Abstract
The presence of ampicillin-resistant Escherichia coli (Ampr E. coli) in the fecal flora of calves was monitored on a monthly basis in seven cohorts of calves. Calves were rapidly colonized by Ampr E. coli, with peak prevalence in cohort calves observed in the 4 months after the calves were born. The prevalence of calves yielding Ampr E. coli in cohorts consistently declined to low levels with increasing age of the calves (P < 0.001).
The presence of bacteria resistant to antimicrobial agents in food animals has serious implications for both veterinary and public health. Clinical treatment of infected animals, together with the potential transmission of resistant zoonotic pathogens to humans through the food chain, may be affected by high background levels of resistance within the intestinal flora of food animals (3, 15). Therefore, the importance of monitoring resistance in commensal indicator bacteria, such as Escherichia coli, has been recognized, and at this time, there is an international effort to organize meaningful schemes (2). However, many of these efforts concentrate on cross-sectional prevalence surveys and do not address the dynamics of antimicrobial agent resistance within the commensal or host populations.
Age appears to be a significant factor affecting gut colonization with antimicrobial-resistant E. coli, with a higher prevalence of resistance demonstrated in young stock than in old stock in surveys of cattle and pig farms (5, 11, 13). While such studies provide valuable point source information about the age distribution of resistance on a farm, they do not address the underlying dynamics determining host age differences in colonization by resistant bacteria. Longitudinal cohort data are required to examine how the prevalence of resistance changes with increasing age. Therefore, the aim of this study was to compare the carriage of ampicillin-resistant E. coli (Ampr E. coli) with age and time in multiple cohorts of calves sampled monthly from birth on four farms. Ampicillin was selected as an example of a beta-lactam agent, the usage of and resistance to which were known to be frequent on Scottish farms (9).
The study was performed on four commercial beef cattle farms, farms A to D, in Scotland, United Kingdom, between 2000 and 2002 (Table 1). Two of the farms (farms A and B) had United Kingdom-registered organic status and adhered to the United Kingdom Advisory Committee on Organic Standards. Under these standards, no animal or group of animals can receive more than three courses of treatment with a veterinary medicinal product or antimicrobial agent within 1 year. Two cohorts were monitored on a farm for three farms (farms A, B, and D). One cohort was monitored on farm C. Only one cohort per year was monitored per farm. Cohorts were of unequal size (10 to 46 calves) and were studied for different lengths of time (4 to 8 months) due to differing management strategies on the farms. Calves were born at different times of the year on the four farms and were kept with their dams as single groups, either at pasture or in straw-bedded barns, according to the season. The calves in this study were born into suckler herds and were therefore able to suckle their dams at will throughout the study period.
TABLE 1.
Prevalence of Ampr E. coli in the herds and calf cohorts and details on the farms and cohort treatments
| Farm | Farm status | No. of cohorts | Cohort yr | Birth seasona | Cohort size (no. of calves) | Cohort Ampr period prevalence (%) | Herd sizeb (no. of animals) | Annual herd Ampr prevalenceb (%) | No. of treatmentsc |
|---|---|---|---|---|---|---|---|---|---|
| A | Organic | 2 | 2001 | Summer | 17 | 88.2 | 38 | 23.7 | 3 (7) |
| 2002 | Summer | 17 | 70.6 | 37 | 2.7 | 1 (2) | |||
| B | Organic | 2 | 2000 | Autumn | 10 | 40.0 | 91 | 8.8 | 0 |
| 2002 | Spring | 25 | 96.0 | 86 | 1.2 | 1 | |||
| C | Nonorganic | 1 | 2002 | Spring | 19 | 94.7 | 136 | 14.7 | 2 |
| D | Nonorganic | 2 | 2000 | Autumn | 25 | 96.0 | 101 | 44.6 | 23 |
| 2001 | Autumn | 46 | 97.8 | 116 | 42.2 | 19 |
Spring, February to April; summer, May to July; autumn, August to October; winter, November to January.
Excluding all animals less than 1 year old.
The total number of treatment events with an antimicrobial agent was recorded for the cohort over the cohort period. The number of additional treatment events with antimicrobial agents, given as topical formulations and not administered systemically, is shown in parentheses.
Individual rectal fecal samples were collected from cohort calves within the first month after birth and then at monthly intervals for up to eight sampling months. Fecal samples were also collected individually from all cattle in the herd (1 year old or older) either before or during the sampling period of each calf cohort. Sample collection was conducted in accordance with United Kingdom Government Home Office license requirements. Records were kept of all antimicrobial treatments received by cohort calves during the study period (Table 1).
Fecal samples (883 samples from cohort calves and 605 samples from herd animals) were kept at 4°C and screened within 48 h of collection on Chromocult tryptone bile X-glucuronide agar (TBX agar; Merck) containing ampicillin at a breakpoint concentration of 16 mg/liter, using recommended guidelines (4). Samples (1 g) were diluted 1:10 in maximum recovery diluent (Oxoid), 10 μl was spread onto nonselective (antibiotic-free) plates and ampicillin-containing plates and incubated overnight at 44°C. Characteristic E. coli colonies ofa dark blue color were recorded (the dark blue color indicating the presence of the glucuronidase enzyme) (7). Reference strains E. coli NCTC 10418 and NCTC 11560 were used to confirm the activity of antibiotic-containing plates.
Statistical analyses were performed using S-Plus (Insightful, Seattle, Wash.). Changes in the prevalence of Ampr E. coli in the cohorts were analyzed by generalized linear mixed-effect models using binomial errors, with Ampr E. coli as the dependent variable, calf identity entered into the model as a random effect, and other variables of interest as fixed factors. To compare the decline from the peak in prevalence in cohorts, the data were aligned so that peaks coincided. Data prior to the peak were not used in this part of the analysis, and data after the proportion of calves yielding Ampr E. coli had been zero for 2 months were also excluded.
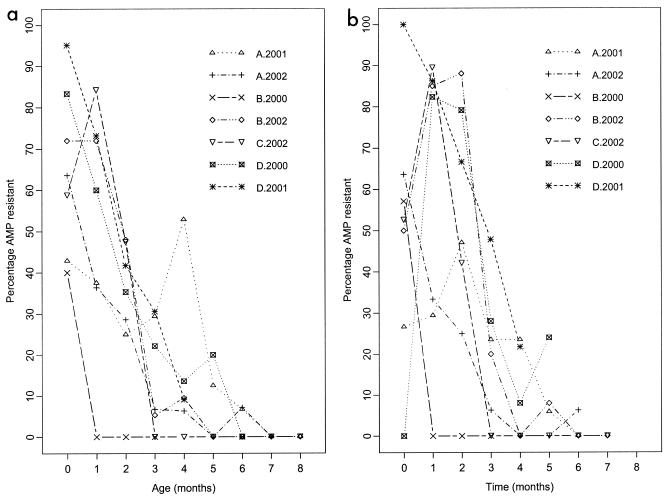
Ampr E. coli bacteria were detected in all calf cohorts and at all annual herd surveys on the four farms (Table 1). The cohort period prevalence (number of calves yielding at least one positive sample during the study period) for Ampr E. coli was high for all cohorts, while the prevalence of Ampr E. coli by age was greater than 40% for all cohorts within the first month of birth. In all but two cohorts (farms A-2001 and C-2002), the prevalence of Ampr E. coli consistently declined with age (Fig. 1a). Overall, the decline in Ampr E. coli with increasing age of the calf was significant on all farms (P < 0.001). A significant decline in Ampr E. coli with sampling time was also observed in all cohorts (P < 0.001) (Fig. 1b).
FIG. 1.
Prevalence of cohort calves yielding ampicillin-resistant (AMP resistant) E. coli on farms A to D, sampled in years 2000, 2001, and 2002, shown by (a) calf age and (b) sampling month.
Ampr E. coli appeared to be ubiquitous on these farms: calves were rapidly colonized by Ampr E. coli shortly after birth, with the prevalence in the cohort subsequently declining to very low levels with increasing age of the calf. However, it should be noted that total, unselected, fecal E. coli counts are also known to decline with age, as the composition of the gut flora changes (14). This may affect the detection of resistance in older animals if E. coli burdens are close to the sensitivity limit of the assays employed. Using viable count analysis, Ampr E. coli bacteria have been shown to decline more rapidly than total (unselected) E. coli with increasing age of the calf (10). This suggests that the decline in prevalence of Ampr E. coli observed in the multiple calf cohorts presented here reflects a true decline in the proportion of total E. coli bacteria that are resistant to ampicillin with increasing age of the calf.
The overall shape of the decline was consistent among all cohorts and occurred within a relatively short age or time frame, although there was a significant difference between the seven cohorts in the peak prevalence values and slopes of the age decline (P < 0.003). Significant differences were also observed in the annual herd and cohort period prevalence according to farm (P < 0.001) and cohort (P < 0.03), but not year (P = 0.380). In a comparison by the type of farm, the period prevalence and peak values of the cohorts on the two organic farms (farms A and B) were lower than those of the cohorts on nonorganic farms (farms C and D) (P < 0.009). A significant difference was also observed in the annual herd prevalence values on organic and nonorganic farms (P < 0.001). While these results suggest that the prevalence of Ampr E. coli was lower on organic farms than on nonorganic farms, further studies are required to determine how representative the animals on these farms are of the general animal populations of organic and nonorganic farms in Scotland.
All cohorts, except B-2000, experienced at least one antimicrobial treatment event during the study period. In general, the frequency of cohort treatment reflected the overall treatment regimen used within the entire herd. Treatment did not appear to directly influence the carriage status of individual animals, with many showing resistance prior to antimicrobial treatment. However, the level of treatment on a farm may have had some impact on the overall selection pressure present within a herd, with farm D showing the highest level of cohort treatment and annual herd prevalence of all the farms. Previous studies of pig herds support this view, demonstrating that individual animal treatment has less influence on resistance status than the level of group medication (6), while herds not exposed to antimicrobial agents show lower overall resistance than those receiving treatment (8, 13). Therefore, it is likely that the source of resistant E. coli for the cohorts in this study was either the respective dam or the environment and that resistance was not the result of specific selection pressure within the guts of individual calves. Early acquisition of resistant E. coli by calves may be due to active selection, with resistance determinants or closely linked genes conferring some colonization advantage in the calf gut (11). A potential advantage of this sort would have serious implications for the elimination of resistant organisms from the farm environment.
In summary, this study has demonstrated a consistent age-related decline in the carriage of Ampr E. coli by seven calf cohorts on four farms within a short age or time frame, regardless of the farm type and when a cohort was studied. The decline in carriage of resistant E. coli with age may be related to a change in competition or niche advantage, the potential impact of fitness cost in an evolving gut environment, or some alteration in the colonization ability of resistant strains (1, 12). The presence of Ampr E. coli in older cattle on all farms suggests that in some calves this decline is transient, with cattle reacquiring resistant bacteria at a later point, possibly due to environmental changes or management events on the farm. Further work to compare phenotypic and molecular profiles of the isolates obtained from the different farms in this study is now being done.
Acknowledgments
We are grateful for assistance from the Molecular Chemotherapy Group, University of Edinburgh.
This study was a component of the International Partnership Research Award in Veterinary Epidemiology (IPRAVE): Epidemiology and Evolution of Enterobacteriaceae Infections in Humans and Domestic Animals (www.vie.gla.ac.uk/wiprave), funded by the Wellcome Trust.
REFERENCES
- 1.Bjorkman, J., I. Nagaev, O. G. Berg, D. Hughes, and D. I. Andersson. 2000. Effects of environment on compensatory mutations to ameliorate costs of antibiotic resistance. Science 287:1479-1482. [DOI] [PubMed] [Google Scholar]
- 2.Caprioli, A., L. Busani, J. L. Martel, and R. Helmuth. 2000. Monitoring of antibiotic resistance in bacteria of animal origin: epidemiological and microbiological methodologies. Int. J. Antimicrob. Agents 14:295-301. [DOI] [PubMed] [Google Scholar]
- 3.Catry, B., H. Laevens, L. A. Devriese, G. Opsomer, and A. Kruif. 2003. Antimicrobial resistance in livestock. J. Vet. Pharmacol. Ther. 26:81-93. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2003. National Antimicrobial Resistance Monitoring System for Enteric Bacteria (NARMS): 2001 annual report. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Atlanta, Ga.
- 5.DeFrancesco, K. A., R. N. Cobbold, D. H. Rice, T. E. Besser, and D. D. Hancock. 2004. Antimicrobial resistance of commensal Escherichia coli from dairy cattle associated with recent multi-resistant salmonellosis outbreaks. Vet. Microbiol. 98:55-61. [DOI] [PubMed] [Google Scholar]
- 6.Dunlop, R. H., S. A. McEwen, A. H. Meek, R. C. Clarke, W. D. Black, and R. M. Friendship. 1998. Associations among antimicrobial drug treatments and antimicrobial resistance of fecal Escherichia coli of swine on 34 farrow-to-finish farms in Ontario, Canada. Prev. Vet. Med. 34:283-305. [DOI] [PubMed] [Google Scholar]
- 7.Frampton, E. W., L. Restaino, and L. Blaszko. 1988. Evaluation of β-glucuronidase substrate 5-bromo-4-chloro-3-indolyl-β-d-glucuronide (X-GLUC) in a 24 hour direct plating method for Escherichia coli. J. Food Prot. 51:402-404. [DOI] [PubMed] [Google Scholar]
- 8.Gellin, G., B. E. Langlois, K. A. Dawson, and D. K. Aaron. 1989. Antibiotic resistance of gram-negative enteric bacteria from pigs in three herds with different histories of antibiotic exposure. Appl. Environ. Microbiol. 55:2287-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gunn, G. J., and J. C. Low. 2003. Analysis of passive surveillance data for antimicrobial resistance from cases of neonatal bovine enteritis. Vet. Rec. 152:537-539. [DOI] [PubMed] [Google Scholar]
- 10.Hoyle, D. V., H. I. Knight, D. J. Shaw, K. Hillman, M. C. Pearce, J. C. Low, G. J. Gunn, and M. E. J. Woolhouse. 2004. The acquisition and epidemiology of antibiotic resistant Escherichia coli in a cohort of new-born calves. J. Antimicrob. Chemother. 53:867-871. [DOI] [PubMed] [Google Scholar]
- 11.Khachatryan, A. R., D. D. Hancock, T. E. Besser, and D. R. Call. 2004. Role of calf-adapted Escherichia coli in maintenance of antimicrobial drug resistance in dairy calves. Appl. Environ. Microbiol. 70:752-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lenski, R. E. 1997. The cost of antibiotic resistance—from the perspective of a bacterium. Ciba Found. Symp. 207:131-140. [DOI] [PubMed] [Google Scholar]
- 13.Mathew, A. G., M. A. Beckmann, and A. M. Saxton. 2001. A comparison of antibiotic resistance in bacteria isolated from swine herds in which antibiotics were used or excluded. J. Swine Health Prod. 9:125-129. [Google Scholar]
- 14.Smith, H. W., and W. E. Crabb. 1961. The faecal bacterial flora of animals and man: its development in the young. J. Pathol. Bacteriol. 82:53-66. [Google Scholar]
- 15.van den Bogaard, A. E., and E. Stobberingh. 2000. Epidemiology of resistance to antibiotics. Links between animals and humans. Int. J. Antimicrob. Agents 14:327-335. [DOI] [PubMed] [Google Scholar]