scholar]
- 55.Rohwer, F., J. J. Breitbart, F. Azam, and N. Knowlton. 2001. Diversity of bacteria associated with the Caribbean coral Montastraea franksi. Coral Reefs 20:85-91. [Google Scholar]
- 56.Rubin, S. J., and R. C. Tilton. 1975. Isolation of Vibrio alginolyticus from wound infections. J. Clin. Microbiol. 2:556-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 58.Santavy, D. L., and E. C. Peters. 1997. Microbial pests: coral diseases in the western Atlantic, p. 607-612. In Proceedings of the 8th International Coral Reef Symposium. Smithsonian Tropical Research Institute, Balboa, Panama.
- 59.Santavy, D. L., E. C. Peters, C. Quirolo, J. W. Porter, and C. N. Bianchi. 1999. Yellow blotch disease outbreak on reefs of the San Blas Islands, Panama. Coral Reefs 18:97. [Google Scholar]
- 60.Smith, G. W., L. D. Ives, I. A. Nagelkerken, and K. B. Ritchie. 1996. Aspergilliosis associated with Caribbean sea fan mortalities. Nature 382:487. [Google Scholar]
- 61.Smith, G. W., and S. S. Hayasaka. 1982. Nitrogenase activity associated with Halodule wrightii roots. Appl. Environ. Microbiol. 43:1244-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Steele, R. D., and N. Goreau. 1977. The breakdown of symbiotic zooxanthellae in the sea anemone Phyllactis (=Oulactis) flosculifera (Actiniara). J. Zool. Lond. 181:421-437. [Google Scholar]
- 63.Suharsono, R. K., and B. E. Brown. 1992. Comparative measurements of mitotic index in zooxanthellae from a symbiotic cnidarian subject to temperature increase. J. Exp. Mar. Biol. Ecol. 158:179-188. [Google Scholar]
- 64.Titlyanov, E. A., T. V. Titlyanova, V. A. Leletkin, J. Tsukahara, R. van Woesik, and K. Yamazato. 1996. Degradation of zooxanthellae and regulation of their density in hermatypic corals. Mar. Ecol. Prog. Ser. 139:167-178. [Google Scholar]
- 65.Trench, R. K. 1971. The physiology and biochemistry of zooxanthellae symbiotic with marine coelenterates. 11. Liberation of fixed 14C by zooxanthellae in vitro. Proc. R. Soc. Lond. B 177:237-250. [Google Scholar]
- 66.Warner, M. E., G. W. Schmidt, and W. K. Fitt. 1999. Damage to photosystem II in symbiotic dinoflagellates: a determinant of coral bleaching, Proc. Natl. Acad. Sci. USA 98:8007-8012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Warner, M. E., W. K. Fitt, and G. W. Schmidt. 1996. The effects of elevated temperature on the photosynthetic efficiency of zooxanthellae in hospice from four species of reef coral: a novel approach. Plant Cell Environ. 19:291-299. [Google Scholar]
- 68.Wilkerson, F. P., D. Kobayashi, and L. Muscatine. 1988. Mitotic index of symbiotic algae in Caribbean reef corals. Coral Reefs 7:29-36. [Google Scholar]
- 69.Williams, E. H., and L. Bunkley-Williams. 1990. The world wide coral reef bleaching cycle and related sources of coral mortality. Atoll Res. Bull. 335:1-71. [Google Scholar]
- 70.Yonge, C. M., and A. G. Nicholls. 1931. Studies on the physiology of corals. IV. The structure, distribution and physiology of the zooxanthellae, p. 135-176. In Scientific Report of the Great Barrier Reef Expedition, 1928-1929.