Abstract
Erwinia chrysanthemi is a phytopathogenic soil enterobacterium closely related to Escherichia coli. Both species respond to hyperosmotic pressure and to external added osmoprotectants in a similar way. Unexpectedly, the pools of endogenous osmolytes show different compositions. Instead of the commonly accumulated glutamate and trehalose, E. chrysanthemi strain 3937 promotes the accumulation of glutamine and α-glucosylglycerate, which is a new osmolyte for enterobacteria, together with glutamine. The amounts of the three osmolytes increased with medium osmolarity and were reduced when betaine was provided in the growth medium. Both glutamine and glutamate showed a high rate of turnover, whereas glucosylglycerate stayed stable. In addition, the balance between the osmolytes depended on the osmolality of the medium. Glucosylglycerate and glutamate were the major intracellular compounds in low salt concentrations, whereas glutamine predominated at higher concentrations. Interestingly, the ammonium content of the medium also influenced the pool of osmolytes. During bacterial growth with 1 mM ammonium in stressing conditions, more glucosylglycerate accumulated by far than the other organic solutes. Glucosylglycerate synthesis has been described in some halophilic archaea and bacteria but not as a dominant osmolyte, and its role as an osmolyte in Erwinia chrysanthemi 3937 shows that nonhalophilic bacteria can also use ionic osmolytes.
Erwinia chrysanthemi is the agent of soft rot disease in a wide range of plants. Various studies suggest that adaptation to osmotic stress is an important factor for the survival of this bacterium in soil (23, 30) and for its pathogenic behavior (17, 25). It is well known that bacterial behavior under hyperosmotic stress is widely influenced by the presence of osmoprotectants in the growth medium. These compounds allow bacteria to strengthen their natural resistance to osmotic stress. Many of them, like glycine betaine, its precursor choline, the imino acid proline, and pipecolic acid, are abundant in plants (21, 43).
Considering that, during pathogenesis, E. chrysanthemi is subjected to osmotic stress in a medium containing osmoprotrectants, most studies focused on identification of the osmoprotectants of E. chrysanthemi and their role in pathogenesis (17, 37). E. chrysanthemi is phylogenetically close to Escherichia coli, so it is not surprising that it behaves like E. coli towards osmoprotectants (19). All osmoprotectants tested so far are actively taken up by two transporters, OusA and OusB, the orthologues of E. coli ProP and ProU, respectively (19), and accumulated at levels equivalent to those previously described in E. coli (18, 19). In addition, they reduce the production of enzymes involved in plant maceration (17).
The pathogenicity of E. chrysanthemi is also linked to its ability to survive in soil during desiccation periods. Osmoprotectants are generally present in small amounts in soil; they are released by plant exudates and the decay of various organisms. Soil microorganisms compete for the same osmoprotectants, so the availability of these compounds for each one remains low. In such conditions, the ability of bacteria to proliferate and survive in osmotically changing media depends highly on their own capacity to face osmotic stress.
Most of the studies on osmotic stress adaptation deal with enterobacteria. A two-step response was described in E. coli and Salmonella enterica serovar Typhimurium (9). The initial response to an osmotic upshift is to rapidly transport and accumulate K+ ions. To counterbalance the potassium charge, glutamate levels increased by de novo synthesis (7, 10). This step allows a transient adaptation, but potassium glutamate enhances intracellular ionic strength proportionally to medium osmolarity, inducing a deleterious effect on cellular metabolism during a prolonged stress (7). Potassium glutamate acts as an intracellular signal of osmotic stress (2, 34), inducing the uptake of osmoprotectants (39) and the synthesis of nonionic organic solutes, which do not alter intracellular macromolecules and replace potassium glutamate (13). Trehalose biosynthesis was described in E. coli and S. enterica serovar Typhimurium strains as related to the strength of the osmotic stress (5, 24). Trehalose not only substitutes for potassium glutamate but also displays protective properties on macromolecules and membranes subjected to an osmotic stress (8, 46). Trehalose can also protect cells against various injuries like desiccation (44) and high temperature (5, 6); it is also essential for survival at low temperatures (28).
Trehalose is the only compatible solute synthesized in enterobacteria, including E. chrysanthemi strains ECC and SR 237 (40). Therefore, trehalose was expected to be a compatible solute in other E. chrysanthemi strains. In most organisms, de novo trehalose synthesis is catalyzed by trehalose-6-phosphate synthase and trehalose-6-phosphate phosphatase, encoded by otsAB in E. coli (27). Two additional ways for trehalose synthesis were described in mycobacteria (11), Corynebacterium glutamicum (45), Sulfolobus solfataricus (31), and Rhizobium spp. (35). One way produces trehalose from maltose by a single transglycosylation catalyzed by the trehalose synthase TreS. The other way catalyzes the conversion of oligo- and polymaltodextrins and glycogen into trehalose in a two-step reaction by maltooligosyltrehalose synthase (TreY) and maltooligosyltrehalose trehalohydrolase (TreZ). Orthologues of otsAB, treS, and treYZ are absent in the E. chrysanthemi strain 3937 genome (https://asap.ahabs.wisc.edu/annotation/php/logon.php). Hence, E. chrysanthemi strain 3937 is unable to synthesize trehalose like other known enterobacteria; however, it may use an unknown pathway. Thus, we analyzed the E. chrysanthemi 3937 endogenous osmolytes and showed that this bacterium does not synthesize trehalose but does synthesize glutamine and α-glucosylglycerate, two uncommon osmolytes in enterobacteria.
MATERIALS AND METHODS
Culture and growth conditions.
E. chrysanthemi 3937 (32) was grown in mineral medium M63 (38) at 30°C. Glucose as a carbon and energy source was added at 10 mM. Low-NH4 medium was M63 containing only 1 mM ammonium sulfate instead of 15 mM. Media of elevated osmotic strength were made by adding NaCl. Their osmotic pressure was determined by measuring the freezing point with an osmometer. The osmoprotectant glycine betaine was supplied at a final concentration of 0.5 mM. Bacterial growth was monitored by optical density measurement at 570 nm.
Extraction of cellular solutes.
Bacterial cells were harvested by centrifugation (8,000 × g, 15 min), and the pellet was extracted twice with 80% (vol/vol) ethanol under vigorous magnetic stirring at room temperature for 15 min. After centrifugation, the combined supernatants were lyophilized. The dried extract was dissolved in water before analysis.
Natural-abundance 13C-nuclear magnetic resonance spectroscopy.
The dried cellular extract prepared from about 2 × 1012 cells (350 mg of protein) was dissolved in 0.8 ml of deuterated water. The natural-abundance 13C nuclear magnetic resonance spectra were recorded in the pulsed Fourier transform mode at an operational frequency of 300 MHz. The spectra of purified glucosylglycerate, hydrolyzed glucosylglycerate fractions, and synthesized glucosylglycerate were performed as for cellular extracts.
Hydrolysis of α-glucosylglycerate.
E. chrysanthemi 3937 was grown to the stationary growth phase in 0.5 M NaCl-M63 medium; bacteria were collected by centrifugation and extracted in 80% (vol/vol) ethanol. The ethanolic extract was partially purified by passage through a cation exchange column of Dowex 50Wx8 in the H+ form. The effluent was concentrated by evaporation and hydrolyzed by 2 N HCl for 75 min at 100°C. The hydrolysate was fractionated with an anion exchange column IRA 400 (HCOO−). The effluent and eluted fractions (in 4 M HCOOH) were concentrated and analyzed by natural-abundance 13C nuclear magnetic resonance spectroscopy.
Chemical synthesis of α-glucosylglycerate.
Glucosylglycerate was synthesized from leucrose according to Hunter et al. (26). Its structure and purity were determined by 13C nuclear magnetic resonance spectroscopy and by chromatography.
Glucosylglycerate isomerization.
Infrared spectra in the attenuated total reflectance mode were performed with a ZnSe crystal at 45°C, and 128 scans were added at 2 cm−1 spectral resolution at room temperature. Samples were dissolved in distilled water prior to acquisition. To determine the anomeric form of the molecule, d-glucose and d-trehalose were analyzed in the same conditions. d-Glucose is present as a mixture of the α and β anomers, whereas d-trehalose exhibits an α anomeric form.
Separation and quantification of osmolytes.
Ethanolic extracts of cells grown in M63 medium containing d-[U-14C]glucose (1.2 MBq mmol−1) were analyzed by a two-dimensional system combining high-voltage electrophoresis (Whatman 3MM paper, 3% formic acid, electric field value of 40 V/cm) as the first dimension and paper chromatography run in butanol-acetic acid-water (12:3:5, vol/vol) as the second dimension. Nonradiolabeled amino acids and glucosylglycerate were added as internal standards. After drying at 60°C, the chromatograms were sprayed with ninhydrin (0.4% in butanol) and heated at 60°C; then they were analyzed by autoradiography. Identified spots were cut out, and their radioactivity was quantified by scintillation counting. A calibration was established between radioactivity and the amount of each compound, taking into account that all the carbon atoms bear an identical radioactivity. Quantification was confirmed for glutamate and glutamine by direct assay as previously described (20). All experiments were repeated three times.
Uptake and catabolism of compatible solutes.
α-[14C]glucosylglycerate was purified from cells grown in 0.5 M NaCl-M63 medium supplemented with d-[U-14C]glucose (1.2 MBq mmol−1). It was used for uptake experiments as previously described (20). The use of α-glucosylglycerate as a growth substrate was analyzed in M63 medium containing 10 mM α-glucosylglycerate as the sole carbon and energy source. Glutamate, glutamine, and betaine uptake and behavior were determined as previously described (19).
[methyl-14C]glycine betaine (2.0 GBq mmol−1 was prepared and its uptake and accumulation were determined as described (17, 20).
RESULTS
Identification of the osmolytes accumulated by E. chrysanthemi grown in high-salt medium.
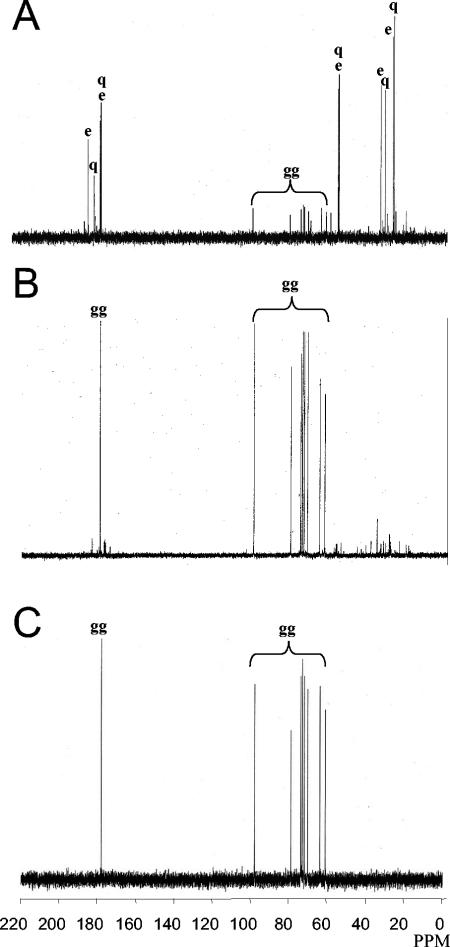
E. chrysanthemi 3937 cells grown in M63 with 0.5 M NaCl were collected in the mid-exponential and in the stationary growth phases. Analysis of 13C nuclear magnetic resonance spectra showed that both extracts contained the same signals, but in different ratios. During the exponential growth phase (Fig. 1A), the main peaks belonged to glutamate and glutamine; other peaks could be attributed collectively to a carbohydrate molecule that did not match the expected trehalose peaks.
FIG. 1.
13C nuclear magnetic resonance spectra of an ethanol extract of E. chrysanthemi 3937 grown in M63 medium with 0.5 M NaCl in the mid-exponential (A) and stationary (B) phases and 13C nuclear magnetic resonance spectrum control of chemically synthesized α-glucosylglycerate (C). The main peaks correspond to carbons of glutamate (e), glutamine (q), and glucosylglycerate (gg).
During the stationary growth phase (Fig. 1B), the peaks of glutamate and glutamine were severely reduced and that of the supposed carbohydrate molecule became the most significant. This compound was purified and hydrolyzed by HCl; the resulting molecules were purified by passage on an anion exchange column. The permeant and eluted fractions were characterized by 13C nuclear magnetic resonance and compared to the nuclear magnetic resonance spectra of commercial molecules to confirm the nature of these two compounds. The permeant fraction spectrum matched α and β glucose and the eluate fraction matched glycerate. These results suggest that the carbohydrate is composed of a glucosyl moiety linked to a glycerate, as in glucosylglycerate. For this molecule, the chemical shifts of carbons with directly bounded protons occurred at 97.6 ppm (C-1′), 72.6 ppm (C-2′), 73.4 ppm (C-3′), 69.7 ppm (C-4′), 71.5 ppm (C-5′), and 60.8 ppm (C-6′) for the glucosyl and at 177.5 ppm (C-1), 78.5 ppm (C-2), and 63.4 ppm (C-3) for the glycerate. Confirmation of the structure as glucosylglycerate was provided by comparison between the 13C chemical shifts of cellular extract and those of glucosylglycerate produced by chemical synthesis (Fig. 1C).
Glucosylglycerate anomerization.
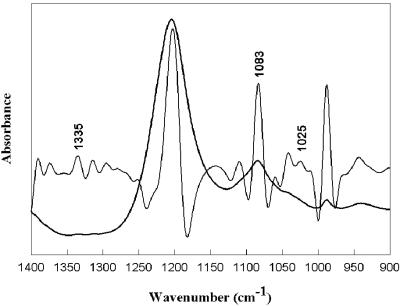
The anomerization of glucosylglycerate purified from E. chrysanthemi was analyzed by infrared spectroscopy. Figure 2 shows the infrared spectrum of the isolated glucosylglycerate along with its inverted second derivative (29) in the 1,400 to 900 cm−1 frequency domain. Peak positions were determined by analyzing the corresponding second derivatives (14) and spectra were band fitted. Indeed, the influence of glucose α- and β-anomerization has been investigated (1). Bands at 1,339 cm−1 and 1,320 cm−1 in distilled water were found to be associated with the α- and β-anomers of glucose, respectively (36). Another study has shown that the bands at 1,020 cm−1 and 1,060 cm−1 in distilled water are associated with the anomeric, C-O stretching of the α and β forms, respectively. d-Glucose in distilled water exhibited a major band at 1,035 cm−1 with a shoulder at 1,066 cm−1 whereas d-trehalose exhibited a band at 1,340 cm−1 and a shoulder at 1,023 cm−1 which is revealed by a second derivative maximum (data not shown). The glucosylglycerate compound (Fig. 2) exhibited a peak at 1,335 cm−1 and a shoulder at 1,025 cm−1 which strongly suggest that it presents the α-anomeric form. Furthermore, a peak at 1,083 cm−1 reinforces this suggestion, since this peak has been assigned to the α-anomeric C-O stretching (36). Hence, it can be assumed that the isolated molecule is α-glucosylglycerate.
FIG. 2.
Infrared spectrum of the isolated glucosylglycerate along with its inverted second derivative (29) in the 1,400 to 900 cm−1 frequency domain.
Evolution of osmolyte content during growth in high-salt medium.
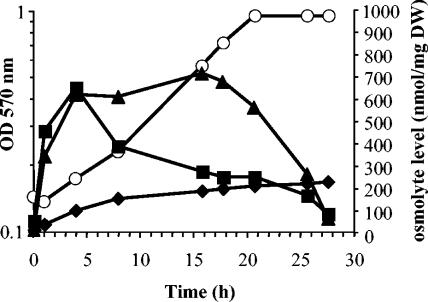
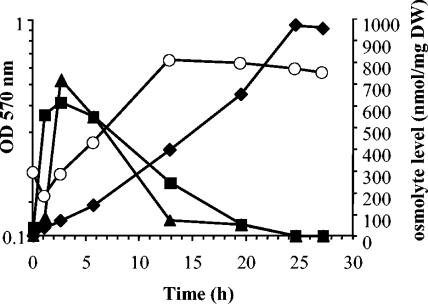
The level of E. chrysanthemi 3937 intracellular osmolytes was analyzed in M63 medium containing 0.5 M NaCl and d-[U-14C]glucose. Accumulated osmolytes were extracted and analyzed by chromatography throughout growth (Fig. 3). The three osmolytes (glutamine, glutamate, and glucosylglycerate) began to accumulate immediately after the osmotic upshift. Glutamate and glutamine reached their maximal level (700 nmol/mg of dry weight) 3 to 4 h after inoculation (Fig. 3). Then the level of glutamate decreased. Glutamine stayed at its maximal level until the late exponential growth phase. Then it decreased throughout the stationary growth phase. Glucosylglycerate appeared as the minor solute during growth; its level increased slowly but continuously to reach a maximum of 220 nmol/mg of dry weight during the stationary growth phase. Since glutamate and glutamine had disappeared by this time, glucosylglycerate became the sole osmolyte.
FIG. 3.
Evolution of osmolyte content during growth. Intracellular levels of glucosylglycerate (diamonds), glutamate (squares), and glutamine (triangles) during growth of E. chrysanthemi 3937 in 0.5 M NaCl-M63 medium with 15 mM (NH4)2SO4. Growth (open circles) was monitored by optical density measurements at 570 nm.
To explain the evolution of osmotic pool components, the turnover of osmolytes was analyzed on cells grown in M63 medium with 0.4 M NaCl and containing [U-14C]glucose. After 6 h of growth, cells were collected and resuspended in the same medium without the radioactive glucose. Cells were collected at regular intervals, and solutes were extracted and analyzed. Spots corresponding to glutamate, glutamine, and glucosylglycerate were excised, and their radioactivity was determined. The radioactivity of glutamate decreased immediately, with a half-life of 23 min. This high turnover is in accordance with its central role in biosynthetic processes. Glutamine radioactivity decreased more slowly than that of glutamate (half-life of 30 min). The labeling of glucosylglycerate remained constant throughout the experiment. Therefore, glucosylglycerate appears to be a stable compound and not an intermediate metabolite. This high metabolic stability could explain why it did not disappear during the stationary growth phase.
Glutamate, glutamine, and glucosylglycerate behave as compatible solutes but are not osmoprotectants.
When a hypoosmotic shock was applied to E. chrysanthemi 3937 cells grown in M63 medium containing 0.5 M NaCl and d-[U-14C]glucose, the three osmolytes were recovered in the surrounding medium whatever the growth phase in which the cells were collected. However, glutamate, glutamine, and glucosylglycerate were not detected in the external medium during growth at high salinity, suggesting that they were not released during growth in hyperosmotic conditions as described for most compatible solutes (10) or that they are efficiently reabsorbed from the medium. To test this hypothesis, 14C-radiolabeled glutamate, glutamine, and glucosylglycerate were added to the E. chrysanthemi growth medium. Glutamate and glutamine were efficiently taken up; in contrast, [U-14C]glucosylglycerate was not taken up by E. chrysanthemi 3937 cells cultivated in M63 medium without or containing 0.4 M NaCl. In addition, E. chrysanthemi 3937 was unable to grow in M63 medium supplemented with α-glucosylglycerate as the sole carbon and energy source. Moreover, when 1 mM glutamate, glutamine, or glucosylglycerate was added to M63 medium with 0.4 M NaCl, none of them improved growth. Therefore, these three compounds are compatible solutes but not osmoprotectants for E. chrysanthemi 3937.
Influence of the medium osmolality on organic solute composition.
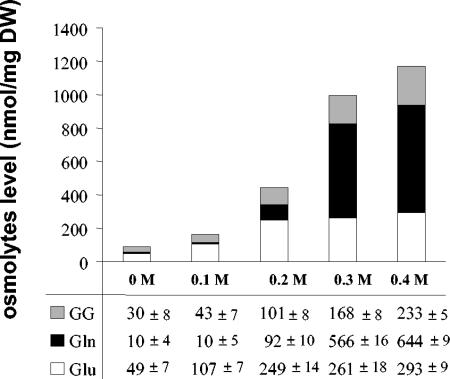
The intracellular osmolyte content was examined in E. chrysanthemi 3937 cells cultivated to the mid-log phase of growth in M63 medium in the presence of d-[U-14C]glucose and various NaCl concentrations (0 to 0.4 M) (Fig. 4). The levels of glutamate, glutamine, and glucosylglycerate increased with medium osmolality; the ratio of these molecules was dependent on the medium osmolality. Thus, glutamate and α-glucosylglycerate were present in similar proportions in M63 medium deprived of salt. When the salt concentration was increased to 0.2 M, glutamate became the main solute. The glutamine content was not significant at low osmolality (0 and 0.1 M NaCl); it began to rise in media containing 0.2 M NaCl. Above this salt concentration, glutamine increased spectacularly reaching more than 60% of the accumulated solutes (Fig. 4). Therefore, during E. chrysanthemi 3937 osmotic adaptation, glutamate and glucosylglycerate are the favorite solutes accumulated at low medium osmolality, whereas glutamine is preferred at high medium osmolality.
FIG. 4.
Influence of medium osmolarity on the ratio of internal solutes. The effect of the NaCl concentration on the intracellular glucosylglycerate (GG, grey), glutamine (Gln, black), and glutamate (Glu, white) content of E. chrysanthemi 3937 grown to exponential phase in M63 medium was determined.
Evolution of osmolyte content during growth in medium of low osmolality.
E. chrysanthemi 3937 cells were grown in M63 medium containing d-[U-14C]glucose. At regular intervals, cells were collected and osmolytes were quantified. Glucosylglycerate and glutamine were estimated to 30 and 10 nmol/mg of dry weight, respectively, throughout growth and did not vary in the stationary growth phase for at least 40 h (data not shown). The glutamate level was estimated to 50 nmol/mg of dry weight all during the exponential growth phase; it decreased to 15 nmol/mg of dry weight during the stationary growth phase. Therefore, glutamine and glucosylglycerate content are not affected by growth phase in M63 medium.
Organic osmolyte ratio depends on medium composition.
Glucose, the carbon and energy source provided at 10 mM in M63, constitutes the growth-limiting nutrient rather than nitrogen. Glutamate and glutamine need both nitrogen and carbon for their biosynthesis, whereas glucosylglycerate is built only from carbon. Therefore, the balance between these two groups of osmolytes could depend on the C/N ratio of the growth medium. To analyze this point, E. chrysanthemi 3937 was grown in modified M63 medium containing 0.5 M NaCl, 1 mM (NH4)2SO4 and d-[U-14C]glucose as the carbon and energy source. The results presented in Fig. 5 are compared to those obtained previously with cells grown in M63 medium containing 15 mM (NH4)2SO4 (Fig. 3). Lowering the level of nitrogen in the medium did not affect the growth rate but, as expected, reduced the growth yield by about 30%. During the first hours of growth, the osmolyte pool composition was identical in low- and high-nitrogen medium (Fig. 3 and 5). Glutamate and glutamine were the main solutes accumulated immediately after the upshock. Glutamate had the same accumulation profile whatever the medium used (Fig. 3 and 5). In contrast, glutamine accumulation depended on the nitrogen concentration and was only transient in low-nitrogen media. Its level decreased simultaneously with that of glutamate during the early steps of exponential growth (Fig. 5). The glucosylglycerate content was maintained at the same level in high and low nitrogen throughout the glutamate and glutamine accumulation phase (Fig. 5).
FIG. 5.
Glucosylglycerate accumulation is favored under NH4 limitation. Intracellular levels of glucosylglycerate (diamonds), glutamate (squares), and glutamine (triangles) were determined during growth of E. chrysanthemi 3937 in M63 medium with 0.5 M NaCl and containing only 1 mM (NH4)2SO4. Growth (open circles) was monitored by optical density measurements at 570 nm.
In contrast, the decrease in glutamine and glutamate content was accompanied by a spectacular increase in the glucosylglycerate level. It became by far the main solute detected at the end of exponential growth, and its level was greater than that observed in high-nitrogen medium (Fig. 5). Its concentration went on increasing after growth arrest, reaching 1,000 nmol/mg of dry weight (Fig. 5), versus 220 nmol/mg of dry weight in high-nitrogen medium (Fig. 3).
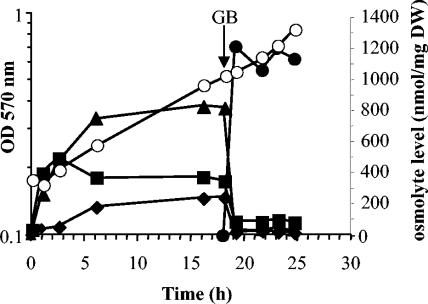
Glycine betaine suppresses the accumulation of glutamate, glutamine, and glucosylglycerate.
In all bacteria, external compatible solutes (i.e., osmoprotectants) replace newly synthesized internal ones (9). Thus, to examine the implication of glutamate, glutamine, and α-glucosylglycerate in osmoprotection, their behavior was followed after addition of 0.5 mM glycine betaine to M63 medium containing 0.5 M NaCl. The levels of glutamate, glutamine, and glucosylglycerate were analyzed before and after the addition of glycine betaine during the exponential growth phase (Fig. 6). Glycine betaine was readily accumulated at a level identical to that reported previously (19). Glycine betaine accumulation abolished the accumulation of glutamate, glutamine, and glucosylglycerate. This result confirms that glutamate, glutamine, and glucosylglycerate are involved in osmoprotection and constitute the internal osmolyte pool of E. chrysanthemi 3937 cells.
FIG. 6.
Suppression of endogenous solute accumulation. Intracellular levels of glucosylglycerate (diamonds), glutamate (squares), glutamine (triangles), and glycine betaine (closed circles) during growth of E. chrysanthemi 3937 in M63 medium with 0.5 M NaCl and 15 mM (NH4)2SO4 was determined; 500 μM [methyl-14C]glycine betaine was added after 18 h of growth. Growth (open circles) was monitored by optical density measurements at 570 nm.
DISCUSSION
Bacteria subjected to a hyperosmotic stress rapidly lose intracellular water and go on to the plasmolysis state. In response to this stress, they must accumulate intracellular solutes, allowing maintenance of their turgor pressure (10). However, not all bacteria produce the same compatible solutes. Halophilic bacteria are able to accumulate salts at high levels and charged organic osmolytes (42). Nonhalophilic bacteria such as E. chrysanthemi cannot support excess intracellular ionic strength (13). Therefore, they accumulate compounds that are not electrically charged at their physiological pH (47).
The analysis of the osmolyte content of E. chrysanthemi 3937 cells cultivated in high-salt media revealed the presence of glutamate, glutamine, and α-glucosylglycerate. Among these three compounds, only glutamine is neutral at physiological pH, which could explain why this amino acid constitutes the main solute accumulated at high osmolality. Glutamine has never been described as a compatible solute in enterobacteria; it is not a common osmolyte in bacteria. It was described as the major endogenous osmolyte accumulated in Corynebacterium spp. (15), and its β form was found in the compatible solute pool of halophilic methanogenic archaea (33).
The other two solutes accumulated by E. chrysanthemi (glutamate and glucosylglycerate) are charged at physiological pH and should increase the ionic strength of the cytoplasm. They represent the only accumulated solutes at low osmolarity and should respond to the entry of potassium into the cell (13), acting as a K+ counterion to maintain electroneutrality. E. chrysanthemi 3937 did not accumulate trehalose like other enterobacteria (5, 24) but glucosylglycerate mainly during stationary growth phase. This absence of trehalose accumulation is intriguing, because this compound was already reported for other E. chrysanthemi strains (40). Accordingly, neither the otsAB genes commonly found in enterobacteria nor the treS and treYZ trehalose biosynthetic genes found in other bacteria (11, 45) were detected on the E. chrysanthemi strain 3937 genome.
E. chrysanthemi strain 3937 is totally without trehalose biosynthetic pathways. Glucosylglycerate, a solute initially identified in Methanohalophilus portucalensis strain FDF1 (41), was recently reported in Halomonas elongata CHR63 (4). In comparison with these strains, the glucosylglycerate content is greater in E. chrysanthemi. Glucosylglycerate is a stable molecule in E. chrysanthemi, while it was considered a metabolic intermediate in Methanohalophilus portucalensis (41), suggesting that in E. chrysanthemi this molecule functions solely as an osmolyte. Glucosylglycerate has only been described in two halophilic strains and so far is not known as a compatible solute in enterobacteria. The presence of this molecule in the endogenous osmolyte pool of E. chrysanthemi strain 3937 is surprising because this bacterium is phylogenetically distant from these halophilic strains. However, glucosylglycerate responds as a classical compatible solute in that its accumulation increased proportionally with the medium osmolality and was suppressed by the addition of the osmoprotectant glycine betaine.
The ratio of E. chrysanthemi 3937 osmolytes varied with medium composition and growth phase. Bacteria able to synthesize various osmolytes adapt their ratio to medium composition; in Ectothiorodospira species, trehalose constitutes a minor solute in high-nitrogen media but becomes the preferred solute under nitrogen-limited conditions (16). Similarly, the ratio of the various osmolytes of Methanohalophilus portucalensis is highly dependent on the growth substrate (41). Glucosylglycerate is the major solute of E. chrysanthemi 3937 in the stationary growth phase; an identical situation was reported for Halomonas elongata, which also synthesizes glucosylglycerate at this stage of growth (4). It is also the case for trehalose, the most frequent organic solute found in bacteria (20, 22).
Glucosylglycerate was believed to be accumulated only by halophilic organisms, while its counterpart glucosylglycerol is accumulated by nonhalophilic ones (42). The present study shows that this distinction is not always true.
Compatible solutes are compounds which are restricted to a small family of molecules that were selected during evolution because they do not affect cellular metabolism (47). While their primary role is to recover turgor, compatible solutes were also shown to improve resistance against other stresses, including desiccation (44) and heat treatment (5, 6); they may act as chemical chaperones favoring native protein folding both in vitro and in vivo (3, 12). This role for glutamate has already been reported (12); such a protective effect could be expected for glutamine and glucosylglycerate and must be confirmed in the future.
Acknowledgments
We thank O. Sire from L2PIC of UBS for determination of infrared glucosylglycerate spectra. We are grateful to S. Georgeault, C. Monnier, M. C. Savary, and M. Uguet for excellent technical assistance and to C. Staines and J. P. Besnard for improving the English usage of the manuscript.
This work was supported by the Ministère de la Recherche and by the CNRS.
REFERENCES
- 1.Back, D. M., and P. L. Polavarapu. 1983. Fourier-transform infrared spectroscopy of sugars. Structural changes in aqueous solutions. Carbohydr. Res. 121:308-311. [DOI] [PubMed] [Google Scholar]
- 2.Booth, I. R., and C. F. Higgins. 1990. Enteric bacteria and osmotic stress: intracellular potassium glutamate as a secondary signal of osmotic stress? FEMS Microbiol. Rev. 75:239-246. [DOI] [PubMed] [Google Scholar]
- 3.Bourot, S., O. Sire, A. Trautwetter, T. Touze, L. F. Wu, C. Blanco, and T. Bernard. 2000. Glycine betaine-assisted protein folding in a lysA mutant of Escherichia coli. J. Biol. Chem. 275:1050-1056. [DOI] [PubMed] [Google Scholar]
- 4.Canovas, D., N. Borges, C. Vargas, A. Ventosa, J. J. Nieto, and H. Santos. 1999. Role of N-γ-acetyldiaminobutyrate as an enzyme stabilizer and an intermediate in the biosynthesis of hydroxyectoine. Appl. Environ. Microbiol. 65:3774-3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Canovas, D., S. A. Fletcher, M. Hayashi, and L. N. Csonka. 2001. Role of trehalose in growth at high temperature of Salmonella enterica serovar Typhimurium. J. Bacteriol. 183:3365-3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carninci, P., Y. Nishiyama, A. Westover, M. Itoh, S. Nagaoka, N. Sasaki, Y. Okazaki, M. Muramatsu, and Y. Hayashizaki. 1998. Thermostabilization and thermoactivation of thermolabile enzymes by trehalose and its application for the synthesis of full length cDNA. Proc. Natl. Acad. Sci. USA 95:520-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cayley, S., B. A. Lewis, H. J. Guttman, and M. T. Record, Jr. 1991. Characterization of the cytoplasm of Escherichia coli K-12 as a function of external osmolarity. Implications for protein-DNA interactions in vivo. J. Mol. Biol. 222:281-300. [DOI] [PubMed] [Google Scholar]
- 8.Crowe, J. H., J. F. Carpenter, and L. M. Crowe. 1998. The role of vitrification in anhydrobiosis. Annu. Rev. Physiol. 60:73-103. [DOI] [PubMed] [Google Scholar]
- 9.Csonka, L. N. 1989. Physiological and genetic responses of bacteria to osmotic stress. Microbiol. Rev. 53:121-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Csonka, L. N., and W. Epstein. 1996. Osmoregulation, p. 1210-1223. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasaki, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 11.De Smet, K. A., A. Weston, I. N. Brown, D. B. Young, and B. D. Robertson. 2000. Three pathways for trehalose biosynthesis in mycobacteria. Microbiology 146:199-208. [DOI] [PubMed] [Google Scholar]
- 12.Diamant, S., D. Rosenthal, A. Azem, N. Eliahu, A. P. Ben-Zvi, and P. Goloubinoff. 2003. Dicarboxylic amino acids and glycine-betaine regulate chaperone-mediated protein-disaggregation under stress. Mol. Microbiol. 49:401-410. [DOI] [PubMed] [Google Scholar]
- 13.Dinnbier, U., E. Limpinsel, R. Schmid, and E. P. Bakker. 1988. Transient accumulation of potassium glutamate and its replacement by trehalose during adaptation of growing cells of Escherichia coli K-12 to elevated sodium chloride concentrations. Arch. Microbiol. 150:348-357. [DOI] [PubMed] [Google Scholar]
- 14.Dong, A., P. Huang, and W. S. Caughey. 1990. Protein secondary structures in water from second-derivative amide I infrared spectra. Biochemistry 29:3303-3308. [DOI] [PubMed] [Google Scholar]
- 15.Frings, E., H. J. Kunte, and E. A. Galinski. 1993. Compatible solutes in representatives of the genera Brevibacterium and Corynebacterium: occurrence of tetrahydropyrimidines and glutamine. FEMS Microbiol. Lett. 109:25-32. [Google Scholar]
- 16.Galinski, E. A., and R. M. Herzog. 1990. The role of trehalose as a substitute for nitrogen-containing compatible solutes (Ectothiorhodospira halochloris). Arch. Microbiol. 153:607-613. [Google Scholar]
- 17.Gouesbet, G., M. Jebbar, S. Bonnassie, N. Hugouvieux-Cotte-Pattate, S. Himdi-Kabbab, and C. Blanco. 1995. Erwinia chrysanthemi at high osmolarity: influence of osmoprotectants on growth and pectate lyase production. Microbiology 141:1407-1412. [DOI] [PubMed] [Google Scholar]
- 18.Gouesbet, G., M. Jebbar, R. Talibart, T. Bernard, and C. Blanco. 1994. Pipecolic acid is an osmoprotectant for Escherichia coli taken up by the general osmoporters ProU and ProP. Microbiology 140:2415-2422. [DOI] [PubMed] [Google Scholar]
- 19.Gouesbet, G., A. Trautwetter, S. Bonnassie, L. F. Wu, and C. Blanco. 1996. Characterization of the Erwinia chrysanthemi osmoprotectant transporter gene ousA. J. Bacteriol. 178:447-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gouffi, K., V. Pichereau, J. P. Rolland, D. Thomas, T. Bernard, and C. Blanco. 1998. Sucrose is a nonaccumulated osmoprotectant in Sinorhizobium meliloti. J. Bacteriol. 180:5044-5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanson, A. D., and C. E. Nelsen. 1978. Betaine accumulation and 14C-formate metabolism in water-stressed barley leaves. Plant Physiol. 62:305-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hengge-Aronis, R., W. Klein, R. Lange, M. Rimmele, and W. Boos. 1991. Trehalose synthesis genes are controlled by the putative sigma factor encoded by rpoS and are involved in stationary-phase thermotolerance in Escherichia coli. J. Bacteriol. 173:7918-7924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoppe, P. E., and A. Kelman. 1969. Bacterial top and stalk rot disease of corn in Wisconsin. Plant. Dis. Rep. 53:66-70. [Google Scholar]
- 24.Howells, A. M., H. L. Bullifent, K. Dhaliwal, K. Griffin, A. Garcia de Castro, G. Frith, A. Tunnacliffe, and R. W. Titball. 2002. Role of trehalose biosynthesis in environmental survival and virulence of Salmonella enterica serovar Typhimurium. Res. Microbiol. 153:281-287. [DOI] [PubMed] [Google Scholar]
- 25.Hugouvieux-Cotte-Pattate, N., H. Dominguez, and J. Robert-Baudouy. 1992. Environmental conditions affect transcription of pectinase genes of Erwinia chrysanthemi 3937. J. Bacteriol. 174:7807-7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hunter, B. K., S. L. Mowbray, and D. J. Walton. 1979. A compound representing the d-glycerate terminus of the methylglucose-containing polysaccharide of Mycobacterium smegmatis. Biochemistry 18:4458-4465. [DOI] [PubMed] [Google Scholar]
- 27.Kaasen, I., P. Falkenberg, O. B. Styrvold, and A. R. Strøm. 1992. Molecular cloning and physical mapping of the otsBA genes, which encode the osmoregulatory trehalose pathway of Escherichia coli: evidence that transcription is activated by katF (AppR). J. Bacteriol. 174:889-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kandror, O., A. DeLeon, and A. L. Goldberg. 2002. Trehalose synthesis is induced upon exposure of Escherichia coli to cold and is essential for viability at low temperatures. Proc. Natl. Acad. Sci. USA 99:9727-9732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kauppinen, J. K., D. J. Moffatt, H. H. Mantsch, and D. G. Cameron. 1981. Fourrier self-deconvolution: methods for resolving intrasically overlapped bonds. Appl. Spectrosc. 35:271-276. [Google Scholar]
- 30.Kelman, A., L. H. Person, and T. T. Herbert. 1957. A bacterial stalk rot of irrigated corn in North Carolina. Plant. Dis. Rep. 41:798-802. [Google Scholar]
- 31.Kobayashi, K., M. Kato, Y. Miura, M. Kettoku, T. Komeda, and A. Iwamatsu. 1996. Gene cloning and expression of new trehalose-producing enzymes from the hyperthermophilic archaeum Sulfolobus solfataricus KM1. Biosci. Biotechnol. Biochem. 60:1882-1885. [DOI] [PubMed] [Google Scholar]
- 32.Kotoujansky, A., M. Lemattre, and P. Boistard. 1982. Utilization of a thermosensitive episome bearing transposon Tn10 to isolate Hfr donor strains of Erwinia carotovora subsp. chrysanthemi. J. Bacteriol. 150:122-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lai, M. C., K. R. Sowers, D. E. Robertson, M. F. Roberts, and R. P. Gunsalus. 1991. Distribution of compatible solutes in the halophilic methanogenic archaebacteria. J. Bacteriol. 173:5352-5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee, S. J., and J. D. Gralla. 2004. Osmo-regulation of bacterial transcription via poised RNA polymerase. Mol. Cell 14:153-162. [DOI] [PubMed] [Google Scholar]
- 35.Maruta, K., K. Hattori, T. Nakada, M. Kubota, T. Sugimoto, and M. Kurimoto. 1996. Cloning and sequencing of trehalose biosynthesis genes from Rhizobium sp. M-11. Biosci. Biotechnol. Biochem. 60:717-720. [DOI] [PubMed] [Google Scholar]
- 36.Michalska, D. F., D. M. Back, and P. L. Polavarapu. 1984. Identification of the characteristic, anomeric bands in the infrared spectrum of lyxose in aqueous solution. Carbohydr. Res. 131:29-38. [DOI] [PubMed] [Google Scholar]
- 37.Midenhall, J. P., B. A. Prior, and L. A. Trollope. 1981. Water relations of Erwinia chrysanthemi: growth and extracellular pectic acid lyase production. J. Gen. Microbiol. 129:3019-3025. [Google Scholar]
- 38.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 39.Prince, W. S., and M. R. Villarejo. 1990. Osmotic control of proU transcription is mediated through direct action of potassium glutamate on the transcription complex. J. Biol. Chem. 265:17673-17679. [PubMed] [Google Scholar]
- 40.Prior, B. A., E. Hewitt, E. V. Brandt, A. Clarke, and J. P. Mildenhall. 1994. Growth, pectate lyase production and solute accumulation by Erwinia chrysanthemi under osmotic stress: effect of osmoprotectants. J. Appl. Bacteriol. 77:433-439. [Google Scholar]
- 41.Robertson, D. E., M. C. Lai, R. P. Gunsalus, and M. F. Roberts. 1992. Composition, variation, and dynamics of major osmotic solutes in Methanohalophilus sp. strain FDF1. Appl. Environ. Microbiol. 58:2438-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roesser, M., and V. Muller. 2001. Osmoadaptation in bacteria and archaea: common principles and differences. Environ. Microbiol. 3:743-754. [DOI] [PubMed] [Google Scholar]
- 43.Storey, R., and R. G. Wyn Jones. 1977. Quaternary ammonium compounds in plants in relation to salt resistance. Phytochemistry 16:447-453. [Google Scholar]
- 44.Welsh, D. T., and R. A. Herbert. 1999. Osmotically induced intracellular trehalose, but not glycine betaine accumulation promotes desiccation tolerance in Escherichia coli. FEMS Microbiol. Lett. 174:57-63. [DOI] [PubMed] [Google Scholar]
- 45.Wolf, A., R. Krämer, and S. Morbach. 2003. Three pathways for trehalose metabolism in Corynebacterium glutamicum ATCC 13032 and their significance in response to osmotic stress. Mol. Microbiol. 49:1119-1134. [DOI] [PubMed] [Google Scholar]
- 46.Xie, G., and S. N. Timasheff. 1997. The thermodynamic mechanism of protein stabilization by trehalose. Biophys. Chem. 64:25-43. [DOI] [PubMed] [Google Scholar]
- 47.Yancey, P. H., M. E. Clark, S. C. Hand, R. D. Bowlus, and G. N. Somero. 1982. Living with water stress: evolution of osmolyte systems. Science 217:1214-1222. [DOI] [PubMed] [Google Scholar]