Abstract
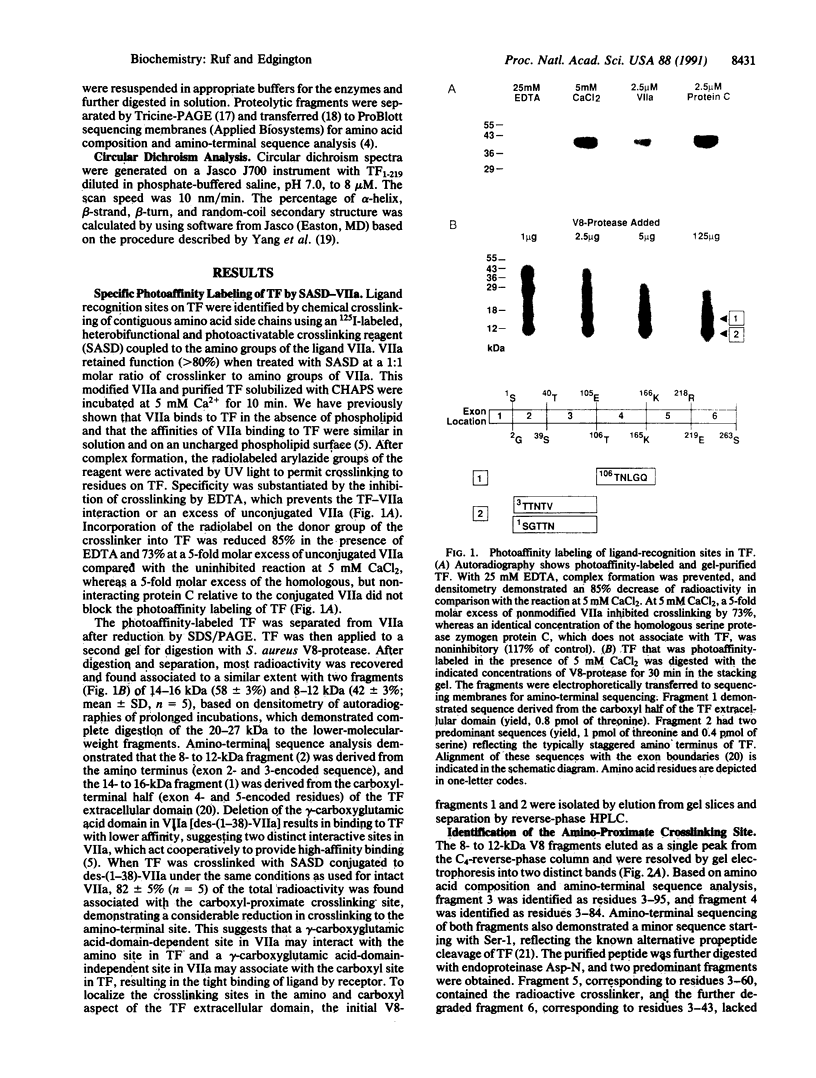
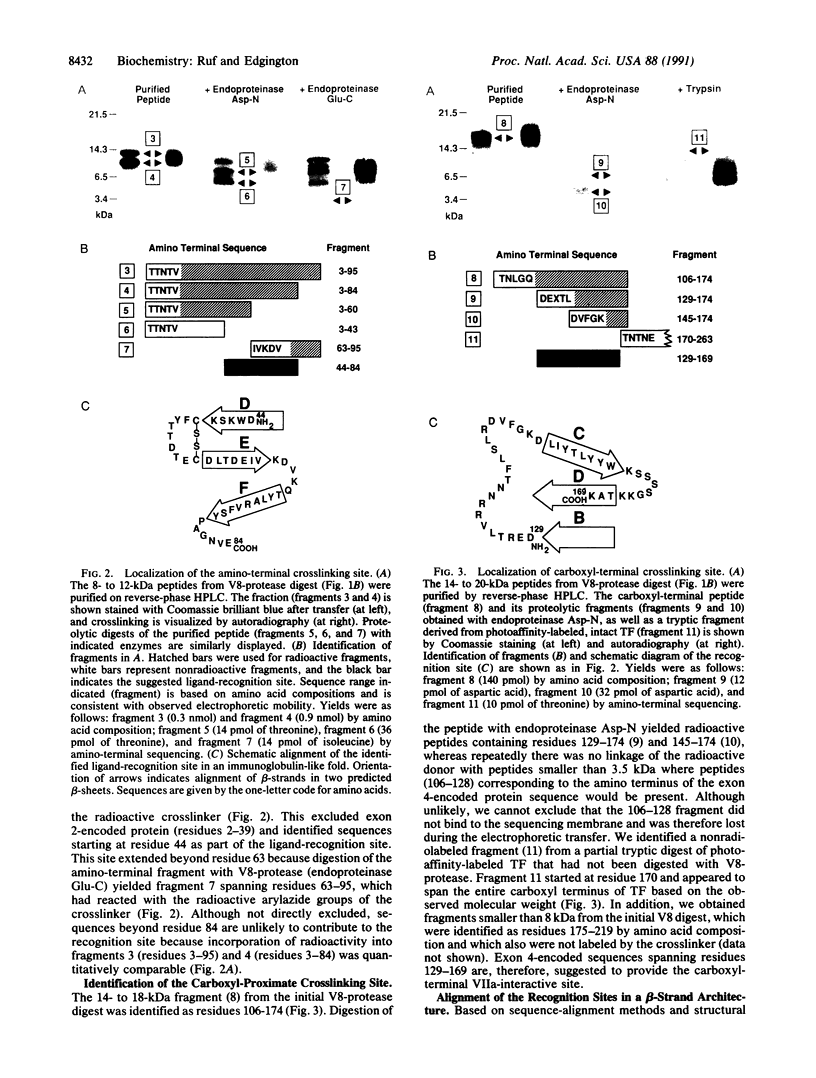
Tissue factor (TF) binds the serine protease coagulation factor VIIa and initiates the coagulation protease cascade by forming a catalytic cofactor-enzyme complex. Using a photoactivatable crosslinking reagent coupled to factor VIIa, we have identified interactive sites in the amino-terminal (residues 44-84) and the carboxyl-terminal (residues 129-169) aspect of the extracellular domain of TF. Epitopes of inhibitory antibodies have previously indicated participation of these regions in TF function. The presence of the gamma-carboxyglutamic acid domain in factor VIIa appears to facilitate the interaction with the negatively charged, amino-proximate site, whereas crosslinking of TF with VIIa or des-(1-38)-VIIa at the positively charged carboxyl-proximate site was similar. Lack of alpha-helical secondary structure in the TF extracellular domain is consistent with the proposed structural similarity of TF with the cytokine receptor family. The interactive sites identified for TF are located in sequence spans that demonstrate a low degree of sequence conservation among the members of this receptor family. Regions with highly conserved residues, such as sequences encoded by exon 2 and 5 in TF, were not implicated in ligand recognition, suggesting that conserved residues in the receptor family may maintain the common beta-strand architecture, and variable regions provide a pair of nonidentical motifs for oriented ligand recognition.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach R. R. Initiation of coagulation by tissue factor. CRC Crit Rev Biochem. 1988;23(4):339–368. doi: 10.3109/10409238809082548. [DOI] [PubMed] [Google Scholar]
- Bach R., Gentry R., Nemerson Y. Factor VII binding to tissue factor in reconstituted phospholipid vesicles: induction of cooperativity by phosphatidylserine. Biochemistry. 1986 Jul 15;25(14):4007–4020. doi: 10.1021/bi00362a005. [DOI] [PubMed] [Google Scholar]
- Bass S. H., Mulkerrin M. G., Wells J. A. A systematic mutational analysis of hormone-binding determinants in the human growth hormone receptor. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4498–4502. doi: 10.1073/pnas.88.10.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan J. F. Structural design and molecular evolution of a cytokine receptor superfamily. Proc Natl Acad Sci U S A. 1990 Sep;87(18):6934–6938. doi: 10.1073/pnas.87.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bom V. J., Bertina R. M. The contributions of Ca2+, phospholipids and tissue-factor apoprotein to the activation of human blood-coagulation factor X by activated factor VII. Biochem J. 1990 Jan 15;265(2):327–336. doi: 10.1042/bj2650327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Drake T. A., Ruf W., Morrissey J. H., Edgington T. S. Functional tissue factor is entirely cell surface expressed on lipopolysaccharide-stimulated human blood monocytes and a constitutively tissue factor-producing neoplastic cell line. J Cell Biol. 1989 Jul;109(1):389–395. doi: 10.1083/jcb.109.1.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D. S., MacDonald M. J. Cooperative interaction between factor VII and cell surface-expressed tissue factor. J Biol Chem. 1987 Aug 25;262(24):11692–11698. [PubMed] [Google Scholar]
- Gerads I., Govers-Riemslag J. W., Tans G., Zwaal R. F., Rosing J. Prothrombin activation on membranes with anionic lipids containing phosphate, sulfate, and/or carboxyl groups. Biochemistry. 1990 Aug 28;29(34):7967–7974. doi: 10.1021/bi00486a027. [DOI] [PubMed] [Google Scholar]
- Godowski P. J., Leung D. W., Meacham L. R., Galgani J. P., Hellmiss R., Keret R., Rotwein P. S., Parks J. S., Laron Z., Wood W. I. Characterization of the human growth hormone receptor gene and demonstration of a partial gene deletion in two patients with Laron-type dwarfism. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8083–8087. doi: 10.1073/pnas.86.20.8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida K., Kitamura T., Gorman D. M., Arai K., Yokota T., Miyajima A. Molecular cloning of a second subunit of the receptor for human granulocyte-macrophage colony-stimulating factor (GM-CSF): reconstitution of a high-affinity GM-CSF receptor. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9655–9659. doi: 10.1073/pnas.87.24.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibi M., Murakami M., Saito M., Hirano T., Taga T., Kishimoto T. Molecular cloning and expression of an IL-6 signal transducer, gp130. Cell. 1990 Dec 21;63(6):1149–1157. doi: 10.1016/0092-8674(90)90411-7. [DOI] [PubMed] [Google Scholar]
- Howell R. M., Rezvan H. Circular dichroic, infrared and other studies on the protein component of pig brain thromboplastin. Biochem J. 1980 Aug 1;189(2):209–218. doi: 10.1042/bj1890209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunkapiller T., Hood L. Diversity of the immunoglobulin gene superfamily. Adv Immunol. 1989;44:1–63. doi: 10.1016/s0065-2776(08)60639-2. [DOI] [PubMed] [Google Scholar]
- Kumar A., Blumenthal D. K., Fair D. S. Identification of molecular sites on factor VII which mediate its assembly and function in the extrinsic pathway activation complex. J Biol Chem. 1991 Jan 15;266(2):915–921. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lesk A. M., Chothia C. Evolution of proteins formed by beta-sheets. II. The core of the immunoglobulin domains. J Mol Biol. 1982 Sep 15;160(2):325–342. doi: 10.1016/0022-2836(82)90179-6. [DOI] [PubMed] [Google Scholar]
- Mackman N., Morrissey J. H., Fowler B., Edgington T. S. Complete sequence of the human tissue factor gene, a highly regulated cellular receptor that initiates the coagulation protease cascade. Biochemistry. 1989 Feb 21;28(4):1755–1762. doi: 10.1021/bi00430a050. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H., Fakhrai H., Edgington T. S. Molecular cloning of the cDNA for tissue factor, the cellular receptor for the initiation of the coagulation protease cascade. Cell. 1987 Jul 3;50(1):129–135. doi: 10.1016/0092-8674(87)90669-6. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H., Gregory S. A., Mackman N., Edgington T. S. Tissue factor regulation and gene organization. Oxf Surv Eukaryot Genes. 1989;6:67–84. [PubMed] [Google Scholar]
- Morrissey J. H., Revak D., Tejada P., Fair D. S., Edgington T. S. Resolution of monomeric and heterodimeric forms of tissue factor, the high-affinity cellular receptor for factor VII. Thromb Res. 1988 May 15;50(4):481–493. doi: 10.1016/0049-3848(88)90197-1. [DOI] [PubMed] [Google Scholar]
- Nemerson Y., Gentry R. An ordered addition, essential activation model of the tissue factor pathway of coagulation: evidence for a conformational cage. Biochemistry. 1986 Jul 15;25(14):4020–4033. doi: 10.1021/bi00362a006. [DOI] [PubMed] [Google Scholar]
- Patthy L. Homology of a domain of the growth hormone/prolactin receptor family with type III modules of fibronectin. Cell. 1990 Apr 6;61(1):13–14. doi: 10.1016/0092-8674(90)90208-v. [DOI] [PubMed] [Google Scholar]
- Ploplis V. A., Edgington T. S., Fair D. S. Initiation of the extrinsic pathway of coagulation. Association of factor VIIa with a cell line expressing tissue factor. J Biol Chem. 1987 Jul 15;262(20):9503–9508. [PubMed] [Google Scholar]
- Ploug M., Jensen A. L., Barkholt V. Determination of amino acid compositions and NH2-terminal sequences of peptides electroblotted onto PVDF membranes from tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis: application to peptide mapping of human complement component C3. Anal Biochem. 1989 Aug 15;181(1):33–39. doi: 10.1016/0003-2697(89)90390-4. [DOI] [PubMed] [Google Scholar]
- Rehemtulla A., Pepe M., Edgington T. S. High level expression of recombinant human tissue factor in Chinese hamster ovary cells as a human thromboplastin. Thromb Haemost. 1991 May 6;65(5):521–527. [PubMed] [Google Scholar]
- Rehemtulla A., Ruf W., Edgington T. S. The integrity of the cysteine 186-cysteine 209 bond of the second disulfide loop of tissue factor is required for binding of factor VII. J Biol Chem. 1991 Jun 5;266(16):10294–10299. [PubMed] [Google Scholar]
- Ruf W., Kalnik M. W., Lund-Hansen T., Edgington T. S. Characterization of factor VII association with tissue factor in solution. High and low affinity calcium binding sites in factor VII contribute to functionally distinct interactions. J Biol Chem. 1991 Aug 25;266(24):15719–15725. [PubMed] [Google Scholar]
- Ruf W., Rehemtulla A., Edgington T. S. Antibody mapping of tissue factor implicates two different exon-encoded regions in function. Biochem J. 1991 Sep 15;278(Pt 3):729–733. doi: 10.1042/bj2780729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruf W., Rehemtulla A., Morrissey J. H., Edgington T. S. Phospholipid-independent and -dependent interactions required for tissue factor receptor and cofactor function. J Biol Chem. 1991 Feb 5;266(4):2158–2166. [PubMed] [Google Scholar]
- Sakai T., Lund-Hansen T., Thim L., Kisiel W. The gamma-carboxyglutamic acid domain of human factor VIIa is essential for its interaction with cell surface tissue factor. J Biol Chem. 1990 Feb 5;265(4):1890–1894. [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Smith K. A. The interleukin 2 receptor. Annu Rev Cell Biol. 1989;5:397–425. doi: 10.1146/annurev.cb.05.110189.002145. [DOI] [PubMed] [Google Scholar]
- Thoreau E., Petridou B., Kelly P. A., Djiane J., Mornon J. P. Structural symmetry of the extracellular domain of the cytokine/growth hormone/prolactin receptor family and interferon receptors revealed by hydrophobic cluster analysis. FEBS Lett. 1991 Apr 22;282(1):26–31. doi: 10.1016/0014-5793(91)80437-8. [DOI] [PubMed] [Google Scholar]
- Wildgoose P., Kazim A. L., Kisiel W. The importance of residues 195-206 of human blood clotting factor VII in the interaction of factor VII with tissue factor. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7290–7294. doi: 10.1073/pnas.87.18.7290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A. F., Barclay A. N. The immunoglobulin superfamily--domains for cell surface recognition. Annu Rev Immunol. 1988;6:381–405. doi: 10.1146/annurev.iy.06.040188.002121. [DOI] [PubMed] [Google Scholar]
- Yang J. T., Wu C. S., Martinez H. M. Calculation of protein conformation from circular dichroism. Methods Enzymol. 1986;130:208–269. doi: 10.1016/0076-6879(86)30013-2. [DOI] [PubMed] [Google Scholar]