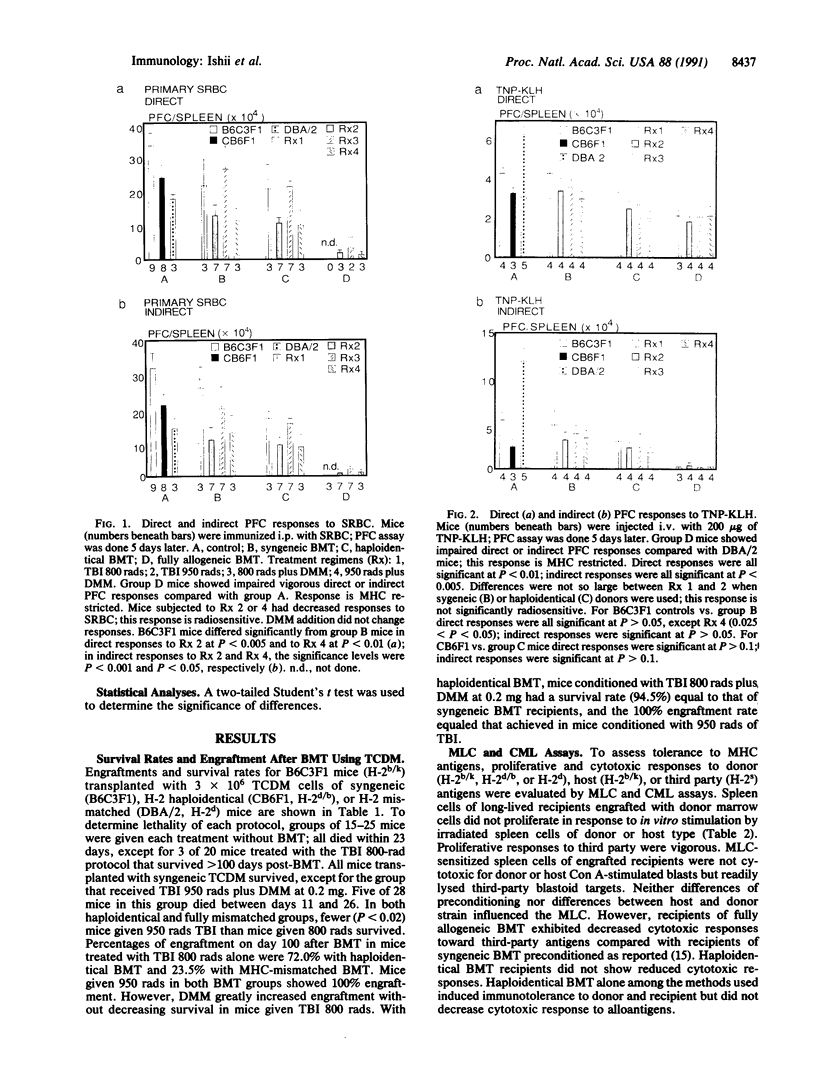
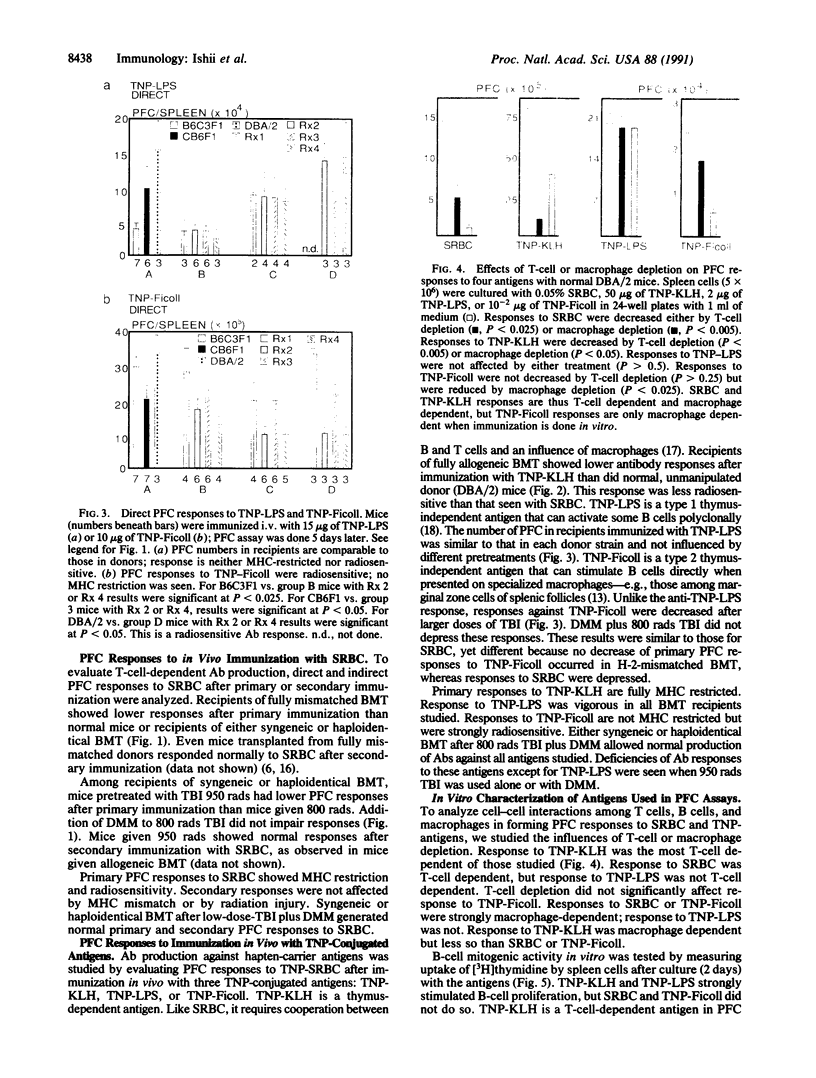
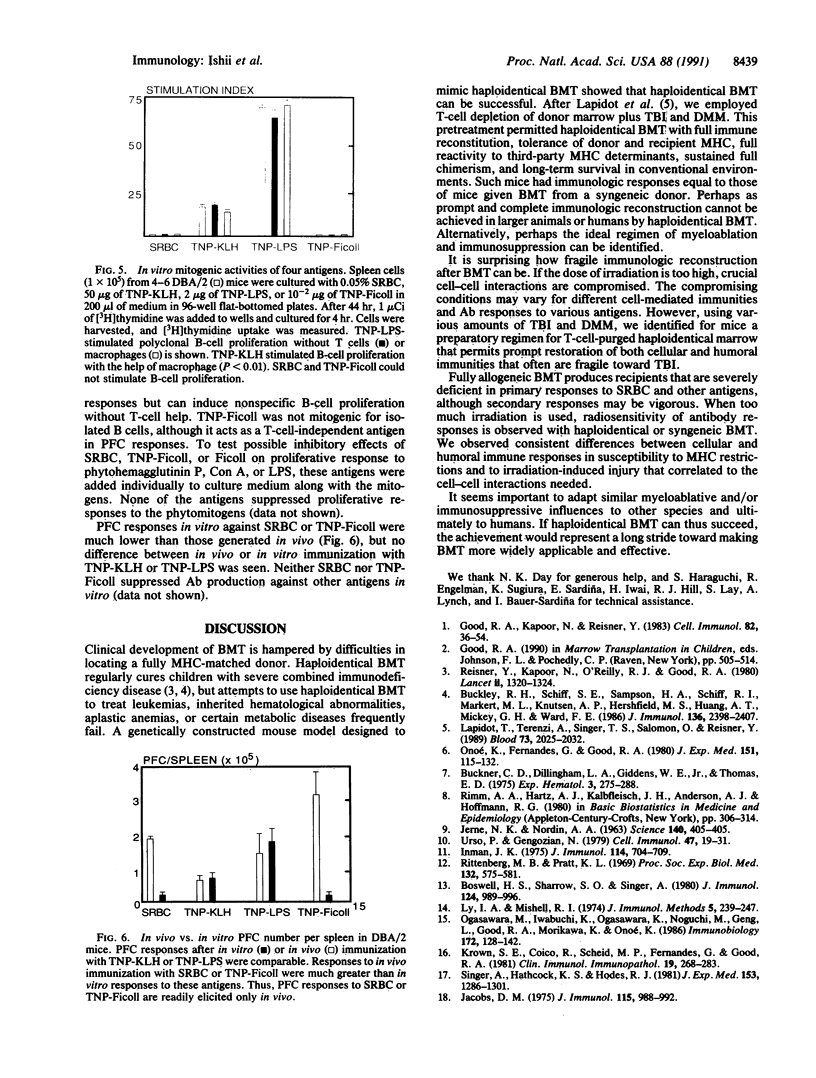
Abstract
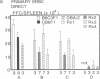
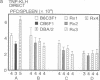
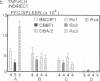
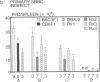
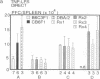
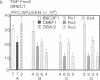
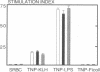
To study murine major histocompatibility complex (MHC)-haploidentical bone-marrow transplantation (BMT), B6C3F1 mice (H-2b/k) underwent BMT using syngeneic [B6C3F1 (H-2b/k)], haploidentical [CB6F1 (H-2d/b)], or fully allogeneic [DBA/2 (H-2d)] donor mice. As pretreatment, dimethyl myleran (DMM), an alkylating agent that produces effective myeloablation but little immunosuppression, was used with total body irradiation (TBI). Four conditioning regimens were studied: TBI 800 rads (1 rad = 0.01 Gy), TBI 950 rads, TBI 800 rads plus DMM (0.2 mg per mouse), and TBI 950 rads plus DMM. Survival rates, chimerism, proliferative responses in mixed-lymphocyte culture, specific cell-mediated lympholysis, and in vivo plaque-forming cell responses to several antigens were compared. TBI 800 rads plus DMM was maximally effective. Haploidentical BMT was as successful in inducing long-term survival and immune and hematologic reconstitution as was syngeneic BMT. This regimen plus haploidentical BMT of T-cell-purged marrow yielded survivors tolerant of donor and recipient major histocompatibility complex. Such myeloablation and immunosuppression prevented graft rejection, immunodeficiency due to histoincompatibility, and damage to a radiosensitive cell population. A microenvironmental influence crucial to some antibody responses was thus revealed. Delayed recovery of antibody production after BMT in humans may be due partly to suboptimal myeloablation or excess irradiation.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boswell H. S., Sharrow S. O., Singer A. Role of accessory cells in B cell activation. I. Macrophage presentation of TNP-Ficoll: evidence for macrophage-B cell interaction. J Immunol. 1980 Feb;124(2):989–996. [PubMed] [Google Scholar]
- Buckley R. H., Schiff S. E., Sampson H. A., Schiff R. I., Markert M. L., Knutsen A. P., Hershfield M. S., Huang A. T., Mickey G. H., Ward F. E. Development of immunity in human severe primary T cell deficiency following haploidentical bone marrow stem cell transplantation. J Immunol. 1986 Apr 1;136(7):2398–2407. [PubMed] [Google Scholar]
- Buckner C. D., Dillingham L. A., Giddens W. E., Jr, Thomas E. D. Toxicologic and marrow transplantation studies in rhesus monkeys given dimethyl myleran. Exp Hematol. 1975 Sep;3(5):275–288. [PubMed] [Google Scholar]
- Good R. A., Kapoor N., Reisner Y. Bone marrow transplantation--an expanding approach to treatment of many diseases. Cell Immunol. 1983 Nov;82(1):36–54. doi: 10.1016/0008-8749(83)90139-9. [DOI] [PubMed] [Google Scholar]
- Inman J. K. Thymus-independent antigens: the preparation of covalent, hapten-ficoll conjugates. J Immunol. 1975 Feb;114(2 Pt 1):704–709. [PubMed] [Google Scholar]
- Jacobs D. M. Structural and genetic basis of the in vivo immune response to TNP-LPS. J Immunol. 1975 Oct;115(4):988–992. [PubMed] [Google Scholar]
- Krown S. E., Coico R., Scheid M. P., Fernandes G., Good R. A. Immune function in fully allogeneic mouse bone marrow chimeras. Clin Immunol Immunopathol. 1981 May;19(2):268–283. doi: 10.1016/0090-1229(81)90069-6. [DOI] [PubMed] [Google Scholar]
- Lapidot T., Terenzi A., Singer T. S., Salomon O., Reisner Y. Enhancement by dimethyl myleran of donor type chimerism in murine recipients of bone marrow allografts. Blood. 1989 May 15;73(7):2025–2032. [PubMed] [Google Scholar]
- Ly I. A., Mishell R. I. Separation of mouse spleen cells by passage through columns of sephadex G-10. J Immunol Methods. 1974 Aug;5(3):239–247. doi: 10.1016/0022-1759(74)90108-2. [DOI] [PubMed] [Google Scholar]
- Ogasawara M., Iwabuchi K., Ogasawara K., Noguchi M., Geng L., Good R. A., Morikawa K., Onoé K. Generation of cytotoxic T lymphocyte responses to allo-H-2 antigens in allogeneic bone marrow chimeras histocompatible at the H-2 subregions. Immunobiology. 1986 Aug;172(1-2):128–142. doi: 10.1016/s0171-2985(86)80059-6. [DOI] [PubMed] [Google Scholar]
- Onoé K., Fernandes G., Good R. A. Humoral and cell-mediated immune responses in fully allogeneic bone marrow chimera in mice. J Exp Med. 1980 Jan 1;151(1):115–132. doi: 10.1084/jem.151.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenberg M. B., Pratt K. L. Antitrinitrophenyl (TNP) plaque assay. Primary response of Balb/c mice to soluble and particulate immunogen. Proc Soc Exp Biol Med. 1969 Nov;132(2):575–581. doi: 10.3181/00379727-132-34264. [DOI] [PubMed] [Google Scholar]
- Singer A., Hathcock K. S., Hodes R. J. Self recognition in allogeneic radiation bone marrow chimeras. A radiation-resistant host element dictates the self specificity and immune response gene phenotype of T-helper cells. J Exp Med. 1981 May 1;153(5):1286–1301. doi: 10.1084/jem.153.5.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urso P., Gengozian N. Nonspecific suppressor elements in murine allogeneic radiation chimeras. Cell Immunol. 1979 Sep 15;47(1):19–31. doi: 10.1016/0008-8749(79)90311-3. [DOI] [PubMed] [Google Scholar]
- Watson L., Moxham J., Fraser P. Hydrocortisone suppression test and discriminant analysis in differential diagnosis of hypercalcaemia. Lancet. 1980 Jun 21;1(8182):1320–1325. doi: 10.1016/s0140-6736(80)91784-5. [DOI] [PubMed] [Google Scholar]