Abstract
Acetate threshold concentrations were determined under chlororespiring and Fe(III)-reducing conditions for Anaeromyxobacter dehalogenans strain 2CP-C. The acetate threshold concentrations measured were 69 ± 4, 19 ± 8, and <1 nM for chlororespiration, amorphous Fe(III) reduction, and Fe(III) citrate reduction, respectively. Residual ΔG values of −75.4 kJ/mol of electrons for chlororespiration and −41.5 kJ/mol of electrons for amorphous Fe(III) reduction were calculated at the acetate threshold concentration. By comparing threshold concentrations for different metabolisms in a single organism, this study provides insight into the metabolic use of energy under different growth conditions.
Efforts to understand the competitive interactions between different electron-accepting processes have resulted in a thermodynamics-based threshold model, which has been useful in explaining the competition for H2 in anaerobic microbial communities (1, 2, 13, 14). The threshold model predicts that microorganisms that couple oxidative metabolism to an energetically more favorable electron acceptor will achieve a threshold H2 concentration that is lower than that attained by organisms using an electron acceptor of lower energy. Analogously, the ability to compete for acetate should be reflected in the acetate threshold concentrations under different electron-accepting conditions. Thus, the acetate threshold values can be used as a means to elucidate the potential competition for acetate between acetotrophic chlororespiration, Fe(III) reduction, and other acetotrophic processes.
The aim of this study was to measure the acetate threshold concentrations in acetotrophic chlororespiration and dissimilatory Fe(III) reduction by using Anaeromyxobacter dehalogenans strain 2CP-C as a model organism. Acetate threshold concentrations were measured in order to evaluate the energetic consequences of competition between electron acceptors, such as different forms of Fe(III) and chlorophenol. In addition, to gain more insight into the physiological significance of threshold phenomena, an energetic analysis of the culture at the acetate threshold concentration was done. This represents the first such comparative analysis of acetate threshold values in anaerobic respiration.
Acetate threshold concentration measurements.
Decreases in the acetate concentration were monitored in cell suspensions amended with excess 2-chlorophenol (2-CP), Fe(III) citrate or amorphous Fe(III) oxyhydroxide, and a limiting amount of acetate. A. dehalogenans strain 2CP-C (ATCC BAA-259) was routinely grown in a chloride-free mineral salt medium (6, 22). To measure acetate threshold concentrations under chlororespiration conditions, strain 2CP-C cultures were grown with 2,6-dichlorophenol. For iron-reducing conditions, fumarate-grown cells were used since Fe(III) reduction is constitutive in strain 2CP-C (5). Cell suspensions (optical density at 600 nm = 0.15) of 20 ml were distributed into 30-ml serum bottles with 100% N2 headspace. Following the addition of 250 μM 2-CP, 5 mM Fe(III) citrate, or 5 mM amorphous Fe(III) oxyhydroxide, the cultures were then starved by incubating at 30°C for 6 h to exhaust endogenous electron donors until no significant reduction of the respective electron acceptor was observed. Low threshold concentrations of acetate were quantified by using [1-14C]sodium acetate (53 mCi · mmol−1; Sigma-Aldrich) as the electron donor, following a procedure adapted from a previously described method (25). Three independent measurements of the threshold concentration were carried out by adding [14C]acetate to triplicate cultures at three different initial acetate concentrations: 10, 20, and 30 or 40 μM. The residual [14C]acetate remaining in the aqueous solution was quantified by scintillation counting following high-performance liquid chromatography separation (5, 25). Cultures were incubated at 30°C in the dark without mixing. Utilization of acetate was monitored until the rate of consumption approached zero.
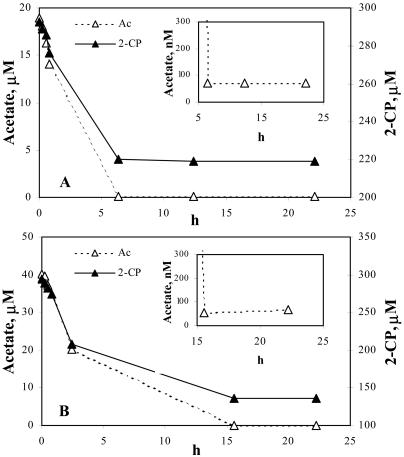
Figure 1 shows the disappearance of [14C]acetate over time with two initial acetate concentrations, 40 and 20 μM. A residual acetate concentration of 69 ± 4 nM was consistently reached in all cell suspensions tested, regardless of the initial acetate concentration. This residual concentration represented the acetate threshold under conditions of chlororespiration by strain 2CP-C. This is more than 2 orders of magnitude lower than the threshold concentrations reported for aceticlastic methanogenesis, which range from 7 to 1,180 μM, depending on the study (8, 9, 17, 26). No consumption of acetate was observed in sterile controls and control cultures without addition of 2-CP.
FIG. 1.
Depletion curves showing levels of acetate and 2-CP present in cell suspensions of strain 2CP-C during acetate threshold measurements, with two initial acetate concentrations of 20 (A) and 40 μM (B). 2-CP was the electron acceptor, and acetate was the electron donor. The insets show the acetate concentration in the vicinity of the threshold value. Note that the scale of the y axis is in nM measurements in the insets. Data points were averages of triplicate cultures. No acetate depletion was observed in control cultures that did not receive 2-CP.
Two forms of ferric iron with significantly different thermodynamic properties, Fe(III) citrate and amorphous Fe(III) oxyhydroxide, had [14C]acetate depletion curves similar to those observed with 2-CP (data not shown). The different Fe(III) forms did, however, have significantly different acetate threshold concentrations. Acetate concentrations dropped below the detection limit of 1 nM with Fe(III) citrate. Thus, the acetate threshold concentration is below 1 nM and cannot be precisely quantified. In contrast, with amorphous Fe(III) oxyhydroxide acetate was depleted to a steady-state concentration of 19 ± 8 nM, which is considerably higher than that observed with soluble Fe(III) as the electron acceptor.
These results support previous observations that dechlorination is suppressed by the presence of soluble Fe(III) species but not by the presence of amorphous Fe(III) oxyhydroxide (5). Nevertheless, the acetate threshold value obtained for the reduction of amorphous Fe(III) oxyhydroxide is of more practical significance due to its broader distribution in natural environments (23). Therefore, it is postulated that insoluble Fe(III) species like those in natural settings would not completely inhibit the coupling of acetate oxidation to reductive dechlorination by A. dehalogenans. It is possible that this will also apply to other chlororespiring organisms capable of using Fe(III) as an alternative electron acceptor (4, 11, 18, 24); however, this needs to be tested.
Energetics at acetate threshold concentration.
To evaluate the residual energy at the acetate threshold concentration for each metabolic reaction (e.g., the reaction with 2-CP shown below), all substrates and products were measured. 2-CP, 2,6-dichlorophenol, and other phenolic compounds, such as the phenol formed in the reaction acetate− + 4[2-CP] + 4[H2O] → 2[HCO3−] + 4[phenol] + 5[H+] + 4[Cl−], were analyzed on a Hewlett-Packard 1090 high-performance liquid chromatograph with a Bio-Rad Hi-Pore reversed-phase column (22). Fe(II) was analyzed using the HCl extraction ferrozine assay as previously described (15, 16). The residual free energy (ΔG) associated with the different acetate threshold concentrations showed that a significant residual negative ΔG remained (Table 1). Notably, the residual ΔG at the acetate threshold was much greater with chlororespiration (−75.4 kJ/mol of electrons) than with Fe(III) reduction (−41.5 kJ/mol of electrons), indicating that a significantly greater amount of energy was required for chlororespiration to proceed.
TABLE 1.
Comparative energetic analysis of chlororespiration and Fe(III) reduction in A. dehalogenans and relevant respiration processes in other anaerobic bacteria
| Process | Organism | Electron donor | Electron acceptora | Electron donor threshold (nM) | Residual ΔG (kJ/mol of electrons) | Reference(s) or source |
|---|---|---|---|---|---|---|
| Chlororespiration | Anaeromyxobacter dehalogenans | Acetate | 2-CP | 69 ± 4 | −75.4 | This study |
| Fe(III) reduction | Anaeromyxobacter dehalogenans | Acetate | Amorphous Fe(III) | 19 ± 8 | −41.5b | This study |
| Chlororespiration | Anaeromyxobacter dehalogenans | H2 | 2-CP | <0.01 | −58.7c,d | 12 |
| Chlororespiration | Desulfitobacterium chlororespirans | H2 | 3Cl-4-HBA | 0.27 | −62.0c | 12, 21 |
| Chlororespiration | Desulfitobacterium sp. strain Viet1 | H2 | PCE | 0.4 | −72.1c | 12 |
| Chlororespiration | Mixed culture | H2 | DCE | 2 | −57.8 | 27 |
| Methanogenesis | Methanothrix soehngenii | Acetate | Acetate | 7,000-69,000 | −3.5c | 9 |
| Methanogenesis | Mixed culture | H2 | CO2 | 11 | −5.8 | 27 |
| Acetogenesis | Acetobacterium woodii | H2 | CO2 | 510 | −6.8 | 19, 27 |
3Cl-4-HBA, 3-chloro-4-hydroxybenzoate; PCE, perchloroethene; DCE, cis-1,2-dichloroethens.
The change in Gibbs free energy in the reduction of amorphous Fe(III) oxyhydroxide was computed with reduction potential data from reference 20.
Calculations of ΔG were based on the following concentrations: acetate and H2, threshold concentrations; chloroethenes, 5 ppm by volume; aromatic species, 1 mM; CH4, 1,000 ppm by volume; Cl−, 1 mM; HCO3−, 30 mM; and pH 7.
Calculated based on a H2 threshold of 0.01 nM.
Although both respiratory processes have substantial available free energy at the acetate threshold concentration, a portion of this could be associated with cell maintenance energy. It would be expected, however, that general maintenance energy costs would be the same for a single population. Thus, it is apparent that there is a higher energy expense associated with chlororespiration than with amorphous Fe(III) reduction. The present study and previous literature suggest that chlororespiration is less efficient than other respiration processes (3, 7). A possible explanation for this inefficiency is that a part of the energy flux is not accounted for by respiration and maintenance requirements alone. One possible additional energy requirement would be the need to keep toxic compounds, such as chlorophenol, out of the cell cytoplasm. The resulting concentration gradient across the cell membrane would require a constant amount of energy to maintain. This chemical gradient is completely analogous to the proton motive force (PMF), with the exception that it cannot be used to drive ATP generation. Bacteria are already known to have PMF-dependent transporters for keeping phenol out of the cell (10), so it would be reasonable that Anaeromyxobacter cells would also adopt a similar strategy with chlorophenol and phenol. In contrast, Fe(III)-reducing cells would not require such a transporter, since amorphous ferric iron does not have the same toxicity. Thus, it is not surprising that the residual ΔG value is lower in the case of Fe(III) reduction.
In support of an energy-dependent transporter associated with chlororespiration, the rate of dechlorination in an acetate-starved culture was shown to be significantly lower than that measured for a nonstarved culture (13.4 versus 52.4 μM/h). Notably, the suppressed dechlorination rates in the acetate-starved cells did not change significantly, even after subsequent repeated additions of 2-CP. Rates managed to only increase gradually (data not shown) but never to levels observed in the nonstarved cultures. These results provide additional evidence of an additional energy expense unrelated to ATP synthesis. It appears that energy is required to maintain chlororespiration activity or prevent toxicity of phenol or chlorophenol, since dechlorination activity is significantly impaired in starved cells.
To determine if the extra energy expense associated with chlororespiration is unique to A. dehalogenans, we examined threshold data reported for chlororespiration and other physiological processes in other microorganisms and calculated the residual ΔG at the threshold electron donor concentration reported (Table 1). Residual ΔG values of more than −58 kJ per mol of electrons transferred are associated with both hydrogenotrophic and acetotrophic chlororespiration, regardless of whether pure cultures or mixed cultures have been evaluated (Table 1). These values are significantly lower than the residual ΔG values found in methanogenesis, which are greater than −10 kJ/mol of electrons. This suggests that energy costs associated with chlororespiration may be higher in general than for other respiration processes and that it might be common to have considerable free energy that is not available for ATP formation.
This study demonstrates the ability of A. dehalogenans to compete for nanomolar-level acetate as the electron donor under different growth conditions. Apparent thermodynamic limitations associated with the different electron acceptors used for growth of A. dehalogenans in this study provide insights into the energetic constraints exerted on these different respiratory processes. Depending on the growth conditions, the difference in the available free energy at the threshold concentration may be accounted for by other energy-requiring processes, such as PMF-dependent transport of toxic compounds, not associated with ATP synthesis. This is the first time such a comparative energetic analysis has been done with different anaerobic metabolisms in a single culture. Future investigations of the biochemistry involved in these processes, chlororespiration and Fe(III) reduction, will reveal how metabolic characteristics affect bioenergetic constraints.
Acknowledgments
We thank Qusheng Jin for reviewing the initial manuscript and providing many helpful suggestions. Joanne Chee-Sanford's review and comments were also greatly appreciated.
REFERENCES
- 1.Conrad, R. 1996. Soil microorganisms as controllers of atmospheric trace gases (H2, CO, CH4, OCS, N2O, and NO). Microbiol. Rev. 60:609-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cord-Ruwisch, R., H.-J. Seitz, and R. Conrad. 1988. The capacity of hydrogenotrophic anaerobic bacteria to compete for traces of hydrogen depends on the redox potential of the terminal electron acceptor. Arch. Microbiol. 149:350-357. [Google Scholar]
- 3.El Fantroussi, S., H. Naveau, and S. N. Agathos. 1998. Anaerobic dechlorinating bacteria. Biotechnol. Prog. 14:167-188. [DOI] [PubMed] [Google Scholar]
- 4.Finneran, K. T., H. M. Forbush, C. V. G. VanPraagh, and D. R. Lovley. 2002. Desulfitobacterium metallireducens sp. nov., an anaerobic bacterium that couples growth to the reduction of metals and humic acids as well as chlorinated compounds. Int. J. Syst. Evol. Microbiol. 52:1929-1935. [DOI] [PubMed] [Google Scholar]
- 5.He, Q., and R. A. Sanford. 2003. Characterization of Fe(III) reduction by chlororespiring Anaeromyxobacter dehalogenans. Appl. Environ. Microbiol. 69:2712-2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He, Q., and R. A. Sanford. 2002. Induction characteristics of reductive dehalogenation in the ortho-halophenol-respiring bacterium, Anaeromyxobacter dehalogenans. Biodegradation 13:306-317. [DOI] [PubMed] [Google Scholar]
- 7.Holliger, C., and W. Schumacher. 1994. Reductive dehalogenation as a respiratory process. Antonie Leeuwenhoek 66:239-246. [DOI] [PubMed] [Google Scholar]
- 8.Jetten, M. S. M., A. J. M. Stams, and A. J. B. Zehnder. 1990. Acetate threshold values and acetate activating enzymes in methanogenic bacteria. FEMS Microbiol. Ecol. 73:339-344. [Google Scholar]
- 9.Jetten, M. S. M., A. J. M. Stams, and A. J. B. Zehnder. 1992. Methanogenesis from acetate: a comparison of the acetate metabolism in Methanothrix soehngenii and Methanosarcina spp. FEMS Microbiol. Rev. 88:181-197. [Google Scholar]
- 10.Kieboom, J., J. J. Dennis, J. A. M. de Bont, and G. J. Zylstra. 1998. Identification and molecular characterization of an efflux pump involved in Pseudomonas putida S12 solvent tolerance. J. Biol. Chem. 273:85-91. [DOI] [PubMed] [Google Scholar]
- 11.Krumholz, L. R. 1997. Desulfuromonas chloroethenica sp. nov. uses tetrachloroethylene and trichloroethylene as electron acceptors. Int. J. Syst. Bacteriol. 47:1262-1263. [Google Scholar]
- 12.Löffler, F. E., J. M. Tiedje, and R. A. Sanford. 1999. Fraction of electrons consumed in electron acceptor reduction and hydrogen thresholds as indicators of halorespiratory physiology. Appl. Environ. Microbiol. 65:4049-4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lovley, D. R. 1985. Minimum threshold for hydrogen metabolism in methanogenic bacteria. Appl. Environ. Microbiol. 49:1530-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lovley, D. R., and S. Goodwin. 1988. Hydrogen concentration as an indicator of the predominant terminal electron-accepting reactions in aquatic sediments. Geochim. Cosmochim. Acta 52:2993-3003. [Google Scholar]
- 15.Lovley, D. R., and E. J. P. Phillips. 1986. Availability of ferric iron for microbial reduction in bottom sediments of the freshwater tidal Potomac River. Appl. Environ. Microbiol. 52:751-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lovley, D. R., and E. J. P. Phillips. 1986. Organic matter mineralization with reduction of ferric iron in anaerobic sediments. Appl. Environ. Microbiol. 51:683-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mladenovska, Z., and B. K. Ahring. 2000. Growth kinetics of thermophilic Methanosarcina spp. isolated from full-scale biogas plants treating animal manures. FEMS Microbiol. Ecol. 31:225-229. [DOI] [PubMed] [Google Scholar]
- 18.Niggemyer, A., S. Spring, E. Stackebrandt, and R. F. Rosenzweig. 2001. Isolation and characterization of a novel As(V)-reducing bacterium: implication for arsenic mobilization and the genus Desulfitobacterium. Appl. Environ. Microbiol. 67:5568-5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peters, V., P. H. Janssen, and R. Conrad. 1998. Efficiency of hydrogen utilization during unitrophic and mixotrophic growth of Acetobacterium woodii on hydrogen and lactate in the chemostat. FEMS Microbiol. Ecol. 26:317-324. [Google Scholar]
- 20.Roden, E. E. 2003. Fe(III) oxide reactivity toward biological versus chemical reduction. Environ. Sci. Technol. 37:1319-1324. [Google Scholar]
- 21.Sanford, R. A., J. R. Cole, F. E. Löffler, and J. M. Tiedje. 1996. Characterization of Desulfitobacterium chlororespirans sp. nov., which grows by coupling the oxidation of lactate to the reductive dechlorination of 3-chloro-4-hydroxybenzoate. Appl. Environ. Microbiol. 62:3800-3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanford, R. A., J. R. Cole, and J. M. Tiedje. 2002. Characterization and description of Anaeromyxobacter dehalogenans gen. nov., sp. nov., an aryl-halorespiring facultative anaerobic myxobacterium. Appl. Environ. Microbiol. 68:893-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Straub, K. L., M. Benz, and B. Schink. 2001. Iron metabolism in anoxic environments at near neutral pH. FEMS Microbiol. Ecol. 34:181-186. [DOI] [PubMed] [Google Scholar]
- 24.Sung, Y., K. M. Ritalahti, R. A. Sanford, J. W. Urbance, S. J. Flynn, J. M. Tiedje, and F. E. Löffler. 2003. Characterization of two tetrachloroethene-reducing, acetate-oxidizing anaerobic bacteria and their description as Desulfuromonas michiganensis sp. nov. Appl. Environ. Microbiol. 69:2964-2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tros, M. E., G. Schraa, and A. J. B. Zehnder. 1996. Transformation of low concentrations of 3-chlorobenzoate by Pseudomonas sp. strain B13: kinetics and residual concentrations. Appl. Environ. Microbiol. 62:437-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Westermann, P., B. K. Ahring, and R. A. Mah. 1989. Threshold acetate concentrations for acetate catabolism by aceticlastic methanogenic bacteria. Appl. Environ. Microbiol. 55:514-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang, Y., and P. L. McCarty. 1998. Competition for hydrogen within a chlorinated solvent dehalogenating anaerobic mixed culture. Environ. Sci. Technol. 32:3591-3597. [Google Scholar]