Abstract
Six characteristic regions (I to VI) were identified in Shiga toxin 2 (Stx2) phages (T. Sato, T. Shimizu, M. Watarai, M. Kobayashi, S. Kano, T. Hamabata, Y. Takeda, and S. Yamasaki, Gene 309:35-48, 2003). Region V, which is ca. 10 kb in size and is located in the upstream region of the Stx operons, includes the most distinctive region among six Stx phages whose genome sequences have been determined. In this study, we developed a PCR-restriction fragment length polymorphism (RFLP) assay for the epidemiological analysis of Shiga toxin-producing Escherichia coli (STEC) on the basis of the diversity of region V. When region V was amplified by long and accurate-PCR (LA-PCR) with five control E. coli strains carrying six different Stx phages such as E. coli strains C600 (Stx1 phage), C600 (933W phage), C600 (Stx2 phage-I), C600 (Stx2 phage-II), and O157:H7 Sakai strain RIMD0509952 (VT1-Sakai phage and VT2-Sakai phage), an expected size of the band was obtained. Restriction digest of each PCR product with BglI or EcoRV also gave the expected sizes of banding patterns and discriminated the RFLPs of five control strains. When a total of 204 STEC O157 strains were analyzed by LA-PCR, one to three bands whose sizes ranged from 8.2 to 14 kb were obtained. Two STEC O157 strains, however, did not produce any bands. Subsequent restriction digest of the PCR products with BglI or EcoRV differentiated the RFLPs of 202 STEC O157 strains into 24 groups. The RFLP patterns of pulsed-field gel electrophoresis (PFGE) of representative strains of STEC O157 divided into 24 groups were well correlated with those of PCR-RFLP when STEC O157 strains were isolated in the same time period and in the close geographic area. To evaluate the PCR-RFLP assay developed here, ten strains, each isolated from four different outbreaks in different areas in Japan (Tochigi, Hyogo, Aichi, and Fukuoka prefecture), were examined to determine whether the strains in each group showed the same RFLP patterns in the PCR-RFLP assay. In accordance with the results of PFGE except for strains isolated in an area (Fukuoka), which did not produce any amplicon, ten strains in each group demonstrated the same RFLP pattern. Taken together, these data suggest that the PCR-RFLP based on region V is as useful as PFGE but perhaps more simple and rapid than PFGE for the molecular epidemiological analysis of STEC strains during sporadic and common source outbreaks.
Infection with Escherichia coli O157:H7 was first recognized in the United States when two outbreaks of an unusual gastrointestinal illness characterized by sudden onset of severe crampy abdominal pain and grossly bloody diarrhea, designated hemorrhagic colitis, occurred in Oregon and Michigan in 1982 (43). This rare serotype, O157:H7, was found to produce a cytotoxin that was toxic to HeLa and Vero cells, and was neutralized by antibody against Shiga toxin (Stx) produced by Shigella dysenteriae type 1 (37). Therefore, the cytotoxin was designated Vero toxin (VT) or Stx and the E. coli strain have since been called as Vero cytotoxin-producing E. coli (VTEC) (22) or Shiga toxin-producing E. coli (STEC) (7).
Several virulence factors of STEC have been reported, such as the locus of enterocyte effacement (32, 41), which has genes for producing both attachment and effacement lesions (9) and a type III secretion system (18), EHEC hemolysins (5), an iron transportation system (28, 34), and Stx (37, 50). Recently, STEC autoagglutinating adhesin was found in an LEE-negative STEC O113:H21 strain isolated from hemolytic-uremic syndrome (HUS) patient (39) and has thus far been detected in only non-O157 STEC strains (19, 27). Among these, Stx is the most important virulence factor in STEC and is broadly divided into two groups, namely, Stx1 and Stx2, on the basis of their immunological properties (37, 50). Stx1 is identical or almost identical to Stx produced by S. dysenteriae type 1 (37, 50). Stx2 is immunologically different from Stx1 but biologically and physicochemically similar to Stx1 (37, 50). Stx1 and Stx2 consist of two subunits that include one molecule of A subunit and five molecules of B subunits. B subunit is responsible for binding to Gb3 which is the receptor molecule of Stx. A subunit is responsible for toxic activity and has an RNA N-glycosidase activity (37, 50). The stx1 and stx2 genes have been reported to be encoded on lambdoid phage, the so-called Stx phage (13, 57).
Since 1982, a number of outbreaks and sporadic cases of STEC infection have been reported all over the world (8, 11, 24, 29, 58, 60), and STEC has become recognized as an emerging human pathogen. Much attention has been focused on this group of organisms because STEC infection can cause life-threatening sequelae such as hemorrhagic colitis, HUS, and neurological disorders, particularly in infants and elderly people (23, 55). In 1996, the largest outbreak caused by STEC O157:H7 occurred in Japan (33). Although the number of large outbreaks caused by STEC has diminished since 1996, the number of sporadic cases, including diffuse outbreaks caused by a single clone in different areas, has increased (52-54). Therefore, it is important to rapidly identify the strain associated with diffuse outbreaks.
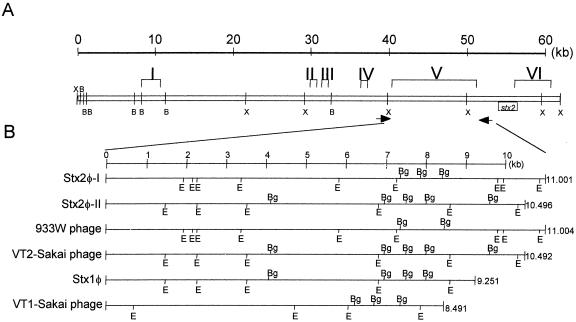
Complete genome sequences of E. coli O157:H7 strains 933W and RIMD0509952 have been determined by two independent groups (12, 40). Ohnishi et al. (38) further analyzed genomic diversity of STEC O157 strains by whole-genome PCR scanning and reported that bacteriophages are the most diverse region in the O157 genome. Thus far, the entire genome sequences of at least seven Stx phages have been determined (30, 35, 42, 47, 48, 62). Comparison of the entire genome sequences of four different Stx2 phages genomes has revealed six characteristic regions (I to VI) (48). Moreover, regions I to VI were also located in Stx1 phage; however, only regions II, V, and VI were found in VT1-Sakai phage (T. Sato and S. Yamasaki, unpublished data). Among these, region V is the most distinct portion in the entire phage genome and is located in the upstream region of the Stx2 operons that is responsible for the immunity and replication. Therefore, targeting region V in Stx phage is the most reasonable for molecular typing.
In the present study, we developed a rapid and simple DNA fingerprinting method by means of a PCR-restriction fragment length polymorphism (RFLP) assay for the molecular epidemiological analysis of STEC strains on the basis of previously reported diversity of region V in Stx phage genome (48). Furthermore, we evaluated whether the PCR-RFLP assay developed here could be utilized for the epidemiological analysis of STEC strains with isolates from recent outbreaks that have occurred in Japan.
(This study was performed in partial fulfillment of the requirements of a Ph.D. thesis for K. Shima from the Graduate School of Agriculture and Biological Sciences, Osaka Prefecture University, Osaka, Japan.)
MATERIALS AND METHODS
Bacterial strains and growth media.
Bacterial strains used in the present study are listed in Table 1. The source of STEC O157 strains used in the present study was either clinical or nonclinical. E. coli strains were grown either on L agar or in L broth.
TABLE 1.
E. coli strains used in this study
| Strain or origin | Serotypea | No. of strainsb | Presence (+) or absence (−) of genec
|
|
|---|---|---|---|---|
| stx1 | stx2 | |||
| C600 | NA | 1 | − | − |
| C600 (Stx 1 φ) | NA | 1 | + | − |
| C600 (Stx2 φ-I) | NA | 1 | − | + |
| C600 (Stx2 φ-II) | NA | 1 | − | + |
| C600 (933W) | NA | 1 | − | + |
| RIMD0509952 (Sakai strain) | O157:H7 | 1 | + | + |
| Okayama, Japan | O157 | 48 | + | + |
| O157 | 1 | − | + | |
| Osaka, Japan | O157 | 37 | + | + |
| O157 | 2 | − | + | |
| Gifu, Japan | O157 | 2 | + | + |
| Gobou, Japan | O157 | 6 | + | + |
| Hiroshima, Japan | O157 | 3 | + | + |
| Nagano, Japan | O157 | 62 | + | + |
| O157 | 1 | − | + | |
| O157 | 1 | + | − | |
| United States | O157 | 26 (6) | + | + |
| O157 | 3 (2) | − | + | |
| O157 | 2 (2) | + | − | |
| Canada | O157 | 7 | + | + |
| O157 | 3 | − | + | |
| Tochigi, Japan | O157:H7 | 10 | + | + |
| Hyogo, Japan | O157:H7 | 10 | + | + |
| Aichi, Japan | O157:H7 | 10 | − | + |
| Fukuoka, Japan | O157:H- | 10 | − | + |
NA, not applicable.
Numbers in parentheses indicate the number of strains isolated from beef or beef products.
The stx1 and stx2 genes were detected by colony hybridization as described in Materials and Methods.
Chemicals and enzymes.
Chemicals were purchased either from Nacalai Tesque (Kyoto, Japan), Wako Pure Chemical Industries (Tokyo, Japan), or Sigma Chemical Co. (St. Louis, Mo.). Restriction enzymes, Takara LA Taq, and LA PCR kit version 2 were purchased from Takara Shuzo (Kyoto, Japan). Bacto tryptone and yeast extract used for preparation of medium were purchased from Difco Laboratories (Detroit, Mich.). Pulsed-field certified agarose, low-melting-point preparative-grade agarose, Seakem GTG agarose and Seakem HGT (for high gelling temperature) agarose were from either Bio-Rad (Hercules, Calif.) or Takara Shuzo. Molecular weight makers were purchased from Bio-Rad, Takara Shuzo, or Invitrogen (Carlsbad, Calif.).
PCR-RFLP.
Long and accurate PCR (LA-PCR) was performed by using an LA-PCR Kit version 2 (Takara Shuzo). The primer sequences were as follows: primer 1, 5′-GACATTGCTCCGTGTATTCACTCGTTGGAA-3′; and primer 2, 5′-ATTTTGCATTTCCTTCGCGCTGGTTTAGCC-3′. The reaction began with denaturation for 1 min at 94°C, followed by 30 cycles of replication (20 s at 98°C and 10 min at 68°C) and final extension at 72°C for 10 min by using a GeneAmp PCR System 2400 (Perkin-Elmer, Wellesley, Mass.). The PCR products were analyzed by 0.4% agarose gel electrophoresis with HGT agarose or by field inversion gel electrophoresis with 1.0% pulsed-field certified agarose gel in 0.5× TBE (45 mM Tris-HCl, 45 mM boric acid, 1.0 mM EDTA [pH 8.0]) buffer for 32.26 h, followed by ethidium bromide staining. Then, a 5-kb DNA ladder (Invitrogen) was used as the molecular mass standard. The run condition was generated by the autoalgorithm mode of CHEF Mapper pulsed-field gel electrophoresis (PFGE) system with a size range of 6 to 15 kb. The PCR products were further restriction digested for 2 h either by 10 U of BglI or 8 U of EcoRV and then analyzed by 1.5% agarose gel electrophoresis or by field inversion gel electrophoresis with 1.0% pulsed-field certified agarose gel in 0.5× TBE buffer for 20.41 h, followed by ethidium bromide staining. Then, a 1-kb DNA ladder (Invitrogen) and λHindIII digest (Takara Shuzo) were used as the molecular mass standard. The run condition was generated by the autoalgorithm mode of CHEF Mapper PFGE system with a size range of 1 to 10 kb for the BglI digest or 1 to 6 kb for the EcoRV digest, followed by ethidium bromide staining. A model 1000 Mini-Chiller (Bio-Rad) was used to maintain the temperature of the buffer at 14°C. The photograph of the ethidium bromide staining gel was taken by Gel-Doc 2000 (Bio-Rad).
PFGE.
PFGE was performed by using essentially the same protocol as described previously (59). Briefly, the genomic DNAs of the various E. coli O157 strains were prepared in agarose plugs. Agarose blocks containing genomic DNA were equilibrated in restriction enzyme buffer for 1 h at room temperature and cleaved in fresh buffer at the appropriate incubation temperature. For complete digests of the DNAs, 50 U of XbaI or ApaI was used. PFGE of the inserts was performed by using the contour-clamped homogenous electric field method on a CHEF Mapper system (Bio-Rad) in 0.5× TBE buffer for 40.24 h. As recommended (45, 49), 50 μM thiourea was included in the PFGE running buffer when smearing bands were obtained. A DNA size standard λ ladder (Bio-Rad) was used as the molecular mass standard, and a model 1000 Mini-Chiller was used to maintain the temperature of the buffer at 14°C. Run conditions were generated by the autoalgorithm mode of the CHEF Mapper PFGE system with a size range of 20 to 300 kb.
Colony hybridization test.
Colony hybridization test was carried out as described previously (61) with nitrocellulose membrane (Schleicher & Schuell, Dassel, Germany) under high-stringency conditions. For stx1- and stx2-specific polynucleotide probes, an internal 0.9-kb HincII-HpaI fragment of the stx1 gene (61) and an internal 0.86-kb PstI-SmaI fragment of the A subunit of the stx2 gene (15) were used. These fragments were labeled by the random priming method by using Multiprime DNA labeling system (Amersham Biosciences Corp. Piscataway, N.J.) and [α-32P]dCTP (Perkin-Elmer). The radioactivity was visualized by using BAS FLA-3000 (Fujifilm, Tokyo, Japan).
RESULTS
PCR-RFLP.
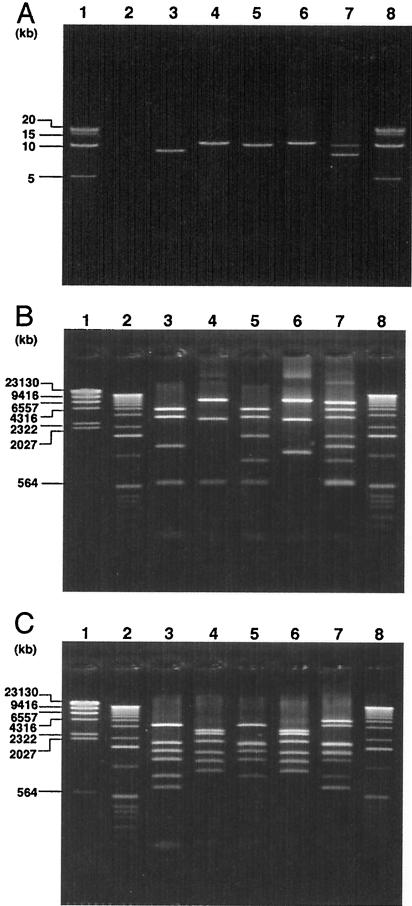
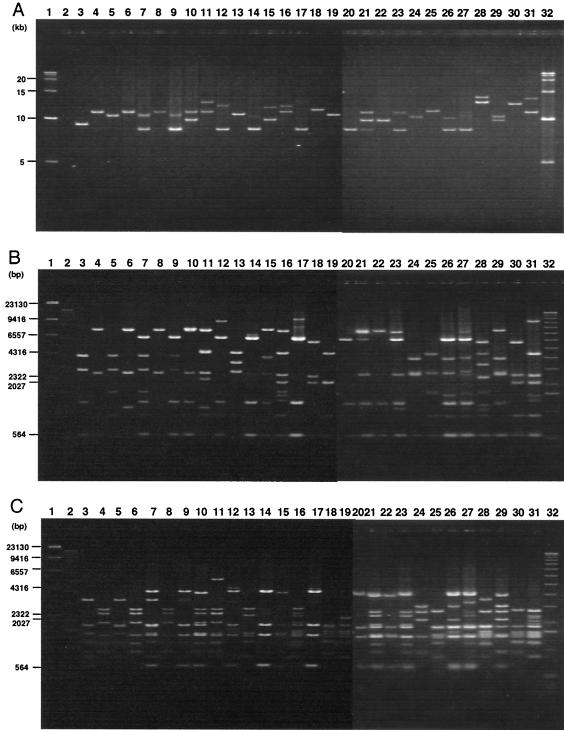
To develop a PCR-RFLP assay for the epidemiological analysis of STEC strains, we focused on the diversity of region V of Stx phages (48), and a set of PCR primers that can amplify region V, located upstream of Stx2 operons, was designed (Fig. 1). When the genomic DNA of five control strains was amplified by LA-PCR and the products were analyzed by 0.4% agarose gel electrophoresis, the five control strains that carry known Stx phages, including E. coli strains C600 (Stx1 phage), C600 (Stx2 phage-I), C600 (Stx2 phage-II), C600 (933W phage), and E. coli O157:H7 Sakai strain RIMD0509952 (VT1-Sakai phage and VT2-Sakai phage) gave expected sizes of the bands such as 9.3, 11, 10.5, 11, and 8.5 and 10.5 kb, respectively (Fig. 2A). No band was obtained with E. coli C600, which was used as the negative control. Since several BglI and EcoRV sites are present in region V as shown in Fig. 1B, the PCR products were further restriction digested by BglI or EcoRV, and the digests were further analyzed by 1.5% agarose gel electrophoresis. When digested by BglI, three to seven bands were visible, and RFLP patterns of the five control strains were different (Fig. 2B). When digested by EcoRV, although C600 (Stx2 phage-I) and C600 (933W phage) showed identical RFLP patterns, five to seven bands were visible and the RFLP patterns of four control strains out of five were different (Fig. 2C). Since the PCR-RFLP assay was able to differentiate five control strains (particularly with BglI) on the basis of their RFLP patterns, a total of 204 STEC O157 strains, including 194 clinical and 10 nonclinical strains isolated in Japan, Canada, and the United States, were analyzed by LA-PCR. When amplified by LA-PCR, one to three bands were obtained from 202 STEC O157 strains (Fig. 3A). However, two strains did not produce any bands. As shown in Fig. 3A, nine STEC strains produced one band whose sizes ranged from 8.25 to 12.5 kb. Two bands whose sizes ranged from 8.2 to 14 kb were obtained from 13 STEC strains. Three bands whose sizes ranged from 8.3 to 11.5 kb were obtained from two STEC strains. The PCR products were further digested by BglI or EcoRV, and the digests were analyzed by field inversion gel electrophoresis. BglI digest gave 3 to 11 bands whose sizes ranged from 500 bp to 9.6 kb (Fig. 3B) and EcoRV digest gave 4 to 13 bands whose sizes ranged from 450 bp to 5.0 kb (Fig. 3C). In some case, the bands were visible on the display but very faint on the photograph. Therefore, the results are summarized in Table 2. Restriction digests of BglI or EcoRV clearly differentiated the RFLP patterns of 202 STEC strains into 24 types.
FIG. 1.
(A) Restriction map of the Stx2φ-I genome and the location of the six characteristic regions (I to VI). B and X represent BamHI and XhoI, respectively. Arrows indicate the location of primers. (B) Restriction map of region V in six Stx phages. Bg and E represent BglI and EcoRV, respectively.
FIG. 2.
(A) LA-PCR products of region V in six Stx-phages. Lanes: 1 and 8, 5-kb DNA ladder; 2, C600; 3, C600 (Stx1φ); 4, C600 (Stx2φ-I); 5, C600 (Stx2φ-II); 6, C600 (933W phage); 7, RIMD0509952 (Sakai strain; VT1-Sakai phage, VT2-Sakai phage). BglI digest (B) and EcoRV digest (C) of LA-PCR products obtained from region V in six Stx phages. Lanes: 1, λ-HindIII digest; 2 and 8, 1-kb DNA ladder; 3, C600 (Stx1φ); 4, C600 (Stx2φ-I); 5, C600 (Stx2φ-II); 6, C600 (933W phage); 7, RIMD0509952 (Sakai strain; VT1-Sakai phage, VT2-Sakai phage).
FIG. 3.
(A) Field inversion gel electrophoresis of LA-PCR products obtained from 6 control strains and 24 representative strains differentiated by the PCR-RFLP assay. Lanes: 1 and 32, 5-kb DNA ladder; 2, C600; 3, C600 (Stx1φ); 4, C600 (Stx2φ-I); 5, C600 (Stx2φ-II); 6, C600 (933W phage); 7, RIMD0509952 (Sakai strain; VT1-Sakai phage, VT2-Sakai phage); 8, 1-A (Okayama, Japan); 9, 2-B (Osaka, Japan); 10, 3-C (Okayama, Japan); 11, 4-D (Okayama, Japan); 12, 5-E (Okayama, Japan); 13, 6-F(Okayama, Japan); 14, 7-G (Osaka, Japan); 15, 8-H (Osaka, Japan); 16, 9-I (Osaka, Japan); 17, 10-J (Osaka, Japan); 18, 11-K (Nagano, Japan); 19, 12-L (Nagano, Japan); 20, 13-M (Nagano, Japan); 21, 14-N (Nagano, Japan); 22, 15-O (Nagano, Japan); 23, 16-P (United States); 24, 17-Q (United States); 25, 18-R (United States); 26, 19-S (United States); 27, 20-T (United States); 28, 21-U (Canada); 29, 22-V (Canada); 30, 23-W (Canada); 31, 24-X (Canada). Field inversion gel electrophoresis of BglI digest (B) and EcoRV digest (C) of LA-PCR products obtained from 6 control strains and 24 representative strains differentiated by the PCR-RFLP assay. Lanes: 1, λ-HindIII digest; 2, 32, 1-kb DNA ladder; 3, C600 (Stx1φ); 4, C600 (Stx2φ-I); 5, C600 (Stx2φ-II); 6, C600 (933W phage); 7, RIMD0509952 (Sakai strain; VT1-Sakai phage, VT2-Sakai phage); 8, 1-A (Okayama, Japan); 9, 2-B (Osaka, Japan); 10, 3-C (Okayama, Japan); 11, 4-D (Okayama, Japan); 12, 5-E (Okayama, Japan); 13, 6-F (Okayama, Japan); 14, 7-G (Osaka, Japan); 15, 8-H (Osaka, Japan); 16, 9-I (Osaka, Japan); 17, 10-J (Osaka, Japan); 18, 11-K (Nagano, Japan); 19, 12-L (Nagano, Japan); 20, 13-M (Nagano, Japan); 21, 14-N (Nagano, Japan); 22, 15-O (Nagano, Japan); 23, 16-P (United States); 24, 17-Q (United States); 25, 18-R (United States); 26, 19-S (United States); 27, 20-T (United States); 28, 21-U (Canada); 29, 22-V (Canada); 30, 23-W (Canada); 31, 24-X (Canada).
TABLE 2.
Results of typing of STEC O157
| PCR-RFLP typing patterna | PCR amplicon size(s) (kb) | No. of isolates analyzedb | No. of strains that originated inb:
|
Colony hybridization
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Japan
|
United States | Canada | stx1 | stx2 | ||||||||
| Okayama | Osaka | Gifu | Gobou | Hiroshima | Nagano | |||||||
| 1-A | 11.0 | 36 | 33 | 1 | 2 | + | + | |||||
| 2-B | 10.5, 8.20 | 52 (4) | 31 | 4 | 3 | 10 (4) | 4 | + | + | |||
| 3-C | 11.0, 9.60 | 41 | 13 | 1 | 3 | 24 | + | + | ||||
| 4-D | 12.8, 11.0 | 24 | 1 | 2 | 21 | + | + | |||||
| 5-E | 12.2, 8.20 | 1 | 1 | + | + | |||||||
| 6-F | 10.5 | 1 | 1 | − | + | |||||||
| 7-G | 10.4, 8.20 | 1 | 1 | + | + | |||||||
| 8-H | 12.0, 9.60 | 1 | 1 | + | + | |||||||
| 9-I | 12.2, 11.0 | 1 | 1 | − | + | |||||||
| 10-J | 13.0, 8.20 | 11 | 2 | 7 | 2 | + | + | |||||
| 11-K | 11.5 | 1 | 1 | − | + | |||||||
| 12-L | 10.5 | 5 | 5 | + | + | |||||||
| 13-M | 8.25 | 3 (2) | 1 | 2 (2) | + | − | ||||||
| 14-N | 11.2, 9.80, 8.40 | 1 | 1 | + | + | |||||||
| 15-O | 9.60 | 1 | 1 | + | + | |||||||
| 16-P | 11.2, 8.40 | 12 (1) | 12 (1) | + | + | |||||||
| 17-Q | 10.5 | 1 | 1 | − | + | |||||||
| 18-R | 11.8 | 2 (1) | 1 (1) | 1 | − | + | ||||||
| 19-S | 10.2, 8.30 | 2 (1) | 1 (1) | 1 | + | + | ||||||
| 20-T | 11.5, 10.5, 8.30 | 1 | 1 | + | + | |||||||
| 21-U | 14.0, 12.8 | 1 | 1 | + | + | |||||||
| 22-V | 10.2, 9.60 | 1 | 1 | + | + | |||||||
| 23-W | 12.5 | 1 | 1 | − | + | |||||||
| 24-X | 13.2, 11.0 | 1 | 1 | − | + | |||||||
| None | 2 (1) | 1 | 1 (1) | − | + | |||||||
| Total | 204 (10) | 49 | 39 | 2 | 6 | 3 | 64 | 31 | 10 | |||
PCR-RFLP typing data are presented as binary combinations: the numeral represents the BglI digest-obtained pattern, and the letter represents the EcoRV digest-obtained pattern.
Values in parentheses are numbers of strains isolated from beef or beef products.
Colony hybridization test.
Colony hybridization test was used to examine if 204 STEC strains carry stx1 and stx2 genes or either the stx1 gene or the stx2 gene. As shown in Table 2, three STEC strains grouped in 13-M harbored only the stx1 gene. Eight STEC strains grouped in 6-F, 9-I, 11-K, 17-Q, 18-R, 23-W, and 24-X harbored only the stx2 gene. Two STEC strains that were not typed by LA-PCR also harbored the stx2 gene only. However, 191 STEC strains clustered into the other 16 groups harbored both the stx1 and the stx2 genes.
PFGE.
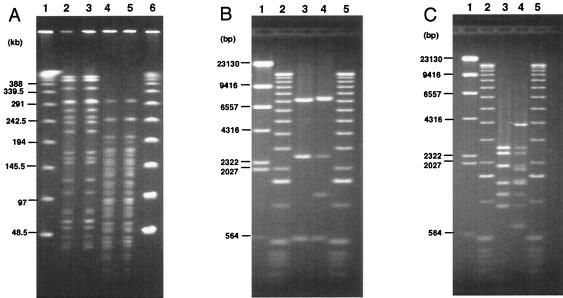
A total of 101 representative strains were randomly selected out of 202 STEC O157 strains differentiated into 24 groups by PCR-RFLP, and the typing result obtained by PFGE was compared to that obtained by PCR-RFLP. First, the genomic DNAs of each representative STEC O157 strain from 24 groups were digested by XbaI, and the RFLP patterns of each strain were analyzed by PFGE. The PFGE pattern of three strains could not be analyzed due to smearing; thus, we reanalyzed the three strains with running buffer containing thiourea. The RFLP patterns of 24 STEC O157 strains obtained by PFGE were clearly differentiated, and the result was well correlated with that of PCR-RFLP (data not shown). To confirm the correlation between PCR-RFLP and PFGE typing results, RFLP patterns obtained by XbaI digestion of additional 77 STEC O157 strains, including 17 strains from 1-A, 24 strains from 2-B, 19 strains from 3-C, 11 strains from 4-D, 4 strains from 10-J, and 2 strains from 12-L, were further analyzed by PFGE. In general, the typing results obtained by PFGE correlated well with those obtained by PCR-RFLP in STEC strains isolated in the same time period and in a nearby geographic area (data not shown). However, there was one case in which the RFLP pattern of PFGE was identical but the PCR-RFLP yielded different results. Namely, one strain isolated in Okayama belonging to 1-A had RFLP patterns identical to those obtained by PFGE of a strain isolated in Nagano which, however, was determined to belong to 3-C when it was digested by either XbaI or ApaI, as shown in Fig. 4. When region V was amplified by LA-PCR, different amplicon sizes were obtained. In addition, a restriction digest of the PCR products by BglI or EcoRV distinguished these two strains as 1-A and 3-C, indicating that the PCR-RFLP assay may give better resolution than PFGE in some cases.
FIG. 4.
Comparison of the typing results between PCR-RFLP and PFGE. (A) PFGE patterns of STEC strains isolated in Okayama and Nagano digested by XbaI or ApaI. Lanes: 1 and 6, lambda ladder; 2, 1-A (Okayama, Japan), XbaI; 3, 3-C (Nagano, Japan), XbaI; 4, 1-A (Okayama, Japan), ApaI; 5, 3-C (Nagano, Japan), ApaI. Field inversion gel electrophoreses of BglI digest (B) and EcoRV digest (C) of LA-PCR products of 1-A (Okayama, Japan) and 3-C (Nagano, Japan). Lanes: 1, λ-HindIII digest; 2 and 5, 1-kb DNA ladder; 3, 1-A (Okayama, Japan); 4, 3-C (Nagano, Japan).
Evaluation of PCR-RFLP with outbreak strains.
We attempted to evaluate the PCR-RFLP assay with STEC strains isolated from four different outbreaks that occurred in Tochigi, Hyogo, Aichi, and Fukuoka prefecture in Japan in 2002 and were identified as caused by a clone when examined by PFGE (J. Terajima and H. Watanabe, unpublished). When the region V in 10 Tochigi strains was amplified by LA-PCR, all strains gave two bands of 8.3 and 12.5 kb (data not shown). Restriction digest of each PCR product with BglI gave an identical banding pattern with six bands, while restriction digestion with EcoRV also gave identical banding pattern with nine bands (data not shown). When another 10 STEC strains isolated during Hyogo outbreak were analyzed by LA-PCR, all strains gave two bands of 8.3 and 10.2 kb. Restriction digests of each PCR product with BglI or EcoRV gave identical banding patterns. A BglI digest gave seven bands, and an EcoRV digest gave nine bands (data not shown). Another 10 STEC strains isolated during the Aichi outbreak were also analyzed by the PCR-RFLP assay. When the genomic DNA of these strains was amplified by the LA-PCR, an 11-kb fragment was specifically obtained from all 10 strains. Restriction digestion of each PCR product with BglI or EcoRV gave identical banding patterns, with a BglI digest yielding five bands and an EcoRV digest yielding seven bands (data not shown). In one case, however, no amplicon was obtained when the LA-PCR was used with 10 STEC strains isolated during Fukuoka outbreak. Although we did not confirm the presence of Stx phage in these strains, the presence of Stx2 gene has been demonstrated in all 10 STEC O157 strains by colony hybridization test (Table 1), suggesting that an Stx2 phage may be present in the strains. These data indicate that there is more diversity in region V in some Stx phages.
DISCUSSION
Molecular epidemiological analyses are very useful to track and identify an epidemic strain during an outbreak. There are several molecular typing methods available such as ribotyping, random amplified polymorphic DNA-PCR, PFGE, etc. (10, 56). PFGE is the most commonly used molecular typing method and has been used for a variety of pathogens, including STEC, because of its high resolution and reproducibility (17, 20, 52-54). However, there are some disadvantages to using PFGE. For example, PFGE requires expensive and elaborate equipment and can only analyze a few strains at a time. If a large outbreak occurs, such as the EHEC outbreak that occurred in Japan in 1996 (33), it may happen that more than a thousand strains may have to be analyzed. In addition, it takes at least 2 days to obtain the complete PFGE results. Moreover, the PFGE is labor-intensive and fairly time-consuming. Apart from this, there is the problem of some strains that give a smear by PFGE, as we experienced in the present study because of strong deoxyribonuclease activity or degradation by free radical or peroxide produced during electrophoresis (17). This type of problem has been reported for E. coli O157:H7, Pseudomonas aeruginosa, Vibrio parahaemolyticus, Salmonella serovars, and Clostridium difficile (20, 25, 26, 31, 44). It has been reported that the addition of thiourea, which is toxic to humans, in Tris-HCl buffer or the use of HEPES buffer instead of Tris-HCl buffer might solve the smearing caused by the scavenger activity of free radical or peroxide by inhibiting the production of free radical and peroxide in some cases (25, 45, 49). In addition, it has also been reported that PFGE alone is not sufficient and that other typing methods may be necessary in epidemiological surveys (6, 10). Therefore, PFGE and another typing method, such as phage typing or ribotyping, were often combined to obtain better resolution in epidemiological surveys (3, 4, 16, 46). However, phage typing can be carried out only at certain laboratories, such as reference laboratories, because of the availability of biologically active phages and control strains. It is important to introduce a more simple method that can be used in an ordinary laboratory in an epidemiological survey.
A delay in identifying a strain responsible for an outbreak would result in many people being affected, and this will increase the number of cases of HUS and neurological disorders in children and elderly people, resulting in death (23, 55). Therefore, it is important to develop a simple and rapid DNA fingerprinting method that can replace PFGE. Extensive comparative analysis of the nucleotide sequences of Stx2 phages has revealed six distinct regions in the Stx phage genome (48). In the present study, we developed a PCR-RFLP for the epidemiological analysis of STEC strains on the basis of the diversity of region V in Stx phages and evaluated this assay in comparison to PFGE with strains isolated from recent outbreaks that occurred in Japan (Terajima and Watanabe, unpublished). We demonstrated that the PCR-RFLP assay is as useful and more discriminative than PFGE for the molecular epidemiological analysis of STEC strains. In addition, some of the disadvantages of PFGE were offset in several ways by the PCR-RFLP assay. First, PCR-RFLP, in contrast to PFGE, does not require special equipment. Second, a large number of strains can be analyzed at the same time. Third, the PCR-RFLP assay can be completed rapidly within a day. Fourth, it is possible to analyze the RFLP without the isolation of strains. For example, when the largest O157 outbreak and sporadic cases of infection occurred in Japan in 1996 and 1997, radish sprouts were suspected to be the contaminant food with E. coli O157 (33). Although we attempted to isolate E. coli O157 not only from radish sprouts but also from radish seeds, we were unable to isolate the organism despite intense efforts. However, PCR with enrichment culture sample as a template DNA, followed by Southern hybridization with stx1, stx2, and O157-specific gene probes demonstrated the specific amplification of each target gene only after cultivation but not before cultivation (T. Shimizu and Y. Takeda, unpublished), indicating that there was a live E. coli O157 strain in the enrichment culture. In such cases, the PCR-RFLP combined with Southern hybridization could be useful for analyzing the DNA fingerprint without the isolation of strains. Fifth, smearing due to strong DNase activity can be avoided. In addition, in the case of PFGE, the changing of a few bands during cultivation of the STEC strain, even between the same clones, has been observed (14, 51). It has been also reported that RFLP patterns of PFGE change even after strain passage through cow or human gastrointestinal tract (1, 2, 21). If the Region V in the Stx phage is stable, this can be averted. This possibility is under the investigation in our laboratory. Sixth, it is not necessary to send STEC strains in order to compare the RFLP patterns at two different laboratories. In the PCR-RFLP assay, it is easier to compare the RFLP patterns obtained at two different laboratories because the numbers and sizes of the bands are expected to be smaller compared to those of PFGE. To compare the RFLP patterns more precisely, however, it will be better if genomic DNA of the strain but not the STEC O157 strain itself, which is safer and easier to ship, can be exchanged.
However, there are some disadvantages in the PCR-RFLP also. For instance, PCR-RFLP is less discriminative than PFGE if the strains were isolated in different time periods and different geographic areas. Because it is possible that a clonal Stx phage can be present in a clonal strain during outbreak, however, it is also possible that the identical Stx phage may be present in different E. coli strains. In addition, there may be a strain that has more diversity in region V. In this case, region V cannot be amplified by the LA-PCR. Region V, which corresponds to the region responsible for immunity and replication, has been reported to be important for the regulation of toxin production (36). Therefore, analysis of region V by PCR-RFLP may give crucial information about the structure-function relationship of the phage genome and virulence.
Taken together, our findings indicate that PCR-RFLP based on region V can be a useful DNA fingerprinting method when it is necessary to rapidly analyze a number of STEC strains for molecular epidemiological analyses during outbreak and that the combined use of PCR-RFLP and PFGE will provide more accurate results regarding DNA finger printing in an epidemiological survey. The PCR-RFLP based on region V will be a useful tool for analyzing structure-function relationships for phage genomes and toxin production. Further investigation with more STEC strains is needed for a thorough evaluation of the PCR-RFLP assay.
Acknowledgments
We thank G. B. Nair, Laboratory Sciences Division, ICDDR, B, for critical reading of the manuscript.
REFERENCES
- 1.Akiba, M., T. Sameshima, and M. Nakazawa. 2000. Clonal turnover of enterohemorrhagic Escherichia coli O157:H7 in experimentally infected cattle. FEMS Microbiol. Lett. 184:79-83. [DOI] [PubMed] [Google Scholar]
- 2.Akiba, M., T. Sameshima, and M. Nakazawa. 1999. The shift of genetic subtypes of Escherichia coli O157:H7 isolates from cattle. Epidemiol. Infect. 122:343-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avery, S. M., E. Liebana, C. A. Reid, M. J. Woodward, and S. Buncic. 2002. Combined use of two genetic fingerprinting methods, pulsed-field gel electrophoresis and ribotyping, for characterization of Escherichia coli O157 isolates from food animals, retail meats, and cases of human disease. J. Clin. Microbiol. 40:2806-2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrett, T. J., H. Lior, J. H. Green, R. Khakhria, J. G. Wells, B. P. Bell, K. D. Greene, J. Lewis, and P. M. Griffin. 1994. Laboratory investigation of a multistate food-borne outbreak of Escherichia coli O157:H7 by using pulsed-field gel electrophoresis and phage typing. J. Clin. Microbiol. 32:3013-3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beutin, L., M. A. Montenegro, I. Orskov, F. Orskov, J. Prada, S. Zimmermann, and R. Stephan. 1989. Close association of verotoxin (Shiga-like toxin) production with enterohemolysin production in strains of Escherichia coli. J. Clin. Microbiol. 27:2559-2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bohm, H., and H. Karch. 1992. DNA fingerprinting of Escherichia coli O157:H7 strains by pulsed-field gel electrophoresis. J. Clin. Microbiol. 30:2169-2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calderwood, S. B., D. W. K. Acheson, G. T. Keusch, T. J. Barrett, P. M. Griffin, N. A. Strockbine, B. Swaminathan, J. B. Kaper, M. M. Levine, B. S. Kaplan, H. Karch, A. D. O'Brien, T. G. Obrig, Y. Takeda, P. I. Tarr, and I. K. Wachsmuth. 1996. Proposed new nomenclature for SLT (VT) family. ASM News 62:118-119. [Google Scholar]
- 8.Caprioli, A., and A. E. Tozzi. 1998. Epidemiology of Shiga toxin-producing Escherichia coli infections in continental Europe, p. 38-48. In J. B. Kaper and A. D. O'Brien (ed.), Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. American Society for Microbiology, Washington, D.C.
- 9.Donnenberg, M. S., S. Tzipori, M. L. Mckee, A. D. O'Brien, J. Alroy, and J. B. Kaper. 1993. The role of eae gene of enterohemorrhagic Escherichia coli in intimate attachment in vitro and in a porcine model. J. Clin. Investig. 92:1418-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grif, K., H. Karch, C. Schneider, F. D. Daschner, L. Beutin, T. Cheasty, H. Smith, B. Rowe, M. P. Dierich, and F. Allerberger. 1998. Comparative study of five different techniques for epidemiological typing of Escherichia coli O157. Diagn. Microbiol. Infect. Dis. 32:165-176. [DOI] [PubMed] [Google Scholar]
- 11.Griffin, P. M. 1998. Epidemiology of Shiga toxin-producing Escherichia coli infections in humans in the United States, p. 15-22. In J. B. Kaper and A. D. O'Brien (ed.), Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. American Society for Microbiology, Washington, D.C.
- 12.Hayashi, T., K. Makino, M. Ohnishi, K. Kurokawa, K. Ishii, K. Yokoyama, C. G. Han, E. Ohtsubo, K. Nakayama, T. Murata, M. Tanaka, T. Tobe, T. Iida, H. Takami, T. Honda, C. Sasakawa, N. Ogasawara, T. Yasunaga, S. Kuhara, T. Shiba, M. Hattori, and H. Shinagawa. 2001. Complete genome sequence of enterohemorrhagic Escherichia coli O157:H7 and genomic comparison with a laboratory strain K-12. DNA Res. 8:11-22. [DOI] [PubMed] [Google Scholar]
- 13.Huang, A., S. D. Grandis, J. Friesen, M. Karmali, M. Petric, R. Congi, and J. L. Brunton. 1986. Cloning and expression of the genes specifying Shiga-like toxin production in Escherichia coli H19. J. Bacteriol. 166:375-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iguchi, A., R. Osawa, J. Kawano, A. Shimizu, J. Terajima, and H. Watanabe. 2002. Effects of repeated subculturing and prolonged storage at room temperature of enterohemorrhagic Escherichia coli O157:H7 on pulsed-field gel electrophoresis profiles. J. Clin. Microbiol. 40:3079-3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ito, H., A. Terai, H. Kurazono, Y. Takeda, and M. Nishibuchi. 1990. Cloning and nucleotide sequencing of Vero toxin 2 variant genes from Escherichia coli O91:H21 isolated from a patient with hemolytic-uremic syndrome. Microb. Pathog. 8:47-60. [DOI] [PubMed] [Google Scholar]
- 16.Izumiya, H., T. Masuda, R. Ahmed, R. Khakhria, A. Wada, J. Terajima, K. Itoh, W. M. Johnson, H. Konuma, K. Shinagawa, K. Tamura, and H. Watanabe. 1998. Combined use of bacteriophage typing and pulsed-field gel electrophoresis in the epidemiological analysis of Japanese isolates of enterohemorrhagic Escherichia coli O157:H7. Microbiol. Immunol. 42:515-519. [DOI] [PubMed] [Google Scholar]
- 17.Izumiya, H., J. Terajima, A. Wada, Y. Inagaki, K. Itoh, K. Tamura, and H. Watanabe. 1997. Molecular typing of enterohemorrhagic Escherichia coli O157:H7 isolates in Japan by using pulsed-field gel electrophoresis. J. Clin. Microbiol. 35:1675-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jarvis, K. G., J. A. Giron, A. E. Jerse, T. K. McDaniel, M. S. Donnenberg, and J. B. Kaper. 1995. Enteropathogenic Escherichia coli contains a putative type III secretion system necessary for the export of proteins involved in attaching and effacing lesion formation. Proc. Natl. Acad. Sci. USA 92:7996-8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jenkins, C., N. T. Perry, T. Cheasty, D. J. Shaw, G. Frankel, G. Dougan, G. J. Gunn, H. R. Smith, A. W. Paton, and J. C. Paton. 2003. Distribution of the saa gene in strains of Shiga toxin-producing Escherichia coli of human and bovine origins. J. Clin. Microbiol. 41:1775-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson, J. M., S. D. Weagant, K. C. Jinneman, and J. L. Bryant. 1995. Use of pulsed-field gel electrophoresis for epidemiological study of Escherichia coli O157:H7 during a food-borne outbreak. Appl. Environ. Microbiol. 61:2806-2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karch, H., H. Russmann, H. Schmidt, A. Schwarzkopf, and J. Heesemann. 1995. Long-term shedding and clonal turnover of enterohemorrhagic Escherichia coli O157 in diarrheal diseases. J. Clin. Microbiol. 33:1602-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karmali, M. A., C. A. Lingwood, M. Petric, J. Brunton, and C. Gyles. 1996. Maintaining the existing phenotype nomenclatures for Escherichia coli cytotoxins. ASM News 62:167-169. [Google Scholar]
- 23.Karmali, M. A. 1989. Infection by verotoxin-producing Escherichia coli. Clin. Microbiol. Rev. 2:15-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan, A., S. Yamasaki, T. Sato, T. Ramamurthy, A. Pal, S. Datta, N. R. Chowdhury, S. C. Das, A. Sikdar, T. Tsukamoto, S. K. Bhattacharya, Y. Takeda, and G. B. Nair. 2002. Prevalence and genetic profiling of virulence determinants of non-O157 Shiga toxin-producing Escherichia coli isolated from cattle, beef, and humans, Calcutta, India. Emerg. Infect. Dis. 8:54-62. [PubMed] [Google Scholar]
- 25.Koort, J. M. K., S. Lukinmaa, M. Rantala, E. Unkila, and A. Siitonen. 2002. Technical improvement to prevent DNA degradation of enteric pathogens in pulsed-field gel electrophoresis. J. Clin. Microbiol. 40:3497-3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kristjansson, M., M. H. Samore, D. N. Gerding, P. C. Degirolami, K. M. Bettin, A. W. Karchmer, and R. D. Arbeit. 1994. Comparison of restriction endonuclease analysis, ribotyping, and pulsed-field gel electrophoresis for molecular differentiation of Clostridium difficile strains. J. Clin. Microbiol. 32:1963-1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar, H. S., I. Karunasagar, I. Karunasagar, T. Tsukamoto, K. Shima, and S. Yamasaki. 2004. Characterization of Shiga toxin-producing Escherichia coli (STEC) isolated from seafood and beef. FEMS Microbiol. Lett. 233:173-178. [DOI] [PubMed] [Google Scholar]
- 28.Law, D., and J. Kelly. 1995. Use of heme and hemoglobin by Escherichia coli O157 and other Shiga-like-toxin-producing E. coli serogroups. Infect. Immun. 63:700-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopez, E. L., M. M. Contrini, and M. F. D. Rosa. 1998. Epidemiology of Shiga toxin-producing Escherichia coli in South America, p. 30-37. In J. B. Kaper and A. D. O'Brien (ed.), Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. American Society for Microbiology, Washington, D.C.
- 30.Makino, K., K. Yokoyama, Y. Kubota, C. H. Yutsudo, S. Kimura, K. Kurokawa, K. Ishii, M. Hattori, I. Tatsuno, H. Abe, T. Iida, K. Yamamoto, M. Onishi, T. Hayashi, T. Yasunaga, T. Honda, C. Sasakawa, and H. Shinagawa. 1999. Complete nucleotide sequence of the prophage VT2-Sakai carrying the verotoxin 2 genes of the enterohemorrhagic Escherichia coli O157:H7 derived from the Sakai outbreak. Genes Genet. Syst. 74:227-239. [DOI] [PubMed] [Google Scholar]
- 31.Marshall, S., C. G. Clark, G. Wang, M. Mulvey, M. T. Kelly, and W. M. Johnson. 1999. Comparison of molecular methods for typing Vibrio parahaemolyticus. J. Clin. Microbiol. 37:2473-2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McDaniel, T. K., K. G. Jarvis, M. S. Donnenberg, and J. B. Kaper. 1995. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc. Natl. Acad. Sci. USA 92:1664-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Michino, H., K. Araki, S. Minami, S. Takaya, N. Sakai, M. Miyazaki, A. Ono, and H. Yanagawa. 1999. Massive outbreak of Escherichia coli O157:H7 infection in schoolchildren in Sakai City, Japan, associated with consumption of white radish sprouts. Am. J. Epidemiol. 150:787-796. [DOI] [PubMed] [Google Scholar]
- 34.Mills, M., and S. M. Payne. 1995. Genetics and regulation of heme iron transport in Shigella dysenteriae and detection of an analogous system in Escherichia coli O157:H7. J. Bacteriol. 177:3004-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miyamoto, H., W. Nakai, N. Yajima, A. Fujibayashi, T. Higuchi, K. Sato, and A. Matsushiro. 1999. Sequence analysis of Stx2-converting phage VT2-Sa shows a great divergence in early regulation and replication regions. DNA Res. 6:235-240. [DOI] [PubMed] [Google Scholar]
- 36.Neely, M. N., and D. I. Friedman. 1998. Functional and genetic analysis of regulatory regions of coliphage H-19B: location of Shiga-like toxin and lysis genes suggest a role for phage functions in toxin release. Mol. Microbiol. 28:1255-1267. [DOI] [PubMed] [Google Scholar]
- 37.O'Brien, A. D., and R. K. Holmes. 1987. Shiga and Shiga-like toxins. Microbiol. Rev. 51:206-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohnishi, M., J. Terajima, K. Kurokawa, K. Nakayama, T. Murata, K. Tamura, Y. Ogura, H. Watanabe, and T. Hayashi. 2002. Genomic diversity of enterohemorrhagic Escherichia coli O157 revealed by whole genome PCR scanning. Proc. Natl. Acad. Sci. USA 99:17043-17048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paton, A. W., P. Srimanote, M. C. Woodrow, and J. C. Paton. 2001. Characterization of Saa, a novel autoagglutinating adhesin produced by locus of enterocyte effacement-negative Shiga-toxigenic Escherichia coli strains that are virulent for humans. Infect. Immun. 69:6999-7009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perna, N. T., G. Plunkett III, V. Burland, B. Mau, J. D. Glasner, D. J. Rose, G. F. Mayhew, P. S. Evans, J. Gregor, H. A. Kirkpatrick, G. Posfai, J. Hackett, S. Klink, A. Boutin, Y. Shao, L. Miller, E. J. Grotbeck, N. W. Davis, A. Lim, E. T. Dimalanta, K. D. Potamousis, J. Apodaca, T. S. Anantharaman, J. Lin, G. Yen, D. C. Schwartz, R. A. Welch, and F. R. Blattner. 2001. Genome sequence of enterohaemorrhagic Escherichia coli O157:H7. Nature 409:529-533. [DOI] [PubMed] [Google Scholar]
- 41.Perna, N. T., G. F. Mayhew, G. Posfai, S. Elliott, M. S. Donnenberg, J. B. Kaper. and F. R. Blattner. 1998. Molecular evolution of a pathogenicity island from enterohemorrhagic Escherichia coli O157:H7. Infect. Immun. 66:3810-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Plunkett III, G., D. J. Rose, T. J. Durfee, and F. R. Blattner. 1999. Sequence of Shiga toxin 2 phage 933W from Escherichia coli O157:H7: Shiga toxin as a phage late-gene product. J. Bacteriol. 181:1767-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Riley, L. W., R. S. Remis, S. D. Helgerson, H. B. Mcgee, J. G. Wells, B. R. Davis, R. J. Hebert, E. S. Olcott, L. M. Johnson, N. T. Hargrett, P. A. Blake, and M. L. Cohen. 1983. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N. Engl. J. Med. 308:681-685. [DOI] [PubMed] [Google Scholar]
- 44.Romling, U., J. Wingender, H. Muller, and B. Tummler. 1994. A major Pseudomonas aeruginosa clone common to patients and aquatic habitats. Appl. Environ. Microbiol. 60:1734-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Romling, U., and B. Tummler. 2000. Achieving 100% typability of Pseudomonas aeruginosa by pulsed-field gel electrophoresis. J. Clin. Microbiol. 38:464-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saari, M., T. Cheasty, K. Leino, and A. Siitonen. 2001. Phage types and genotypes of Shiga toxin-producing Escherichia coli O157 in Finland. J. Clin. Microbiol. 39:1140-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sato, T., T. Shimizu, M. Watarai, M. Kobayashi, S. Kano, T. Hamabata, Y. Takeda, and S. Yamasaki. 2003. Genome analysis of a novel Shiga toxin 1 (Stx1)-converting phage which is closely related to Stx2-converting phages but not to other Stx1-converting phages. J. Bacteriol. 185:3966-3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sato, T., T. Shimizu, M. Watarai, M. Kobayashi, S. Kano, T. Hamabata, Y. Takeda, and S. Yamasaki. 2003. Distinctiveness of the genomic sequence of Shiga toxin 2-converting phage isolated from Escherichia coli O157:H7 Okayama strain as compared to other Shiga toxin 2-converting phages. Gene 309:35-48. [DOI] [PubMed] [Google Scholar]
- 49.Silbert, S., L. Boyken, R. J. Hollis, and M. A. Pfaller. 2003. Improving typability of multiple bacterial species using pulsed-field gel electrophoresis and thiourea. Diagn. Microbiol. Infect. Dis. 47:619-621. [DOI] [PubMed] [Google Scholar]
- 50.Takeda, Y., H. Kurazono, and S. Yamasaki. 1993. Vero toxins (Shiga-like toxins) produced by enterohemorrhagic Escherichia coli (verocytotoxin-producing E. coli). Microbiol. Immunol. 37:591-599. [DOI] [PubMed] [Google Scholar]
- 51.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Terajima, J., H. Izumiya, S. Iyoda, K. Tamura, and H. Watanabe. 2002. High genomic diversity of enterohemorrhagic Escherichia coli isolates in Japan and its applicability for the detection of diffuse outbreak. Jpn. J. Infect. Dis. 55:19-22. [PubMed] [Google Scholar]
- 53.Terajima, J., H. Izumiya, A. Wada, K. Tamura, and H. Watanabe. 2000. Molecular epidemiological investigation of enterohaemorrhagic Escherichia coli isolates in Japan. J. Appl. Microbiol. 88:99-105. [DOI] [PubMed] [Google Scholar]
- 54.Terajima, J., H. Izumiya, S. Iyoda, K. Tamura, and H. Watanabe. 1999. Detection of a multi-prefectural E. coli O157:H7 outbreak caused by contaminated Ikura-Sushi ingestion. Jpn. J. Infect. Dis. 52:52-53. [PubMed] [Google Scholar]
- 55.Tesh, V. L., and A. D. O'Brien. 1991. The pathogenic mechanisms of Shiga toxin and the Shiga-like toxins. Mol. Microbiol. 5:1817-1822. [DOI] [PubMed] [Google Scholar]
- 56.Watanabe, H., J. Terajima, H. Izumiya, and S. Iyoda. 2003. Molecular typing methods for STEC. Methods Mol. Med. 73:55-65. [DOI] [PubMed] [Google Scholar]
- 57.Watarai, M., T. Sato, M. Kobayashi, T. Shimizu, S. Yamasaki, T. Tobe, C. Sasakawa, and Y. Takeda. 1998. Identification and characterization of a newly isolated Shiga toxin 2-converting phage from Shiga toxin-producing Escherichia coli. Infect. Immun. 66:4100-4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Waters, J. R., J. C. M. Sharp, and V. J. Dev. 1994. Infection caused by Escherichia coli O157:H7 in Alberta, Canada, and in Scotland: a five-year review, 1987-1991. Clin. Infect. Dis. 19:834-843. [DOI] [PubMed] [Google Scholar]
- 59.Yamasaki, S., G. B. Nair, S. K. Bhattacharya, S. Yamamoto, H. Kurazono, and Y. Takeda. 1997. Cryptic appearance of a new clone of Vibrio cholerae serogroup O1 biotype El Tor in Calcutta, India. Microbiol. Immunol. 41:1-6. [DOI] [PubMed] [Google Scholar]
- 60.Yamasaki, S., and Y. Takeda. 1997. Enterohemorrhagic Escherichia coli O157:H7 episode in Japan with a perspective on Vero toxins (Shiga-like toxins). J. Toxicol. Toxin Rev. 16:229-240. [Google Scholar]
- 61.Yamasaki, S., Z. Lin, H. Shirai, A. Terai, Y. Oku, H. Ito, M. Ohmura, T. Karasawa, T. Tsukamoto, H. Kurazono, and Y. Takeda. 1996. Typing of verotoxins by DNA colony hybridization with poly- and oligonucleotide probes, a bead-enzyme-linked immunosorbent assay, and polymerase chain reaction. Microbiol. Immunol. 40:345-352. [DOI] [PubMed] [Google Scholar]
- 62.Yokoyama, K., K. Makino, Y. Kubota, M. Watanabe, S. Kimura, C. H. Yutsudo, K. Kurokawa, K. Ishii, M. Hattori, I. Tatsuno, H. Abe, M. Yoh, T. Iida, M. Ohnishi, T. Hayashi, T. Yasunaga, T. Honda, C. Sasakawa, and H. Shinagawa. 2000. Complete nucleotide sequence of the prophage VT1-Sakai carrying the Shiga toxin 1 genes of the enterohemorrhagic Escherichia coli O157:H7 strain derived from the Sakai outbreak. Gene 258:127-139. [DOI] [PubMed] [Google Scholar]