Abstract
A freeze-dried human serum preparation containing immunoglobulin G (IgG) to Toxoplasma gondii was assessed for its suitability as an international reference reagent in an international collaborative study by 24 laboratories from 17 countries. This candidate standard was compared with the third international standard (IS) for human anti-Toxoplasma serum, TOXM, with the previous second IS, TOXS, and with a range of other serum samples. Samples were tested with the Sabin-Feldman dye test and a range of agglutination assays and enzyme immunoassays. This study emphasizes the need for appropriate standards if intermethod agreement of estimates is to be achieved. On the basis of the results of this study, the preparation was established by the World Health Organization as the first IS for human anti-Toxoplasma IgG, with an assigned potency of 20 IU per ampoule of total anti-Toxoplasma antibodies.
The third international standard (IS) for human anti-Toxoplasma serum (TOXM) was established by the Expert Committee of Biological Standardization (ECBS) of the World Health Organization in 1994 and assigned a potency of 1,000 IU on the basis of its comparison with the second IS TOXS in an international collaborative study (2). Stocks of TOXM are declining, and a new preparation is needed. TOXM is used by reference laboratories, diagnostic laboratories, and manufacturers of diagnostic immunoassays. The assigned potency of TOXM is for the total amount of specific antibodies and was based on results of the Sabin-Feldman test (dye test). This test is a complement-mediated cell-killing assay, utilizing toxoplasma trophozoites, and does not distinguish between immunoglobulin classes. Because of developments in assays for anti-Toxoplasma antibodies, the activities of specific immunoglobulin G (IgG), IgM, and IgA antibodies were calculated for TOXM (2). However, the ECBS did not assign unitages for these antibodies and suggested that different and more specific reference materials may be required (5). One of the proposals of the ECBS was the use of late-stage convalescent-phase sera as a source for the candidate standard. These sera contain specific IgG but lack specific IgM, so that even in assays that detect multiple immunoglobulin classes, the component of the assay that detects IgG can be compared directly to assays that detect IgG only. Such immunoglobulin-specific assays are now used commonly in many diagnostic laboratories. Further, specific IgM and IgG antibodies interact and may cause false-positive or false-negative results in some immunoassays that measure specific immunoglobulin classes (3). Although the dye test is an in-house test and is generally restricted to reference or specialist laboratories, it is considered the reference method for the serodiagnosis of toxoplasmosis (4). The test is able to detect antibody at low levels and is also used to confirm results of commercial immunoassays, which often incorporate different antigen preparations. The potential disadvantage caused by the presence of multiple specific antibody classes and the recommendation of the ECBS that attempts should be made to rectify inconsistencies in the comparison of sera led to a replacement for TOXM based on a pool of human sera that have a lower level of specific IgG than TOXM but lack specific IgM. The level of IgG falls within the linear range of most assays and will assist laboratories in determining whether their assays can distinguish between background and diagnostic levels of IgG. This degree of sensitivity in quantifying and monitoring the IgG response associated with acute toxoplasmosis is important for supporting appropriate clinical management.
An international collaborative study was carried out to assess the suitability of this candidate preparation both in calibrating IgG assays and as a replacement for TOXM. The aims of the study were (i) to assess the suitability of the candidate as a reference standard for complement-mediated cell-killing assays and to calibrate the candidate in terms of the current IS TOXM by dye test, (ii) to confirm the continuity of unitage of the candidate with the second IS TOXS, (iii) to assess the reactivity of the candidate and the third IS TOXM in various assays currently in use, and (iv) to assess the performance of the candidate and high- and low-titer sera in these assays.
MATERIALS AND METHODS
Participants.
Twenty-four laboratories from 17 countries, including national reference laboratories, took part in the study and are listed in Acknowledgments. Throughout the study, participating laboratories were identified by a randomly assigned code number to maintain confidentiality.
Samples.
Each participant received two sets comprising nine coded ampoules and an ampoule each of TOXM and TOXS. The study codes, National Institute for Biological Standards and Control (NIBSC) codes, the assigned unitage, and a brief characterization of the samples are given in Table 1. The samples tested negative for antibodies to human immunodeficiency virus types 1 and 2, hepatitis C virus, and hepatitis B virus surface antigen. Duplicates of three samples were included in the sample set to provide an independent measure of intra-assay variability. All samples were distributed as lyophilized preparations at room temperature by courier. The progress of delivery was tracked online via the courier's home page on the worldwide web.
TABLE 1.
Samples used in the collaborative study
| Study code(s) | NIBSC code | Sample description | Unitage by dye test (IU ml−1)a |
|---|---|---|---|
| TOXM | TOXM | Third IS preparation, 1994 | 1,000 |
| TOXS | TOXS | Second IS preparation, 1980 | 1,000b |
| B, F | 01/600 | Candidate standard preparation, representing 23 pooled sera | 64 |
| H | 01/576 | IgG-negative sample, representing 7 pooled sera | <2 |
| C | 97/682 | Low-IgG-positive serum taken from 1 donor | 32 |
| D | 98/706 | IgG-positive serum taken from 1 donor | 64 |
| E, G | 83/571 | Normal serum taken from 1 donor | 32 |
| A, I | 82/585 | Normal serum taken from 1 donor | 16 |
Unitage of samples A to I was established in laboratory 7.
TOXS was suspended in 2 ml of distilled water; other samples were suspended in 1 ml of distilled water.
Participants were requested to reconstitute samples according to the instructions supplied with the vials and to test these within 7 days. Samples were reconstituted with 1 ml of sterile distilled water, with the exception of TOXS, which was reconstituted in 2 ml of sterile distilled water and stored at 2 to 8°C until further use. Further dilutions of the reconstituted samples with the appropriate assay buffer solutions were made by participants to ensure that the concentrations used covered the sensitivity range of the assay. Each set of samples was tested in duplicate on two different days.
Characterization of proposed IS preparation 01/600.
Informed consent was obtained from 23 individuals to retain serum. Fifteen samples were collected at Swansea Hospital (Swansea, United Kingdom), and eight samples were collected and supplied by the Blood Transfusion Service (Birmingham, United Kingdom). The average volume was 198 ± 68 ml per sample. Anti-Toxoplasma IgM titers were determined by immunosorbent agglutination assay (ISAGA; BioMerièux). All samples were positive by the dye test but found to be negative for IgM. Samples were stored at −20°C. At NIBSC, samples were pooled (4.6 liters) and dispensed in 1-ml aliquots into glass vials coded 01/600. The mean fill weight for 42 vials was 1.0063 g, with a coefficient of variation of 0.06%. On the same day, freeze-drying under vacuum conditions started, and the process was completed after 4 days. Vials were backfilled with pure N2 (moisture content, <10 ppm). Residual moisture was measured by the Karl-Fischer method in 6 vials and ranged from 0.1838 to 0.1924%. Eighty-two vials were rejected during the production process, 150 vials were held for accelerated degradation studies, and 3,979 vials were stored at −20°C. These are available for distribution by NIBSC.
Diagnostic assays.
The assays used in this study are summarized in Table 2. Two types of assay were distinguished. Titration methods yield an end point titer, and participants who used this type of assay were requested to report four titers in total for each sample. Enzyme immunoassays (EIAs) produce a numerical value such as an absorbance or a fluorescence value. Participants who used an EIA were requested to report four sequential dilutions (e.g., 1/2, 1/4, 1/8, and 1/16) for each sample, and thus, 16 data points were obtained for each sample. Participants using in-house tests followed standard procedures, and participants who used commercially available tests followed procedures described by the manufacturer. A brief description of the procedures accompanied the raw data, which were returned to NIBSC for analysis.
TABLE 2.
Assays used in laboratories that participated in this study
| Test format | Test type | Manufacturer and name of test | Laboratory code(s)a | Total no. of assays |
|---|---|---|---|---|
| Titration assay | Dye test | In-house | 2, 6, 7, 8, 10, 11, 17, 18, 21, 23 | 10 |
| Immunofluorescence assay | BioMérieux IIFT or in-house | 1, 2, 13, 14 | 4 | |
| Agglutination assay | Eiken toxoreagent or in-house | 3, 16, 17 | 3 | |
| Complement fixation assay | In-house | 12 | 1 | |
| EIA | Enzyme-linked immunosorbent assay or enzyme fluorescence assay | BioMérieux VIDAS Toxo IgG II | 1, 2, 11, 14, 16, 18, 20, 22, 24 | 9 |
| Biorad Platelia Toxo IgG TMB | 4, 9, 12, 13 | 4 | ||
| In-house | 3, 8, 15 (×2) | 4 | ||
| Behring Enzygnost Toxoplasmosis IgG | 5 | 1 | ||
| Abbott IMX Toxo IgG EIA | 12 | 1 | ||
| DiaSorin ETI- TOXO-G Plus | 19 | 1 |
The majority of laboratories carried out more than one assay.
Data analysis.
The results for all samples were calculated in international units per milliliter relative to TOXM and TOXS. For titration methods, end point titers for all samples were compared to those obtained for TOXM and TOXS, both known to contain 1,000 IU ml−1. For EIAs, assays were analyzed by the principles of multiple-parallel line bioassay, with the transformed assay response compared to the log concentration (1). Responses were transformed to percentages relative to the estimated upper and lower limits of the dose-response curve and weighted regression of logit response on log concentration was used. All mean potencies shown in this report are unweighted geometric mean potencies.
RESULTS
A summary of the results obtained from titration methods is given in Table 3, and a summary of EIA results is given in Table 4. Preparation 01/576 (coded H), which did not contain specific IgG, was reported negative by all laboratories, and no results are presented for this sample. Lab 24 (BioMérieux VIDAS Toxo IgG II) did not report results in a format that could be converted to international units per milliliter, so results from this laboratory do not contribute to the summary.
TABLE 3.
Summary of results by titration method
| Sample | Geometric mean potency (IU ml−1) (95% confidence limits) calculated relative to TOXM or TOXS by: |
|||||||
|---|---|---|---|---|---|---|---|---|
| Dye test (n = 10) |
Immunofluorescence assay (n = 4) |
Agglutination assay (n = 3) |
Complement fixation assay (n = 1) |
|||||
| TOXM | TOXS | TOXM | TOXS | TOXM | TOXS | TOXM | TOXS | |
| TOXS | 927.2 (781.1-1,100.7) | 1,033.0 (840.8-1,269.1) | 2,181.0 (1,003.8-4,738.9) | 707.1 | ||||
| TOXM | 1,078.5 (908.6-1,280.2)a | 968.0 (787.9-1,189.3) | 458.5 (211.0-996.3) | 1,414.2 | ||||
| 01/600 (B and F) | 18.0 (10.9-29.8) | 19.5 (11.3-33.6) | 37.3 (10.8-128.8) | 36.1 (12.3-106.3) | 131.7 (23.3-745.9) | 60.4 (23.1-158.3) | 11.0 | 15.6 |
| 97/682 (C) | 7.0 (4.0-12.3) | 7.6 (4.2-13.6) | 16.8 (3.7-77.2) | 16.3 (4.2-63.4) | 52.2 (7.1-384.3) | 23.9 (7.1-81.1) | 5.5 | 7.8 |
| 98/706 (D) | 32.8 (24.4-44.1) | 35.3 (26.7-46.7) | 67.1 (30.7-146.9) | 65.0 (36.4-116.1) | 134.7 (1.0-20,359.0) | 61.8 (1.0-4,610.0) | 13.1 | 18.5 |
A mean potency by dye test of 679.4 (315.8 to 1,461.8) was found by five laboratories in the study, establishing TOXM as the third IS. The potency assigned was 1,000 IU per ampoule (2).
TABLE 4.
Summary of results by EIA methods
| Sample | Geometric mean potency (IU ml−1) (95% confidence limits) calculated relative to TOXM or TOXS by test |
|||||
|---|---|---|---|---|---|---|
| VIDAS (n = 8) |
Platelia Toxo IgG (n = 4) |
In-house (n = 4) |
||||
| TOXM | TOXS | TOXM | TOXS | TOXM | TOXS | |
| TOXS | 2,055.9 (1,836.7-2,301.3) | 2,188.2 (638.8-7,495.5) | 2,850.83 (2,119.4-3,834.7) | |||
| TOXM | 486.4 (434.5-544.5)a | 457.0 (133.5-1,565.2)a | 350.8 (260.7-471.9)a | |||
| 01/600 (B and F) | 114.8 (91.1-144.6) | 55.8 (46.6-66.8) | 135.3 (38.0-481.1) | 61.8 (54.5-70.1) | 392.4 (198.5-775.4) | 137.7 (76.2-248.8) |
| 97/682 (C) | 53.0 (42.2-66.4) | 25.8 (21.5-30.9) | 57.7 (14.1-237.2) | 26.4 (21.8-31.9) | 286.3 (133.9-612.0) | 100.4 (54.1-186.5) |
| 98/706 (D) | 212.9 (175.6-258.2) | 103.5 (88.9-120.7) | 217.0 (59.2-795.6) | 99.2 (80.9-121.7) | 473.3 (358.4-625.2) | 166.0 (110.6-249.3) |
A mean potency by EIA of 904.3 (522.2 to 1,565.9) was found by seven laboratories in the study to establish TOXM as the third IS.
Intra- and interassay variability.
Intra-assay variability was assessed by using the potencies of coded duplicates (A and I, B and F, and E and G) relative to one another. From 109 potencies, only two exceeded 2.0 (laboratories 10 and 21, dye test) and only one was below 0.5 (laboratory 10, dye test). Furthermore, these potencies were in the range of 0.7 to 1.4 in 100 of the 109 cases (92%). Interassay variability was assessed in a similar way by using the potencies of identical samples from different ampoule sets relative to one another. Potencies were in the range of 0.5 to 2.0 in 310 of 327 cases (95%), which is comparable to the intra-assay variability.
Presence of specific IgM and IgA in study samples.
Laboratories 1 and 24 calculated the IgM content of samples by VIDAS (bioMerièux). The IgA content was determined by laboratory 1 by using Platelia Toxo IgA (Bio-Rad). All coded samples (A to I) were reported to be negative for IgM and IgA, whereas TOXM and TOXS were reported positive. The calculated ratio of IgM content per ampoule for TOXM relative to TOXS was found to be 1.30 by laboratory 1 and 1.13 by laboratory 24. These show good agreement with the value of 1.5 reported previously (2). For IgA, laboratory 1 found the ratio to exceed 1.0, but responses to TOXM were outside the measurable range in this assay. Nevertheless, this is consistent with the previously reported value of 2.5 (2).
Dye test results.
The results from 10 laboratories performing the dye test are expressed in international units per milliliter and are summarized in Table 3. The mean potency of 1,078.5 IU ml−1 (95% confidence limit, 908.6 to 1,280.2) calculated for TOXM relative to TOXS is consistent with the 1,000-IU-per-ampoule unitage previously assigned to TOXM for total anti-Toxoplasma antibodies (2). This confirms continuity of unitage with the second IS TOXS.
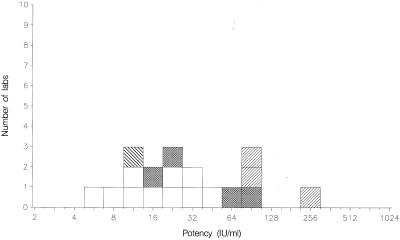
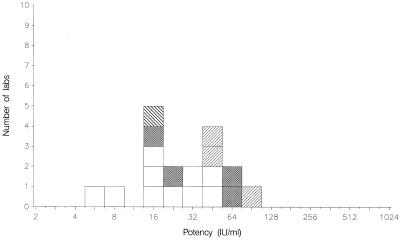
The candidate standard 01/600 has been compared with both TOXM and TOXS (Table 3 and Fig. 1 and 2). Potency estimates for the candidate standard relative to TOXM ranged from 5.2 to 48.9 IU ml−1 (n = 10 laboratories), with a mean potency of 18.0 IU ml−1 (95% confidence limit, 10.9 to 29.8). The geometric coefficient of variation between laboratories was 101%, larger than the value of 69% calculated for the coded duplicate ratios, indicating additional sources of variability between laboratories. When calculated relative to TOXS, the mean potency for the candidate standard was 19.5 IU ml−1 (95% confidence limit, 11.3 to 33.6). A potency of 20 IU per ampoule was consistent with these estimates, and it was proposed that 01/600 be assigned this unitage for total anti-Toxoplasma antibodies.
FIG. 1.
Mean potencies of 01/600 relative to TOXM obtained by laboratories performing titration assays. Dye test, □; immunofluorescence assay, ▩; agglutination assay, ▨; complement fixation assay, ▧.
FIG. 2.
Mean potencies of 01/600 relative to TOXS obtained by laboratories performing titration assays. Dye test, □; immunofluorescence assay, ▩; agglutination assay, ▨; complement fixation assay, ▧.
Dilution steps used in titration assays.
The dilution steps used in the titration assays were not consistent between laboratories. Laboratories 3, 7, 8, 12, 13, 16, 17, 18, 21, and 23 used twofold dilution steps, whereas laboratories 1, 2, 6, and 10 used fourfold dilution steps. Fivefold steps were used by laboratory 11. Laboratory 14 did not specify the dilution steps used. These differences are a possible contributory factor to the variability in the results, with twofold dilution steps allowing a greater degree of precision for estimating the potency of the samples. However, there was no indication in the results that the choice of dilution steps had an effect on the magnitude of the potency estimates obtained. Furthermore, the relative potency estimates for the coded duplicates, included to quantify within-assay variability, indicated no significant difference between those laboratories using twofold dilution steps and the other laboratories.
Results from other assays.
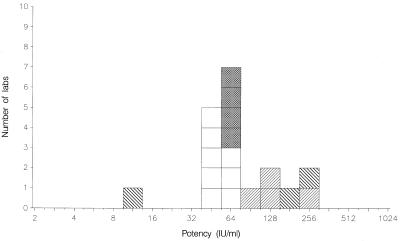
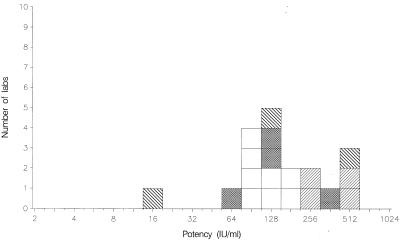
The results from other assays are summarized in Tables 3 and 4. All assays, excluding the complement fixation assay used by laboratory 12, detected positive IgG activities in all samples. The ratio of IgG content per ampoule for TOXM relative to TOXS was 0.48 by indirect immunofluorescence assay, which is in agreement with the value of 0.5 reported previously (2). For the agglutination assays, VIDAS, Platelia Toxo IgG, and in-house EIAs, these ratios were 0.23, 0.24, 0.23, and 0.18, respectively, approximately 50% of the value reported previously (2). Hence, for these methods, the potency estimates given for the coded samples are approximately 50% lower when calculated against TOXS. For the candidate standard 01/600, these results are illustrated in Fig. 1 to 4. Nevertheless, whichever of the standards is selected, the mean potency estimates calculated by the agglutination assays, VIDAS, and Platelia Toxo IgG show good agreement with each other in general.
FIG. 4.
Mean potencies of 01/600 relative to TOXS obtained by laboratories performing EIAs. VIDAS, □; Platelia, ▩; in-house assays, ▨; other assays, ▧.
The potency estimates have been recalculated for all assay methods relative to the candidate standard 01/600, now assumed to contain 20 IU per ampoule. A summary of these is shown in Table 5. For the coded samples A, E, G, I, (normal sera), C (low-IgG positive serum), and D (IgG positive serum), which appear to be negative for IgM and IgA, the mean potency estimates suggest that all methods show good agreement with each other (results for normal sera are not shown).
TABLE 5.
Calculated potency of study samples relative to 01/600 (assumed to contain 20 IU ml−1)
| Sample | Geometric mean potency (IU ml−1) (95% confidence limits) determined by: |
||||||
|---|---|---|---|---|---|---|---|
| Dye test (n = 10) | Immunofluorescence assay (n = 4) | Agglutination assay (n = 3) | Complement fixation assay (n = 1) | VIDAS (n = 8) | Platelia TOXO IgG (n = 4) | In-house EIA (n = 4) | |
| TOXS | 1,030.2 (867.9-1,223.0) | 553.9 (450.8-680.5) | 331.2 (152.4-719.7) | 1,285.6 | 358.2 (320.0-400.9) | 323.5 (94.4-1,108.0) | 145.3 (108.0-195.6) |
| TOXM | 1,106.2 (931.9-1,313.0) | 536.3 (436.5-658.9) | 151.8 (69.9-329.9) | 1,813.1 | 174.3 (155.7-195.2) | 147.9 (43.2-506.5) | 51.0 (37.9-68.6) |
| 97/682 (C) | 7.8 (4.4-13.7) | 9.0 (2.0-41.4) | 7.9 (1.1-58.4) | 10.0 | 9.2 (7.4-11.6) | 8.5 (2.1-35.1) | 14.6 (6.8-31.2) |
| 98/706 (D) | 36.4 (27.1-49.0) | 36.0 (16.5-78.8) | 20.5 (0.2-3091.7) | 23.8 | 37.1 (30.6-45.0) | 32.1 (8.8-117.6) | 24.1 (18.3-31.9) |
By contrast, calculation of the IgG potency of TOXS and TOXM showed that these differed twofold by VIDAS and Platelia Toxo IgG and threefold with the in-house EIAs (Table 6). This discrepancy is likely to result from the presence of IgA and IgM in these samples. The relative ratio of these antibodies compared to IgG is three to five times higher in TOXM than TOXS. Hence, the competition for antigen sites on the enzyme-linked immunosorbent assay surface between IgA, IgM, and IgG is increased in the TOXM sample, leading to artificially lower results and a lower potency for IgG.
TABLE 6.
Stability of anti-Toxoplasma IgG in sample 01/600 after 16 months of storage at various temperatures, measured by dye test
| Storage temperature (°C) | Test | Potency relative to sample stored at −20°C |
|||
|---|---|---|---|---|---|
| Laboratory 7 |
Laboratory 17 |
||||
| Day 1 | Day 2 | Day 1 | Day 2 | ||
| +4 | 1 | 1.00 | 1.00 | 1.00 | 1.00 |
| 2 | 1.00 | 1.00 | 1.00 | 1.00 | |
| +20 | 1 | 1.00 | 1.00 | 1.00 | 1.00 |
| 2 | 0.50 | 0.50 | 1.00 | 1.00 | |
| +37 | 1 | 0.25 | 0.25 | 0.50 | 0.50 |
| 2 | 0.50 | 0.50 | 0.50 | 0.50 | |
Stability of 01/600.
Two months after storage at −20°C, one vial of 01/600 was reconstituted and tested by the dye test in six independent assays. The unitage of the sample in these assays was 32 IU ml−1. To assess the stability of 01/600, 100 vials were stored at various temperatures (−20, 4, 20, and 37°C) for 15 months. The unitage of these samples was determined and compared to that of vials taken from stock, which are stored continuously at −20°C. Laboratories 7 and 17 tested two sets of samples in duplicate on two different dates by the dye test. The results are given in Table 6. The preparation showed no loss of activity after storage at temperatures ranging from −20 to 20°C, but the unitage declined after storage at 37°C. Previously, samples of preparation D had been stored at 45°C, but these dissolved poorly and the potency could not be determined by dye test. Hence, no samples of 01/600 were laid down at this temperature. At these high temperatures, proteins are likely to denature, resulting in poor resuspension of antibodies as well as loss of immunoreactivity. Our findings strongly suggest that the unitage of the preparation will remain stable at −20°C.
DISCUSSION
Preparation 01/600 was intended as a replacement for the third IS TOXM. Following discussion at the ECBS meeting in November 2003, the World Health Organization decided to establish the preparation as the first IS for human anti-Toxoplasma IgG, with an assigned potency of 20 IU per ampoule of total anti-Toxoplasma antibodies (6). The name reflects the quantitative and qualitative differences with TOXM. Since the candidate preparation contains no detectable levels of specific IgM or IgA, it permits direct comparison of the sensitivities of assays for specific IgG whether those assays are IgG specific themselves or not.
Three of the participating laboratories raised concerns about the establishment of the candidate preparation as a single IS with a low IgG concentration, all suggesting that additional standard preparations are needed to cover the range of requirements of reference laboratories and manufacturers. One suggested that, although the candidate preparation was suitable as a low standard, there were also requirements for mid and high standards and that a preparation containing only 20 IU should not be considered the sole world standard. One participant requested that TOXM or another high standard remain available so that manufacturers can continue to develop kits that can express results in IU for high levels of specific IgG. One further comment from a single laboratory was that a panel of at least five different IgG serum samples is needed. They also did not think it acceptable to define the unitage primarily on the basis of the dye test results, as they believe this test to be less sensitive than any EIA for the detection of slight IgG changes in samples with low IgG concentrations.
The candidate preparation had been designed specifically in response to widespread concerns that the previous IS contained relatively high levels of IgM as well as IgG. This resulted in difficulties in standardization between assays detecting IgG only or IgG and IgM. Higher levels of IgG, like those in the previous IS, are found only in acute (IgM positive) infections. Thus, either the standard would be required to contain IgM or IgM would have to be removed chemically, a process that may affect properties of the IgG. Neither of these options was considered desirable when taking into account the views of both laboratories and manufacturers. The proposed standard had been chosen specifically to provide a value within the linear range of most commercial and in-house assays and was not intended as a means of determining high-end accuracy of assays. The ECBS recognized these concerns of the participants and recommended that the development of a replacement for TOXM be considered.
We are in agreement with the need for a high-IgG standard as well as an IgM-positive standard. An additional IS should be established that will contain both anti-Toxoplasma IgM and IgG. This additional standard should address criticisms of the previous IS and should be calibrated both in terms of IgG and IgM levels. The first IS for human anti-Toxoplasma IgG will be crucial in determining levels of IgG in the candidate anti-Toxoplasma IgM and IgG standard preparation. With a combination of these two standards, laboratories and manufacturers would have access to an IS containing IgG only and to an IS that would contain the higher levels of IgG required for high-end assay calibration.
FIG. 3.
Mean potencies of 01/600 relative to TOXM obtained by laboratories performing EIAs. VIDAS, □; Platelia, ▩; in-house assays, ▨; other assays, ▧.
Acknowledgments
We thank T. Garrad (Standards Processing Division) for coding and packaging the samples and organizing the distribution.
We also thank all of the participants for their helpful contributions to the study: G. Street and A. Jenkins, Queensland Medical Laboratory, Westend, Australia; W. Apt and L. Sandoval, Universidad de Chile, Santiago, Chile; E. Petersen, Statens Serum Institut, Copenhagen, Denmark; M. Lappalainen, Helsinki University Central Hospital, Helsinki, Finland; M. Dardé, Centre Hospitalier Universitaire Dupuytren, Limoges, France; H. Pelloux, Centre Hospitalier Universitaire de Grenoble, Grenoble, France; P. Thulliez, Institut de Puériculture, Paris, France; U. Gross, University Hospital of Göttingen, Göttingen, Germany; I. Reiter-Owona, Institute für Medizinische Parasitologie der Universität Bonn, Bonn, Germany; Z. Szénási, Johan Béla National Center for Epidemiology, Budapest, Hungary; F. Irwin, University College Dublin, Dublin, Ireland; I. Riklis, National Public Health Laboratory, Tel Aviv, Israel; L. Kortbeek, National Institute for Public Health and the Environment, Bilthoven, The Netherlands; M. Paul, University of Medical Sciences, Poznan, Poland; B. Evengård, Huddinge University Hospital, Stockholm, Sweden; Y. Sukthana, Mahidol University, Bangkok, Thailand; A. Y. Gürüz and M. Korkmaz, Ege University, Bornova-Izmir, Turkey; O. Markov and I. Markov, Children Clinic for Immunodeficiency Vitacell, Kiev, Ukraine; E. Guy and J. Francis, Singleton Hospital, Swansea, United Kingdom; D. Ho-Yen and J. Chatterton, Raigmore Hospital, Inverness, United Kingdom; M. Golightly, State University of New York, Stony Brook; H. Kapoor and W. Crafts, Michigan Department of Community Health, Lansing; J. Remington and C. Press, Palo Alto Medical Foundation Research Institute, Palo Alto, Calif.; M. Wilson, Centers for Disease Control and Prevention, Atlanta, Ga.
REFERENCES
- 1.Finney, D. J. 1978. Statistical method in biological assay, 3rd ed. Charles Griffin, London, United Kingdom.
- 2.Hansen, G. A., J. Lyng, and E. Petersen. 1994. Calibration of a replacement preparation for the second international standard for anti-Toxoplasma serum, Human. W.H.O. Expert Committee on Biological Standardization BS/94.1761. World Health Organization, Geneva, Switzerland.
- 3.Hofgartner, W. T., S. R. Swanzy, R. M. Bacina, J. Condon, M. Gupta, P. E. Matlock, D. L. Bergeron, J. J. Plorde, and T. R. Fritsche. 1997. Detection of immunoglobulin G (IgG) and IgM antibodies to Toxoplasma gondii: evaluation of four commercial immunoassay systems. J. Clin. Microbiol. 35:3313-3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reiter-Owona, I., E. Petersen, D. Joynson, H. Aspock, M. L. Darde, R. Disko, O. Dreazen, H. Dumon, R. Grillo, U. Gross, M. Hayde, R. Holliman, D. O. Ho-Yen, K. Janitschke, P. A. Jenum, K. Naser, M. Olszewski, P. Thulliez, and H. M. Seitz. 1999. The past and present role of the Sabin-Feldman dye test in the serodiagnosis of toxoplasmosis. Bull. W. H. O. 77:929-935. [PMC free article] [PubMed] [Google Scholar]
- 5.WHO Tech. Rep. Ser. 1995. WHO Expert Committee on Biological Standardization, 45th report. WHO Tech. Rep. Ser. 858:1-101. [PubMed] [Google Scholar]
- 6.WHO Tech. Rep. Ser. WHO Expert Committee on Biological Standardization, 54th report. WHO Tech. Rep. Ser., in press.