Abstract
Since 1993, all Mycobacterium tuberculosis isolates recovered in the province of Manitoba, Canada, have been genotyped by the standard IS6110-restriction fragment length polymorphism (RFLP) method for routine surveillance, prevention, and control purposes. To date, our laboratory has collected 1,290 isolates, from which we have identified approximately 390 unique fingerprint patterns or “types.” Although the standard method is well known for being a lengthy and labor-intensive procedure, a more efficient alternative for typing tuberculosis isolates, the mycobacterial interspersed repetitive unit-variable number tandem repeat (MIRU-VNTR) method, has recently gained acceptance. Consequently, all isolates acquired in 2003 (n = 126) were typed by both methods in order to determine the utility of replacing the RFLP method with MIRU typing for all future isolates. Application of Hunter's discriminatory index to the available study population showed that the MIRU method was close in discriminatory power (D) to the RFLP method (DMIRU = 0.831 to 0.984 versus DRFLP = 0.821 to 0.997). Clustering of isolates by using MIRU data correlated with RFLP-derived clustering, lending useful information for either an investigation or confirmation of an incidence of recent transmission. In addition, it was determined that each predominant RFLP type in Manitoba had a corresponding, recognizable MIRU type. It is conceivable that in the future RFLP typing can be replaced with MIRU for real-time, ongoing tuberculosis surveillance in the province.
Despite the best efforts to track transmission of Mycobacterium tuberculosis between individuals, in many instances different patients sharing the same DNA fingerprint cannot be confirmed by a documented epidemiological link, whether due to the possibilities of reactivation, convergence, or other factors (11, 18, 22). In contrast, when links are suggested, there is always the possibility that a different, rather than expected, fingerprint will be observed (3). To irrefutably prove that a cluster derived from molecular data is due to recent transmission, contact data should be available for correlation, and the two investigative tools should not be thought of as mutually exclusive (8, 14, 16, 18).
One way to track transmission within a district is to acquire the fingerprint data from all cases in a defined jurisdiction, in this case Manitoba, into a comprehensive database. The potential benefits of doing so are numerous: a well-described population of patients providing defined strains to be characterized will allow for an infinite amount of study material and starting points in the ongoing attempt to battle the disease in the region. Therefore, Manitoba provincial health authorities instigated routine fingerprinting of all recovered M. tuberculosis isolates commencing in 1993.
Although the current “gold standard” of tuberculosis (TB) strain typing is restriction fragment length polymorphism (RFLP) fingerprinting utilizing the IS6110 element (27), there are numerous, well-known disadvantages inherent in the technique that are limiting to a TB control program (5, 16, 20). The method is both slow (requiring a large culture biomass, technical expertise, and time) with subjective analysis (i.e., band numbering and gel mobility differences), making interlaboratory comparisons difficult, if not impossible. As the amount of RFLP data increases, comparison parameters used must become less stringent to allow for correct clustering among isolates with small band shifts, which could ultimately result in incorrect clustering (29).
In a progression toward creating a universally comparable database, it would be preferable to have a more efficient, unlimited, numerical typing system by using rapid PCR-based tests, such as spoligotyping or variable number tandem repeat (VNTR) typing (18, 29). Spoligotyping is less discriminatory than RFLP and should be used as secondary typing method to confirm clusters established by another method, as well as to type strains with low-copy number RFLP patterns (12). VNTR typing (10), is a more rapid method, requires little culture growth, provides easy-to-compare numerical data, and can be performed by standard agarose gel electrophoresis or, alternatively, developed as a high-throughput system. Mycobacterial interspersed repetitive unit (MIRU) typing utilizes 12 VNTR loci and has been found to have similar discriminatory power to RFLP depending on the sample population, particularly in cases where isolates have zero to five copies of the IS6110 element, such as with M. bovis strains (2, 7, 15, 18, 25). It is plausible that MIRU typing may become the predominant method of choice for TB genotyping (15).
Consequently, we at the National Reference Centre for Mycobacteriology (NRCM) implemented a transitional period for which both RFLP and MIRU typing were performed on all incoming isolates in order to examine whether MIRU can be considered the primary typing methodology and be correlated with previously acquired RFLP types.
MATERIALS AND METHODS
Designated medical staff at the Respiratory Clinic, Health Sciences Centre (HSC), in addition to other health care centers across Manitoba, collected clinical specimens that were forwarded to the Provincial Clinical Mycobacteriology Laboratory at the Health Sciences Centre for isolation and identification of M. tuberculosis. The Accuprobe M. tuberculosis complex kit (Gen-Probe, Inc., San Diego, Calif.) was used to identify M. tuberculosis from positive cultures. M. tuberculosis isolates were forwarded to the NRCM and subsequently processed for DNA genotyping according to the internationally standardized methodology of IS6110-RFLP (27).
The NRCM has an existing RFLP database managed by using Bionumerics software version 3.0 (Applied Maths, Kortrijk, Belgium). The cases evaluated in the present study have been acquired over a 10-year period and consist of 1,290 entries. All cases were from Manitoba from January 1993 to December 2003. Of these, there are approximately 390 unique fingerprint types or patterns. Fingerprints are defined as the same type if they are identical or share a one-band difference. A one-band difference is noted as a lowercase letter (a, b, c, etc.) next to the number assigned for the type. Fingerprint pattern comparisons are routinely calculated by using the Dice coefficient with a band tolerance of 1.5% and an optimization value of 1.5%.
All isolates recovered from 1 January to 31 December 2003 were subjected to MIRU typing. In all, 126 isolates were recovered from 122 individual patients (four samples were serial isolates). Twelve MIRU loci were amplified with the primers and protocol published by Cowan et al. (7). Briefly, 12 master mixes were made and distributed to 96-well plates. For each sample, 20 ng of template DNA (originally extracted for the RFLP procedure [27]) was added across the row of 12, and the plate was sealed and placed in an MJ Research 96-well block thermocycler. After the thermocycling step, the PCR products were electrophoresed on a 2% agarose gel and sized with a 50-bp ladder (Novagen). The H37Rv control was added in each plate to confirm clinical isolate product sizes. All MIRU patterns were then entered into Bionumerics for sizing, visually confirmed, and then added as a character set for analysis. Comparisons of the resulting numerical values are calculated by considering each loci value as a variable in categorical analysis.
To compare isolates combining both methods, a multiexperiment composite data set with MIRU character data and RFLP fingerprint data was created by using available tools in Bionumerics. Each method was weighed as equal. The composite data set utilized the “take from experiments” parameter for cluster analysis.
Evaluation of the discriminatory power of the two typing methods both separately as well as in combination was undertaken by using the Hunter-Gaston index (HGI) (12). For RFLP, the discrimination index was calculated in two ways: defining clusters as sharing identical fingerprint patterns versus defining clusters that contain identical fingerprint patterns plus or minus a one-band difference. The calculation was applied to both the entire sample population and a discrete sample set comprised of unique isolates. Unique isolates are defined as strains that did not share identical RFLP and MIRU patterns.
RESULTS
A total of 126 isolates were acquired for 2003 and typed by both IS6110 RFLP and MIRU. Clustering results from each method were compared to each other, as well as against a combined typing approach to determine the discriminatory power of the typing methods applied in Manitoba. As shown in Table 1, there are fewer unique isolates and more clustering with MIRU (72% of total) than with RFLP (65 to 70%). MIRU typing gave resolving power close to that of RFLP: when applied to the entire population, MIRU (D = 0.831) seemed more apt to discriminate between strains than RFLP (D = 0.821), if using the definition that each type can be comprised of strains with an identical or a one-band difference in fingerprint. If the definition of a type is limited to contain only identical fingerprints, D for RFLP increases for the population to 0.836.
TABLE 1.
HGI values obtained by using the different typing methods and definitions of a “cluster” applied in this studya
| Set and methodb | No. of isolates | No. of unique types | No. of unique isolates | No. of clusters (ranges) | No. of clustered isolates (%) | HGI |
|---|---|---|---|---|---|---|
| Set 1 | ||||||
| MIRU | 126 | 48 | 35 | 11 (2-50) | 91 (72) | 0.831 |
| RFLP 1 | 126 | 46 | 38 | 9 (2-51) | 88 (70) | 0.821 |
| RFLP 2 | 126 | 55 | 44 | 11 (2-45) | 82 (65) | 0.836 |
| Both | 126 | 60 | 51 | 9 (2-44) | 75 (60) | 0.870 |
| Set 2 | ||||||
| MIRU | 60 | 45 | 37 | 8 (2-6) | 23 (38) | 0.984 |
| RFLP 1 | 60 | 47 | 40 | 7 (2-5) | 20 (33) | 0.987 |
| RFLP 2 | 60 | 55 | 51 | 4 (3-2) | 9 (15) | 0.997 |
| Both | 60 | 60 | 60 | 0 | 0 | 1.00 |
The first set includes the entire sample population for 2003 (n = 126), while the second set is calculated from a unique, discrete set of isolates (n = 60).
RFLP 1, clustered fingerprints are defined as having identical or a one-band difference between patterns; RFLP 2, clustered fingerprints are defined as having identical patterns.
Application of the HGI to a limited number of isolates (n = 60, representative of unique isolates only as shown in Fig. 1) demonstrated the same: there were fewer unique isolates and more clustering when using MIRU (38%) rather than with RFLP (15 to 33%). The resolving power between the strains was highest for RFLP (0.997), again using the definition that a cluster or type must share identical fingerprints. The combined approach is normalized to 1.0, since these 60 isolates were chosen as representatives of unique patterns when both methods were combined.
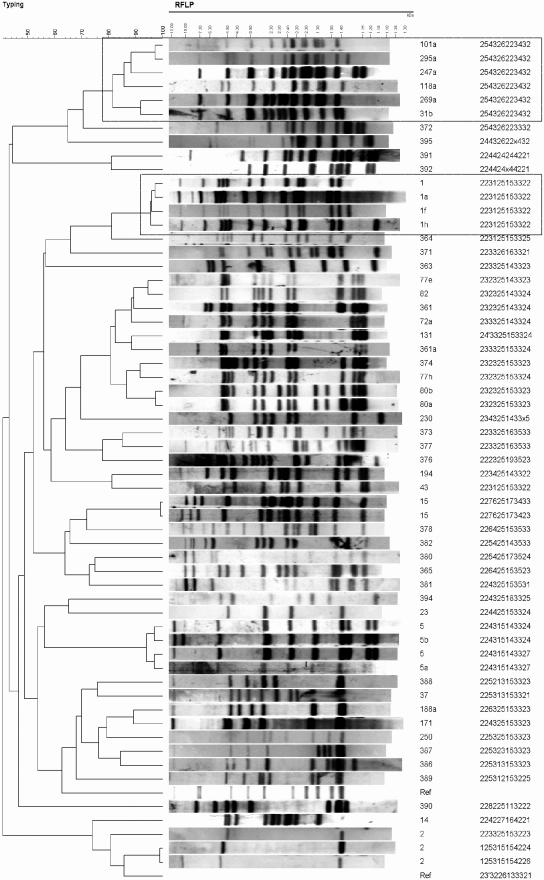
FIG. 1.
Unique isolates (n = 60) obtained in the present study as delineated by RFLP and MIRU typing. A dendrogram was created with Bionumerics v3.0 software (Applied Maths) by using the “take from experiments” parameter for multiexperiment comparisons. Boxed isolates are those mentioned in text: the type 1′ group with variable one-band differences among RFLP patterns but invariable MIRU patterns and a group of six isolates that share similar RFLP patterns and yet identical MIRU patterns.
Clustering of the isolates by RFLP was correlated with MIRU-VNTR typing, with major clusters defined for types 1, 5, and 15 with either method (data not shown). The type 1 cluster contained 51 fingerprints with identical or one-band-different patterns, which was perfectly clustered by one MIRU pattern. The cluster of 12 identical fingerprints for type 15 showed a single isolate whose MIRU pattern differed by one loci (loci 39). MIRU typing also revealed one difference in loci 40 within the cluster of 10 type 5s (5, 5a, and 5b), which split the cluster into two groups: one group consisting of 5 and 5a and the other containing 5 and 5b. These isolates were defined as unique due to a different MIRU pattern and can be visualized in Fig. 1. In contrast, there was one cluster of six isolates delineated by MIRU (sharing identical MIRU patterns) that did not share an identical or a one-band difference between RFLP patterns. These isolates were grouped together by RFLP, sharing some level of banding similarities (72.5 to 96.0%), with common bands at 4.5, 3.5, 2.8, 2.2, 2.0, 1.8, and 1.45 kDa, as seen in Fig. 1.
DISCUSSION
This study examined the possibility of utilizing MIRU typing to replace RFLP typing for the province. The goal was to take a year of submissions (i.e., 2003), perform both typing methodologies on all isolates to correlate, if possible, recognizable MIRU pattern types that correspond to predominant RFLP types, and ultimately to determine the validity of implementing the MIRU method as the first-line procedure for genotyping in Manitoba.
The ability of determining whether MIRU types correspond to RFLP types is underpinned by the fact that there currently exists an accumulation of 10 years of RFLP data for the province. These data have already shown which predominant strains are circulating (for example, the arbitrarily named “type 1”) that are either endemic or part of an ongoing transmission chain (4). The epidemiology of Manitoba TB strains has already been extensively studied and, without unique MIRU profiles corresponding to each RFLP type already established, the potential for inclusive study of prior data may not be maximized. Advantageously, the MIRU patterns accrued for 2003 closely correlate with that of RFLP data. For example, the predominant strain in Manitoba, the aforementioned type 1′, accounts for 25% of the isolates obtained overall, with ca. 75% of isolates obtained from cases in Aboriginal individuals (4). Although the fingerprint pattern for these isolates can differ by one band, the MIRU pattern was identical for all isolates (see Fig. 1). No other isolate with a different fingerprint patterns used in the present study shared this unique type 1 MIRU pattern. This observation was evident in other type clusters as well (i.e., types 5 and 15), leading to clusters of MIRU types that correlated with clusters of RFLP types. The one cluster of six isolates that shared an identical MIRU pattern, but not the same RFLP pattern, did share some commonalities within banding patterns. These isolates were grouped together in a separate cluster exhibiting band similarities of 72.5 to 96.0% when compared against the whole 126 isolates. These isolates could be related, and band differences simply may be due to the mobility of the IS6110 element over a long period of time. Thus, it can be assumed with some certainty that future isolates sharing the same MIRU pattern as one of those identified in the 2003 group would share a similar fingerprint pattern and likely would cluster by RFLP as well. This result infers that if RFLP were replaced by MIRU as a rapid, front-line typing method, it would be beneficial to validate MIRU-derived clusters by RFLP as a secondary-typing measure to confirm potential chains of transmission and to compare to strains isolated prior to 2003.
Although clustering was shown to be analogous with either typing method, the second part of the present study was to determine whether this method can replace RFLP as a first-line typing method, i.e., if it is as discriminatory and will indicate a circumstance of ongoing transmission within a group to alert epidemiologists to further investigate. The validity of a new method can be ascertained by addressing the following aspects: (i) typeability, (ii) reproducibility, (iii) stability, and (iv) discriminatory power (12, 24). Typeability (T) is defined as the proportion of strains that are assigned a type by the method in use (24). To test this aspect of a typing method in an ideal setting would require a sample of unique, well-characterized, epidemiologically unlinked strains that would result in a calculated value of T = 1. When two or more methods are compared in the more realistic, nonideal situation without a completely unique sample population, a typing system that provides a value closer to approaching 1 is the better system. Reproducibility (R) described the ability of a method to assign the same strain to the same group or type in independent testing. Stability (S) represents the ability of a marker or pattern to remain stable after serial passages. Finally, discriminatory power (D), is the average probability that a typing system will assign a different type to two unrelated strains randomly sampled in a population. This calculation was an application of Simpson's index of diversity by Hunter and Gaston that has been valuable for comparison of bacterial typing systems (12). Again, in an ideal situation, with a set of completely unrelated strains, D would equal 1.
In regard to typeability (where T = number of isolates assigned a type [Nt]/number of isolates tested [N]) (24), both methods can assign a type to every isolate, giving equal values of T = 1. Reproducibility of methods was not tested in the present study since it has been documented previously for both methods as being 100% (13, 25). This also applied for stability testing due to short period of time the present study encompassed, as well as the multitude of reference material available reporting the ambiguous stability of the IS6110 element (1, 6, 9, 19, 28, 30) and, to a lesser extent, MIRU typing (15, 21, 25).
Applying the HGI to the entire sample population from 2003 (n = 126), the index for DNA fingerprinting, while still higher than for MIRUs, is low (0.836, Table 1). This is due to the inherent assumption of the calculation that n is the number of unrelated strains and the weight of a predominant type (such as type 1) that skews the data. However, if the principle of the index is adhered to and only unique isolates or types are chosen to represent a unique sample population, the sample size is reduced to 60 unique types, and DMIRU = 0.984 versus DRFLP = 0.987 to 0.997. We chose the 60 unique types based on both methods: one representative isolate was taken per cluster of isolates that shared both identical RFLP and MIRU patterns.
The predicament in using this approach to calculate discriminatory power lies in the aforementioned marker stability. The IS6110 element is thought to be sufficiently stable to allow the interpretation that a cluster of strains sharing an identical IS6110 RFLP pattern reflects an event of recent transmission (6, 9, 28). However, another report suggests that the rate of change of this marker may be too fast to be reliably used for outbreak investigations (1). There is a great deal of discussion on the many factors that can affect the marker stability of the IS6110 transposable element, such as disease state, i.e., pulmonary, extrapulmonary, or both, with possible bacterial dissemination (1). Other factors that may affect marker stability are duration of disease process, IS6110 element copy number, or specific genotype, among others, leading to strain variants that are descendants of the same clone (1, 28, 30). Serial patient isolates have shown up to three-band RFLP pattern differences from the originally acquired sample (9). As a result, using a calculation where only identical RFLP patterns are considered the same type, and isolates with one-band differences that are potentially involved in the same transmission chain are classified as a different type, the value for D may be overinflated. Accordingly, the extent of transmission would be underestimated. Thus, the higher discriminatory power attained with RFLP may be misleading as the better tool for outbreak investigations.
MIRU loci have been reported to have a slower molecular clock than the IS6110 element (15, 21, 26), a predictable fact since this method is considered to be less discriminatory that RFLP. MIRUs, which have shown a greater marker stability resulting in identical patterns between isolates with slightly different RFLP patterns, could potentially more accurately reflect a cluster, although resulting in a lower calculated value for D. This was correlated with our findings of clusters of type 1, 5, and 15, as well as of a cluster of six isolates with nonidentical RFLP patterns and yet an identical MIRU profile. These six isolates clustered together in branch separate from the other 120 isolates examined. In addition, types 5 and 15 were found to have two MIRU patterns within their respective clusters, with a difference in loci 40 and 39, respectively. These loci have both been shown to show considerable allelic diversity in separate studies, depending on the population studied (15, 17, 23). Similar one-loci differences were reported in prior studies: at locus 26, with serial patient isolates that shared identical IS6110 patterns (21) and, at locus 4, within the M. bovis BCG genealogy (26).
Thus, in order to do an entirely accurate comparison, it is necessary to acquire a set of unique, characterized strains from different locations, confirmed to be unlinked epidemiologically. Regardless, when applying this formula to the available sample population, it was concluded that the RFLP method is only marginally of greater discriminatory power than that of the MIRU method. This is not surprising when one considers the study sample only included three low-copy IS6110 containing strains and that the proportion of low-copy band number isolates (being strains containing five or fewer bands) in Manitoba accounts for <10% of the isolates.
Nonetheless, it is these low-copy IS6110 containing strains that would benefit from replacing RFLP with MIRU. There are many other advantages to the use of a PCR-based test such as MIRU as a first-line method, such as the small amount of starting material necessary (20 ng versus 4.5 μg for RFLP), as well as faster results obtained by the procedure alone (1 day versus 4 days for RFLP). This reduces technician time and allows the processing of more samples at a lower cost. Although RFLP typing has a slightly higher discriminatory rate (and this can be partially attributed to the stability of the IS6110 element), one questions if it is significant in the global scheme of subtyping. MIRU has proven invaluable for rapidly determining potential outbreak situations (unpublished data). To reiterate, whichever typing method is utilized in defining clusters, all are only a guesstimate of recent transmissions in a population. Even calculations aimed at reducing the guesswork such as the n or the n − 1 methods (29) do not guarantee that every clustered strain is a result of ongoing transmission as opposed to reactivation or convergence. Beyond investigation of every isolate with an entire battery of testing to attain maximum specificity, typing methods are accepted as a valuable tool for epidemiologists and prevention and control authorities. These data allow the NRCM to implement MIRU as a rapid, first-line typing procedure, with RFLP as a secondary method for delineating clusters of concern.
Acknowledgments
We are grateful for the technical support provided by Alisa Thompson and April Powell at the NRCM and Nancy Smart at the Health Sciences Center (HSC).
REFERENCES
- 1.Alito, A., N. Morcillo, S. Scipioni, A. Dolmann, M. I. Romano, A. Cataldi, and D. van Soolingen. 1999. The IS6110 restriction fragment length polymorphism in particular multidrug-resistant Mycobacterium tuberculosis strains may evolve too fast for reliable use in outbreak investigation. J. Clin. Microbiol. 37:788-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barlow, R. E. L., D. M. Gascoyne-Binzi, S. H. Gillespie, A. Dickens, S. Qamer, and P. M. Hawkey. 2001. Comparison of variable number tandem repeat and IS6110-restriction fragment length polymorphism analyses for discrimination of high- and low-copy-number IS6110 Mycobacterium tuberculosis isolates. J. Clin. Microbiol. 39:2453-2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Behr, M. A., P. C. Hopewell, E. A. Paz, L. M. Kawamura, G. F. Schecter, and P. M. Small. 1998. Predictive value of contact investigation for identifying recent transmission of Mycobacterium tuberculosis. Am. J. Respir. Crit. Care Med. 158:465-469. [DOI] [PubMed] [Google Scholar]
- 4.Blackwood, K. S., Al-Azem, A., L. J. Elliott, Hershfield, E. S., and A. M. Kabani. 2003. Conventional and molecular epidemiology of tuberculosis in Manitoba. BMC Infect. Dis. 3:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braden, C. R., J. T. Crawford, and B. A. Schable. 2002. Assessment of Mycobacterium tuberculosis genotyping in a large laboratory network. Emerg. Infect. Dis. 8:1210-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cave, M. D., K. D. Eisenach, G. Templeton, M. Salfinger, G. Mazurek, J. H. Bates, and J. T. Crawford. 1994. Stability of DNA fingerprint pattern produced with IS6110 in strains of Mycobacterium tuberculosis. J. Clin. Microbiol. 32:262-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cowan, L. S., L. Mosher, L. Diem, J. P. Massey, and J. T. Crawford. 2002. Variable-number tandem repeat typing of Mycobacterium tuberculosis isolates with low copy numbers of IS6110 by using mycobacterial interspersed repetitive units. J. Clin. Microbiol. 40:1592-1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crawford, J. T. 2003. Genotyping in contact investigations: a CDC perspective. Int. J. Tuberc. Lung Dis. 7:S453-S457. [PubMed] [Google Scholar]
- 9.de Boer, A. S., M. W. Borgdorff, P. E. de Haas, N. J. Nagelkerke, J. D. van Embden, and D. van Soolingen. 1999. Analysis of rate of change of IS6110 RFLP patterns of Mycobacterium tuberculosis based on serial patient isolates. J. Infect. Dis. 180:1238-1244. [DOI] [PubMed] [Google Scholar]
- 10.Frothingham, R., and O. W. Meeker. 1998. Genetic diversity in the Mycobacterium tuberculosis complex based on variable numbers of tandem DNA repeats. Microbiology 144:1189-1196. [DOI] [PubMed] [Google Scholar]
- 11.Gillespie, S. H., A. Dickens, and T. D. McHugh. 2000. False molecular clusters due to nonrandom association of IS6110 with Mycobacterium tuberculosis. J. Clin. Microbiol. 38:2081-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunter, P. R., and M. A. Gaston. 1988. Numerical index of the discriminatory ability of typing systems: an application of Simpson's index of diversity. J. Clin. Microbiol. 26:2465-2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kremer, K., D. van Soolingen, R. Frothingham, W. H. Haas, P. W. Hermans, C. Martin, P. Palittapongarnpim, B. B. Plikaytis, L. W. Riley, M. A. Yakrus, J. M. Musser, and J. D. van Embden. 1999. Comparison of methods based on different molecular epidemiological markers for typing of Mycobacterium tuberculosis complex strains: interlaboratory study of discriminatory power and reproducibility. J. Clin. Microbiol. 37:2607-2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mathema, B., P. J. Bifani, J. Driscoll, L. Steinlein, N. Kurepina, S. L. Moghazeh, E. Shashkina, S. A. Marras, S. Campbell, B. Mangura, K. Shilkret, J. T. Crawford, R. Frothingham, and B. N. Kreiswirth. 2002. Identification and evolution of an IS6110 low-copy-number Mycobacterium tuberculosis cluster. J. Infect. Dis. 185:641-649. [DOI] [PubMed] [Google Scholar]
- 15.Mazars, E., S. Lesjean, A. L. Banuls, M. Gilbert, V. Vincent, B. Gicquel, M. Tibayrenc, C. Locht, and P. Supply. 2001. High-resolution minisatellite-based typing as a portable approach to global analysis of Mycobacterium tuberculosis molecular epidemiology. Proc. Natl. Acad. Sci. USA 98:1901-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McNabb, S. J., C. R. Braden, and T. R. Navin. 2002. DNA fingerprinting of Mycobacterium tuberculosis: lessons learned and implications for the future. Emerg. Infect. Dis. 8:1314-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mokrousov, I., O. Narvskaya, E. Limeschenko, A. Vyazovaya, T. Otten, and B. Vyshnevsky. 2004. Analysis of the allelic diversity of the mycobacterial interspersed repetitive units in Mycobacterium tuberculosis strains of the Beijing family: practical implications and evolutionary considerations. J. Clin. Microbiol. 42:2438-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mostrom, P., M. Gordon, C. Sola, M. Ridell, and N. Rastogi. 2002. Methods used in the molecular epidemiology of tuberculosis. Clin. Microbiol. Infect. 8:694-704. [DOI] [PubMed] [Google Scholar]
- 19.Niemann, S., S. Rusch-Gerdes, E. Richter, H. Thielen, H. Heykes-Uden, and R. Diel. 2000. Stability of IS6110 restriction fragment length polymorphism patterns of Mycobacterium tuberculosis strains in actual chains of transmission. J. Clin. Microbiol. 38:2563-2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roring, S., A. Scott, D. Brittain, I. Walker, G. Hewinson, S. Neill, and R. Skuce. 2002. Development of variable-number tandem repeat typing of Mycobacterium bovis: comparison of results with those obtained by using existing exact tandem repeats and spoligotyping. J. Clin. Microbiol. 40:2126-2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Savine, E., R. M. Warren, G. D. van der Spuy, N. Beyers, P. D. Van Helden, C. Locht, and P. Supply. 2002. Stability of variable-number tandem repeats of mycobacterial interspersed repetitive units from 12 loci in serial isolates of Mycobacterium tuberculosis. J. Clin. Microbiol. 40:4561-4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Small, P. M., P. C. Hopewell, M. D. Samir, P. Singh, A. Paz, J. Parsonnet, D. C. Ruston, B. S. Gisela, F. Schecter, C. L. Daley, and G. Schoolnik. 1994. A population-based study using conventional and molecular methods. N. Engl. J. Med. 330:1703-1709. [DOI] [PubMed] [Google Scholar]
- 23.Sola, C., I. Filliol, E. Legrand, S. Lesjean, C. Locht, P. Supply, and N. Rastogi. 2003. Genotyping of the Mycobacterium tuberculosis complex using MIRUs: association with VNTR and spoligotyping for molecular epidemiology and evolutionary genetics. Infect. Genet. Evol. 3:125-133. [DOI] [PubMed] [Google Scholar]
- 24.Struelens, M. J. 1996. Consensus guidelines for appropriate use and evaluation of microbial epidemiologic typing systems. Clin. Microbiol. Infect. 2:2-11. [DOI] [PubMed] [Google Scholar]
- 25.Supply, P., S. Lesjean, E. Savine, K. Kremer, D. van Soolingen, and C. Locht. 2001. Automated high-throughput genotyping for study of global epidemiology of Mycobacterium tuberculosis based on mycobacterial interspersed repetitive units. J. Clin. Microbiol. 39:3563-3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Supply, P., E. Mazars, S. Lesjean, V. Vincent, B. Gicquel, and C. Locht. 2000. Variable human minisatellite-like regions in the Mycobacterium tuberculosis genome. Mol. Microbiol. 36:762-771. [DOI] [PubMed] [Google Scholar]
- 27.van Embden, J. D., M. D. Cave, J. T. Crawford, J. W. Dale, K. D. Eisenach, B. Gicquel, P. Hermans, C. Martin, R. McAdam, T. M. Shinnick, et al. 1993. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J. Clin. Microbiol. 31:406-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Warren, R. M., G. D. van der Spuy, M. Richardson, N. Beyers, C. Booysen, M. A. Behr, and P. D. Van Helden. 2002. Evolution of the IS6110-based restriction fragment length polymorphism pattern during the transmission of Mycobacterium tuberculosis. J. Clin. Microbiol. 40:1277-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang, Z. 2003. Molecular epidemiology of tuberculosis. Front Biosci. 8:d440-d450. [DOI] [PubMed] [Google Scholar]
- 30.Yeh, R. W., L. A. Ponce-de, C. B. Agasino, J. A. Hahn, C. L. Daley, P. C. Hopewell, and P. M. Small. 1998. Stability of Mycobacterium tuberculosis DNA genotypes. J. Infect. Dis. 177:1107-1111. [DOI] [PubMed] [Google Scholar]