Abstract
Infection by Penicillium marneffei in human immunodeficiency virus-positive patients in India has recently been described; the aim of our study was to survey wild rodents and their associated environment in order to identify the natural populations of this fungus. Surveys recovered P. marneffei from the internal organs of 10 (9.1%) of 110 bamboo rats (Cannomys badius) examined from Manipur state, India, an area endemic for penicilliosis marneffei. Identification of the isolates was based on a detailed study of their morphological characteristics, in vitro conversion to fission yeast form, and exoantigen tests. Multilocus microsatellite typing (MLMT) of the isolates revealed five genotypes. No genotypes were shared between sample sites, and all bamboo rats were infected with a single genotype within sample sites, demonstrating spatial genetic heterogeneity. One MLMT genotype was identical to that seen in a human isolate, suggesting that either coinfection from a common source or host-to-host transmission had occurred. This demonstrates the utility of an MLMT-based approach to elucidating the epidemiology of P. marneffei.
Penicillium marneffei is the only dimorphic species of the genus Penicillium and is the etiological agent of penicilliosis marneffei. This opportunistic fungal infection occurs among human immunodeficiency virus (HIV)-infected and other immunocompromised patients in several regions of southeast Asia. Areas where P. marneffei infection is known to be endemic include Thailand, southern China, Taiwan, Hong Kong, Malaysia, Indonesia, Viet Nam, Myanmar (Burma), and Manipur state in India (10, 11, 23, 27, 28). A single case of the disease in an African from Ghana, who had no history of travel to Asia, has also been described (21). Several cases of penicilliosis marneffei have been reported from Europe, the United States, Australia, and Singapore in patients who had prior history of visits to areas where the infection is endemic (17, 28).
Initial isolation of P. marneffei was from a captive bamboo rat (Rhizomys sinensis) used for laboratory experiments in South Vietnam (3). The native bamboo rat had been experimentally inoculated with the scrub typhus bacterium Rickettsia orientalis (now designated Rickettsia tsutsugamushi). At autopsy, the rodent was found to have an enlarged liver and spleen, viscous ascitic fluid, and epiploic nodules. Cultures from all the organs yielded a Penicillium species, which proved pathogenic to hamsters (Mesocricetus auratus). The fungus was subsequently described as a new species by Segretain (25), who named the fungus Penicillium marneffei in honor of Hubert Marneffei, then-director of the Pasteur Institute in French Indochina. In later decades, several workers investigated the prevalence of P. marneffei in bamboo rats in different geographic areas (1, 5, 9, 20, 29, 33). Four species of bamboo rats, R. sinensis, Rhizomys pruinosus, Rhizomys sumatrensis, and Cannomys badius, were identified as natural hosts of P. marneffei (1, 5, 9). The aim of the present study was to investigate whether P. marneffei occured in bamboo rats in Manipur state, an area where several human cases of penicilliosis marneffei have recently been described (23, 27).
MATERIALS AND METHODS
Description of the study area.
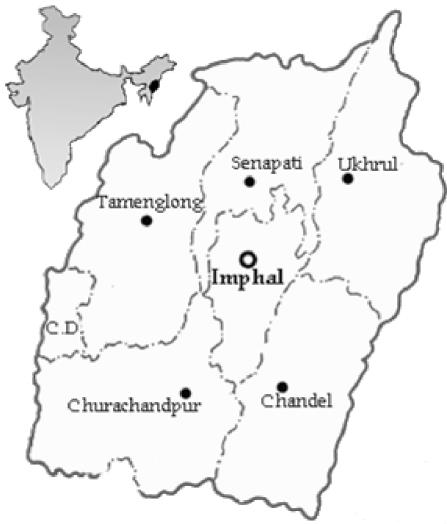
The sites where bamboo rats were trapped are located in the Senapati district, about 62 km north of the state capital of Imphal, and the Tamenglong district, approximately 150 km northwest of Imphal in Manipur state (Fig. 1). Manipur is situated from 23.80°N to 25.68°N and from 93.03°E to 94.78°E; it is predominantly a hill state in the northeast of India, with an elevation ranging from 800 to 3,000 m above mean sea level. Geologically, the region is a part of the Manipur-Nagaland orogenic belt, which in turn forms the northern part of the Indo-Burmese range. The region has a subtropical climate, with temperatures ranging between a mean maximum of 32°C and a mean minimum of 0°C. The rainy season lasts from May to October, with an average rainfall of 1,980 mm. The Senapati district is located from 93.29°E to 94.15°E and 24.37°N. The climate is humid and subtropical, with an annual rainfall ranging from 671 to 1,454 mm. The temperature ranges from a minimum of 3.36°C to a maximum of 34.14°C. The Tamenglong district is situated from 93.30°E to 24.59°N. It is entirely composed of hills, ranges, and narrow valleys (altitude, 1,260 m above mean sea level). The temperature ranges from a minimum of 4°C to a maximum of 31°C. The climate is humid and subtropical with heavy rainfall, with an annual mean rainfall of 3,135 mm. Tropical evergreen forest, subtropical forest, and virgin forest are represented in this district. The soil in both districts is clay loam with patches of clay and loam soil. The predominant species of bamboo trees in these places are Bambusa arundinacea and Melocanna bambusoides. The bushy bamboo species commonest in the Senapati and Tamenglong districts are Cephalostachyum capitatum and Bambusa tulda, respectively.
FIG. 1.
Map of Manipur state, showing the locations of the Senapati and Tamenglong districts where bamboo rats were trapped. Bamboo rats were trapped on four separate bamboo plantations within the Tamenglong district.
Investigation of rodents and soil samples.
Professional trappers were contracted to capture the bamboo rats. One hundred and ten bamboo rats of the species C. badius, the so-called bay bamboo rat, were trapped on bamboo plantations in Manipur state and subsequently examined. Thirty-five of the animals were trapped on two different bamboo plantations in the Senapati district. The remaining 75 rats were trapped on four different bamboo plantations in the Tamenglong district. All the captured rats were brought to the laboratory, euthanized within 1 to 3 days with ether, and aseptically dissected. Portions of lungs, liver, spleen, and pancreas of the animals were removed and minced. These were then inoculated on multiple slopes of Sabouraud dextrose agar (SDA) and brain heart infusion (BHI) agar (HIMedia Laboratories, Mumbai, India), both supplemented with chloramphenicol (0.05 mg ml−1). In 30 of the animals, portions of kidneys and intestines were also similarly processed for culture. Small portions of the internal organs of 25 of the animals were preserved in 10% formalin for histology. Cultures were incubated at 25 to 28°C for 3 to 4 weeks and periodically examined for growths suggestive of P. marneffei and other potentially pathogenic fungi. Pure cultures were obtained by subculturing onto SDA slants.
Seventy-two rodents of five other species, Bandicota bengalensis, Rattus norvegicus, Rattus rattus, Rattus nitidus, and Mus musculus, were trapped on bamboo plantations in Guwahati (Assam), Bara Pani, Umeam, near Shillong (Meghalaya), Imphal (Manipur), and Tandong (Sikkim), India. These species were all investigated for infection by P. marneffei by the methods described above.
Twenty-five soil samples collected from the burrows of C. badius were also examined for occurrence of P. marneffei by dilution plating and the mouse passage technique. Triplicate plates of SDA containing chloramphenicol (0.05 mg ml−1), mold inhibitory agar, yeast extract phosphate agar, and Dichloran Rose Bengal agar (HIMedia Laboratories) supplemented with chloramphenicol (0.05 mg ml−1) were streaked with 0.2-ml quantities of a 1:10 dilution of the suspension of each soil sample. The mouse passage technique was essentially as that described by Beneke and Rogers (2). Three male, 4-week-old Swiss mice were injected intraperitoneally with 0.5-ml quantities of a suspension from each environmental sample. The mice were sacrificed after a period of 4 to 5 weeks, and portions of their internal organs (lungs, liver, and spleen) were cultured on multiple slopes of SDA and BHI agar containing chloramphenicol. Ten samples each of bamboo leaves and shoots were collected from the sites where bamboo rats were trapped and were similarly processed. All the inoculated plates and/or slopes were incubated at 28°C for a period of 3 weeks. Fungal colonies growing in the cultures were purified by subculture and subsequently identified. An additional 120 soil samples were collected and screened from the burrows of other rodent species and from non-rodent-associated sites in bamboo plantations.
Identification of isolates.
Isolates were identified as P. marneffei by a detailed examination of their gross and microscopic features. Identification was confirmed by (i) in vitro conversion to the fission yeast form on BHI agar (HIMedia) at 37°C and (ii) the exoantigen test. For the latter test, the antigenic extract from 1-week-old SDA slant cultures of each isolate was tested by immunodiffusion against rabbit anti-P. marneffei serum with the appropriate controls (26). Proteinase activity of mycelial and yeast forms of all the isolates was tested with 0.4% albumin as a substrate, by using the method of Ruchel et al. (24) with minor modifications recommended by Chakrabarti et al. (4). Mycelial form and yeast form cultures for testing for proteinase were incubated for 10 days at 28 and 37°C, respectively. Both mycelial and yeast forms of all the isolates were also tested for urea hydrolysis with Christensen's urea agar (8). Gelatin and casein hydrolysis was tested by standard procedures (2). Three isolates of P. marneffei from cases in Thailand that were obtained from A. Chindamporn and N. Vanittanakom were included in each test series.
Multilocus microsatellite typing (MLMT) of isolates.
DNA was extracted from 7-day-old cultures of each isolate as described previously (32). Multilocus genotypes for each isolate were then generated by scoring polymorphisms at 23 microsatellite-containing loci by the protocol described previously (13). Briefly, 1 μl of a 1/10 dilution of the DNA from each isolate was amplified with the QIAGEN Multiplex PCR kit with a working primer concentration of 0.2 μM. Cycling conditions were as follows: 95°C for 15 min; followed by 35 cycles each consisting of 94°C for 30 s, 57°C for 90 s, and 72°C for 60 s; followed by a final extension step of 60°C for 30 min. Subsequently, the PCR products were electrophoresed through a capillary sequencer with a POP6 gel and a ROX-500 internal size standard (Applied Biosystems). Alleles were scored using Genotyper software (Applied Biosystems), and unique genotypes were then assigned a specific microsatellite type (MT) identifier according to the P. marneffei MLMT scheme (13). These multilocus genotypes are held in an SQL Server relational database at http://pmarneffei.multilocus.net/. Two clinical isolates, CBS 101038 and MT30, were also typed with the MLMT scheme. CBS 101038 was obtained from the Centraalbureau voor Schimmelcultures (CBS) and was recovered by A. Chakrabarti in 1998 from one of the first four autochthonous cases of penicilliosis marneffei detected in Manipur state (27). Isolate MT30 was isolated in 2000 from an HIV-positive patient in Chiang Mai, Thailand, by N. Vanittanakom.
RESULTS
Prevalence of P. marneffei in bamboo rats (C. badius).
Ten (9.1%) of 110 C. badius rats examined over a period of 10 months (from June 2002 to April 2003) were positive for P. marneffei (Table 1). All the P. marneffei-positive bamboo rats appeared healthy. At autopsy, no gross lesions were observed on any of the internal organs of the rats. Two of the isolates were recovered from rats trapped in the Senapati district, and the remaining eight isolates were from rats trapped in the Tamenglong district in Manipur state. In 2 of the 10 positive rats, P. marneffei was cultured from the liver and spleen. Cultures of the liver, spleen, and pancreas from three rats yielded P. marneffei; cultures of the fungus from lungs, liver, and spleen of another two animals also produced the fungus. For the remaining three P. marneffei-positive animals, only spleen tissue cultures yielded the fungus. Histopathological examination of lungs, liver, and spleen of 15 C. badius rats (including 5 of the P. marneffei-positive animals) did not reveal any fungal elements. Unfortunately, we were unable to perform histological analyses of the internal organs of the remaining 10 animals.
TABLE 1.
Prevalence of P. marneffei in bamboo rats (C. badius) in Manipur state, India, from June 2002 to April 2003
| Mo and yr | Locality | No. of rats investigated | No. of rats (%) testing positive |
|---|---|---|---|
| June 2002 | Senapati district | 8 | 0 |
| November to December 2002 | Senapati district | 15 | 2 (13.3) |
| January 2003 | Senapati district | 12 | 0 |
| February 2003 | Tamenglong district | 14 | 0 |
| March 2003 | Tamenglong district | 48 | 7 (14.6) |
| April 2003 | Tamenglong district | 13 | 1 (7.7) |
| Total | 110 | 10 (9.1%) |
P. marneffei was not recovered from any of the 25 samples of soil from the burrows of C. badius or from 10 samples (each) of bamboo shoots and leaves from the surrounding areas. Further sampling of soil samples from the immediate areas surrounding the burrows of bamboo rats and from the burrows of other rodent species (120 soil samples in total) were also negative for P. marneffei.
None of the 72 rodents of the other five species (B. bengalensis, R. norvegicus, R. rattus, R. niditus, and M. musculus) trapped on bamboo plantations from other areas of northeast India were found to harbor P. marneffei.
Characteristics of the isolates.
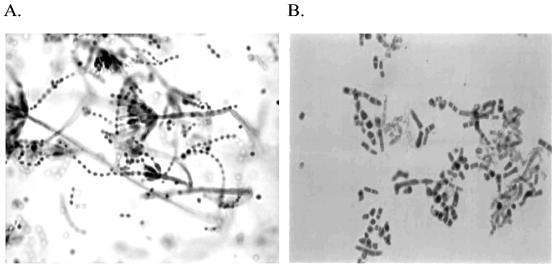
The characteristics of the isolates were compatible with the standard description of P. marneffei (1, 8, 10, 22). The diffusible red pigment could be easily observed on the reverse of the colonies in young cultures on SDA, later turning the entire medium wine red. Colonies of the isolates on corn meal agar (HIMedia) were relatively slow growing with reduced red pigmentation. Regarding the microscopic features, all the 10 isolates had both symmetrical biverticillate and monoverticilliate penicilli (Fig. 2A) with the former generally predominant; occasionally, both types of penicilli were observed in the same branch in some of the isolates. Microscopic examination of growth on BHI at 37°C revealed cylindrical (arthroconidia-like) or ellipsoidal yeast cells, interspersed with a few hyphal fragments (Fig. 2B). The exoantigen extracts of all the isolates gave positive results, as evidenced by multiple precipitation lines of identity with rabbit anti-P. marneffei antiserum and a reference antigen. The results were negative against antigenic extracts of Aspergillus fumigatus, Aspergillus flavus, and non-marneffei Penicillium species. All the tested isolates of P. marneffei demonstrated marked proteolytic activity in both mycelial and yeast form growth, as evidenced by the zone of clearing of substrate (bovine serum albumin) around the growth. There was no significant difference in the proteinase activity of bamboo rat and human isolates. Results with the urease test were also positive in both mold and yeast forms of the isolates. However, the tests for the hydrolysis of casein and 10% gelatin were negative.
FIG. 2.
(A) Lactophenol blue mount of 7 days' growth of P. marneffei (isolate VPCI 214) showing characteristic biverticillate and monverticillate penicilli. Magnification, ×425. (B) Lactophenol blue mount of 3 days' growth of P. marneffei isolate (VPCI 214), showing fission yeast form. Magnification, ×425.
Genotypes of the isolate.
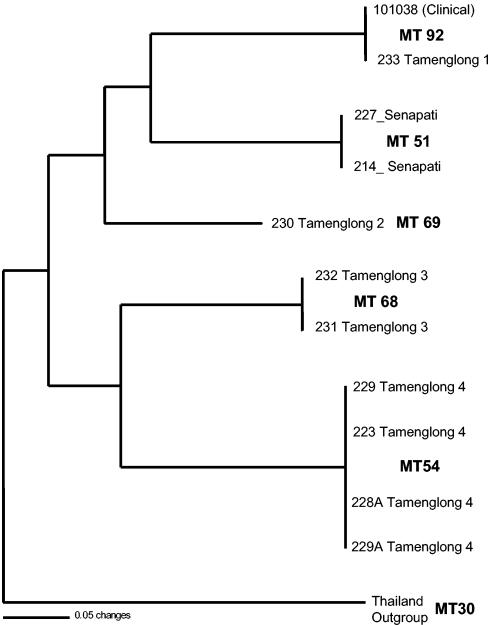
MLMT analyses of the 10 isolates recovered five distinct multilocus MTs (Fig. 3). A single isolate, VPCI 230, was found to have a unique genotype. Otherwise, genotypes were shared by two or more isolates. Where multiple isolates were recovered from a single geographic area, all isolates were found to have identical multilocus genotypes (Fig. 3). No MT was recovered from more than a single trapping site, showing that genotypes are geographically patchy. However, one bamboo rat isolate, VPCI 233, had identical alleles at all 23 loci to the human clinical isolate from Manipur state, CBS 101038 (27).
FIG. 3.
Neighbor-joining tree showing the relationships between the 10 recovered isolates and the CBS 101038 clinical isolate. Numbers following the districts refer to the bamboo plantations where the bamboo rats were trapped.
DISCUSSION
The present report establishes the bamboo rat C. badius as a natural host of P. marneffei in India. Earlier studies have shown that P. marneffei naturally infects C. badius in Thailand. However, the prevalence of infection observed in the present study (9.1%) is much lower than that reported in surveys of C. badius in Thailand (1, 5, 29). C. badius rats are divided into two groups, primarily on the basis of the color of their fur, which is greyish black or reddish brown. A study by Chariyalertsak et al. (5) found that all 51 greyish-black C. badius specimens studied were negative for P. marneffei, while 3 of the 10 rats (30%) in the reddish-brown group were positive for P. marneffei. All C. badius rats investigated in the present study were reddish brown and are given a subspecies designation in the Indian zoological literature. In the present study, P. marneffei was recovered more frequently from the spleen than from other organs. This contrasts with previous surveys of C. badius in Thailand, where the rate of isolation of the fungus was highest from lungs (1, 29).
The prevalence of infection in several bamboo rat species varies widely across their ranges, reviewed here in Table 2. C. badius is found in the states of Arunachal Pradesh, Assam, Manipur, Meghalaya, Mizoram, and Nagaland, India, and also in Nepal, Bhutan, Bangladesh, Myanmar, Laos, Thailand, and northern Vietnam (7, 12, 19, 27). R. sinensis occurs from southern China to northern Myanmar through Thailand to Malaysia (19). The distribution of R. pruinosus and R. sumatrensis ranges from southern China through Thailand to Malaysia, extending to Indonesia in the case of R. sumatrensis (19). R. pruinosus is found in the Indian states of Assam, Manipur, Meghalaya, Mizoram, and Nagaland, while R. sumatrensis is not found in India (7, 12). Geographical and pathological variations in the prevalence of infection in these species suggests that either there are regional variations in the endemicity of infection or there are geographic variations in the predisposition to infection within different species and subspecies of bamboo rats. It would be worthwhile to carry out comprehensive studies of the prevalence of P. marneffei in C. badius and R. pruinosus in the northeastern and eastern states of India to further test these hypotheses. However, that we found no sign of infection by P. marneffei in the tissues of 72 rodents of five other rodent species is strongly supportive of the hypothesis that there are species-specific variations in susceptibility to P. marneffei. Whether this observed variation in susceptibility to infection is driven by ecological, behavioral, or genetic factors awaits further investigation.
TABLE 2.
Prevalence of P. marneffei in bamboo rats in Southeast Asia
| Country (province, state) | Species of rat (no. of rats examined) | No. (%) of rats positive | Reference |
|---|---|---|---|
| China (Guangxi, Churag) | R. pruinosus (19) | 18 (94.7) | 9 |
| China (Guangxi, Churag) | R. pruinosus (179) | 14 (63.7) | 32 |
| China (Guangxi, Churag) | R. pruinosus (16) | 15 (93.8) | 19 |
| Thailand (Kanchanaburi) | C. badius (31) | 6 (19.4) | 1 |
| Thailand (Lopburi, Prachuap Khri Khan) | R. pruinosus (8) | 6 (75.0) | 1 |
| Thailand (Chiang Mai) | C. badius (10) | 3 (33.3) | 5 |
| R. sumatrensis (14) | 13 (92.8) | 5 | |
| Thailand (Chiang Mai) | C. badius (27) | 6 (22.2) | 28 |
| R. pruinosus (8) | 6 (75.0) | 28 | |
| India (Manipur) | C. badius (110) | 10 (9.1) | This study |
Several investigators have reported the use of various molecular techniques in the differentiation of P. marneffei strains. Vanittanakom et al. (32) employed restriction endonuclease with HaeII to differentiate P. marneffei isolates from northern Thailand. The 22 human isolates in that study were classified into two DNA types: type I comprised 16 (72.7%) isolates, and type II comprised 6 (27.3%) isolates. Of the 23 bamboo rat isolates, 20 isolates from R. sumatrensis were type I, and 3 isolates from C. badius were type II; the solitary soil isolate investigated was type II. In a study of 20 P. marneffei isolates from Taiwanese patients, Hsueh et al. (14) found eight distinct patterns by randomly amplified polymorphic DNA analysis, and two types (types I and II) based on chromosomal DNA restriction fragment-length polymorphism. Imwidthaya et al. (15) carried out fingerprinting of 30 P. marneffei isolates recovered from Chiang Mai in northern Thailand and Bangkok in central Thailand by single-nucleotide pattern primers [(GACA)4] and the phage M13 core sequence. Four types were distinguishable, based on differences in the major bands: type A (6.7% prevalence in Chiang Mai), type B (6.7% prevalence in Chiang Mai), type C (3.3% prevalence in Bangkok), and type D (83.3% prevalence in both Chiang Mai and Bangkok). A separate study analyzing genomic DNA of 64 P. marneffei isolates from different regions of Thailand by pulsed-field gel electrophoresis with restriction enzyme NotI (30) revealed two macrorestriction patterns (MPI and MPII) that could be grouped into nine subprofiles (MPIa to MPIf and MPIIa to MPIIc). No correlation between macrorestriction patterns of P. marneffei isolates and geographic region or specimen source was observed in these studies. Recently, a study by Lasker and Ran (18) using three microsatellite-containing loci suggested that there was geographic isolation between Chinese and Thai populations of P. marneffei.
In the present study, MLMT of the 10 isolates of P. marneffei from C. badius revealed five genotypes, thus showing that genetically diverse isolates have infected these animals. Due to the large number of microsatellite loci typed (n = 23) and the multiallelic nature of the microsatellites (mean number of alleles locus−1 = 5), the chances of finding identical genotypes by chance alone within a recombining population are vanishingly small (∼523) (13). However, that we see isolates with identical genotypes within this data set suggests that that there is extensive clonal reproduction within the local fungal populations. The Talaromyces sexual stage (teleomorph) that characterizes other biverticilliate Penicillium species has never been observed for P. marneffei, and this suggests that there will be a strong asexual component to the population structure of P. marneffei. Our data are in concordance with this observation. Within our study populations, isolates with identical MT types invariably came from bamboo rats that were trapped at the same site. These data show that P. marneffei clones are spatially localized and that the fungal populations are not homogenized over the geographic distances studied here. This finding suggests that long-distance dispersal of P. marneffei spores is a relatively rare event; however, larger sample sizes are necessary to corroborate the generality of this finding.
The detection of a genotype that occurs in one of the C. badius isolates (VPCI 233) and one of the four human isolates (CBS 101038) of penicilliosis marneffei reported in Manipur state (27) suggests that either coinfection from a common source or host-to-host transmission has occurred. This is the first conclusive genetic evidence that bamboo rats and HIV-positive patients share genetically similar strains of P. marneffei. Hypotheses as to how the infection event occurred are at best speculative until the natural reservoir of P. marneffei is better understood. So far, it has not been established whether bamboo rats are important natural reservoirs for the transmission of P. marneffei to humans in areas of endemicity or whether the fungus resides in soil, and bamboo rats and/or humans are only incidental hosts. However, the evolution to a pathogenic lifestyle may have occurred if death of the host results in the production of large quantities of spores, thus increasing the lifetime reproductive success of the fungus. If this is the case, then bamboo rats may prove an important amplifying host for human P. marneffei infections.
The ecology of P. marneffei remains enigmatic. Deng et al. (10) isolated P. marneffei from three soil samples collected from the burrows of bamboo rats (R. pruinosus). Chariyalertsak et al. (5) were able to recover P. marneffei from 1 (3.6%) of 28 soil samples collected from the burrows of the bamboo rat, R. sumatrensis. A case-control study performed in northern Thailand by Chariyalertsak et al. (6) did not indicate that bamboo rats were a reservoir of infection for humans. Rather, age (16 to 30 years) and an occupation involving exposure to plants or animals were found to be factors that were independently associated with an increased risk to infection by P. marneffei. These investigators postulated that soil exposure, especially during the rainy season, is a critical risk factor associated with penicilliosis marneffei. In the present study, P. marneffei could not be isolated from any of the 25 soil samples collected from the burrows of the bamboo rats (C. badius), nor could it be recovered from any of the 120 soil samples collected from burrows of other species of rats or from sites other than rodent burrows in bamboo plantations in different parts of northeast India. Vanittanakom et al. (31) demonstrated 80 to 85.1% recovery of CFU after 3 days of incubation from sterilized soil seeded with P. marneffei; however, recovery from nonsterile soil seeded with the fungus was only 6%. A recent study has demonstrated that P. marneffei can survive in sterile soil for several weeks but can survive in nonsterile soil for only a few days (16). Thus, definite evidence of the natural occurrence of P. marneffei in soil is still lacking. Naturally infected bamboo rats may seed the soil with P. marneffei, thus possibly creating new foci of the organism. Further investigations are required to determine the role of bamboo rats in the epidemiology of human infections due to P. marneffei.
Acknowledgments
The study was supported by a financial grant from the Indian Council of Medical Research, New Delhi, India, and a Wellcome Trust Biodiversity Fellowship to M.C.F.
We thank A. K. Mandal, Zoological Survey of India, for identification of the bamboo rats. The assistance of S. Sharma and D. Singh of the Department of Life Sciences, Manipur University, in dissection of the bamboo rats is gratefully acknowledged. We also thank V. K. Vijayan, Director, and H. S. Randahwa, Emeritus Scientist, Vallabhbhai Patel Chest Institute, for their encouragement. Sybren de Hoog, Centraalbureau voor Schimmelculture, provided DNA from isolate CBS 101038.
REFERENCES
- 1.Ajello, L., A. A. Padhye, S. Sukroongreung, C. H. Nilakul, and S. Tantimavanic. 1995. Occurrence of Penicillium marneffei infections among wild bamboo rats in Thailand. Mycopathologia 131:1-8. [DOI] [PubMed] [Google Scholar]
- 2.Beneke, E. S., and A. L. Rogers. 1996. Medical mycology and human mycoses, p. 40-48. Star Publishing Co., Belmont, Calif
- 3.Capponi, M. P., G. Segretain, and G. Sureau. 1956. Penicilliosis de Rhizomys sinensis. Bull. Soc. Pathol. Exot. Filiales 49:418-421. [PubMed] [Google Scholar]
- 4.Chakrabarti, A., N. Nayak, and P. Talwar. 1991. In vitro proteinase production by Candida species. Mycopathologia 114:163-168. [DOI] [PubMed] [Google Scholar]
- 5.Chariyalertsak, S., P. Vanittanakom, K. E. Nelson, T. Sirisanthana, and N. Vanittanakom. 1996. Rhizomys sumatrensis and Cannomys badius, new natural animal hosts of Penicillium marneffei. J. Med. Vet. Mycol. 34:105-110. [PubMed] [Google Scholar]
- 6.Chariyalertsak, S., T. Sirisanthana, K. Supparatpinyo, J. Praparattanapan, and K. E. Nelson. 1997. Case control study of risk factors for Penicillium marneffei in human immunodefficiency virus-infected patients in northern Thailand. Clin. Infect. Dis. 24:1080-1086. [DOI] [PubMed] [Google Scholar]
- 7.Chauhan, N.S., and R. N. Saxena. 1985. The phenomenon of bamboo flowering and associated increase in rodent population in Mizoram. J. Bombay Nat. Soc. 83:644-647. [Google Scholar]
- 8.de Hoog, G. S., J. Guarro, J. Gené, and M. J. Figueras. 2000. Atlas of clinical fungi, p. 50, 52, and 833. Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands.
- 9.Deng, Z., M. Yun, and L. Ajello. 1986. Human penicilliosis and its relation to the bamboo rat (Rhizomys pruinosus) J. Med. Vet. Mycol. 24:383-389. [DOI] [PubMed] [Google Scholar]
- 10.Deng, Z., Ribas, J. L., D. W. Gibson, and D. H. Connor. 1988. Infection caused by Penicillium marneffei in China and southeast Asia. Review of 18 cases and report of four more Chinese cases. Rev. Infect. Dis. 10:640-652. [DOI] [PubMed] [Google Scholar]
- 11.Duong, T. A. 1996. Infection due to Penicillium marneffei, an emerging pathogen: review of 155 reported cases. Clin. Infect. Dis. 23:125-130. [DOI] [PubMed] [Google Scholar]
- 12.Elerman, J. R. 1961. The fauna of India including Pakistan, Burma and Ceylon: mammalia. Manager of Publications, Government of India, New Delhi, India.
- 13.Fisher, M. C., G. S. de Hoog, and N. Vanittanakom. 2004. A highly discriminatory multilocus microsatellite typing system (MLMT) for Penicillium marneffei. Mol. Ecol. Notes 5:231-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsueh, P.-R., L. J. Teng, C.-C. Hunh, J.-H. Hsu, P.-C. Yang, S.-W. Ho, and K. W. Luh. 2001. Molecular evidence for strain differentiation of Penicillium marneffei: an emerging pathogen in Taiwan. J. Infect. Dis. 181:1706-1712. [DOI] [PubMed] [Google Scholar]
- 15.Imwidthaya, P., K. Thipsuvan, A. Chaiprasert, S. Danchaivijitra, R. Sutthent, and J. Jearanaisilavong. 2001. Penicillium marneffei: types and drug susceptibility. Mycopathologia 149:109-115. [DOI] [PubMed] [Google Scholar]
- 16.Joshi, A., H. C. Gugnani, and V. K. Vijayan. 2003. Survival of Penicillium marneffei in sterile and unsterile soil. J. Med. Mycol. 13:211-212. [Google Scholar]
- 17.Kurup, A., Y. S. Leo, and A. L. Tan. 1999. Disseminated Penicillium marneffei infections: a report of five cases in Singapore. Ann. Acta Med. Singapore 28:605-609. [PubMed] [Google Scholar]
- 18.Lasker, B. A., and Y. Ran. 2004. Analysis of polymorphc microsatellite markers for typing Penicillium marnefei isolates. J. Clin. Microbiol. 42:1483-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lekagul, B., and J. A. McNeely (ed.). 1988. Mammals of Thailand, p. 388-396. Association for the Conservation of Wildlife. Saha Karn Bhaet Co., Bangkok, Thailand.
- 20.Li, J. C., L. Q. Pan, and S. X. Wu. 1989. Mycologic investigation on Rhizomys pruinosus senex in Guangxi as natural carrier with Penicillium marneffei. Chin. Med. J. 102:477-485. [PubMed] [Google Scholar]
- 21.Lo, Y., K. Tintelnot, U. Lippert, and T. Hoppe. 2000. Disseminated Penicillium marneffei infection in an African AIDS patient. Trans. R. Soc. Trop. Med. Hyg. 95:187. [DOI] [PubMed] [Google Scholar]
- 22.Pracharktam, R., S. Sriurairatna, and P. Jayanetra. 1992. Morphological variation in pathogenic strains of Penicillium marneffei. J. Med. Assoc. Thai. 75:172-179. [PubMed] [Google Scholar]
- 23.Ranjana, K. H., K. Priyaokumar, T. J. Singh, C. C. Gupta, L. Sharmila, P. N. Singh, and A. Chakrabarti. 2002. Disseminated Penicillium marneffei infection in HIV-infected patients in Manipur state, India. J. Infect. 45:268-271. [DOI] [PubMed] [Google Scholar]
- 24.Ruchel, R., R. Tegeler, and M. A. Trost. 1982. A comparison of secretory proteases from different strains of Candida albicans. Sabouraudia 10:233-234. [DOI] [PubMed] [Google Scholar]
- 25.Segretain, G. 1959. Description d'une nouvelle espece de Penicillium Penicillium marneffei n. sp. Bull. Soc. Mycol. Fr. 75:412-416. [Google Scholar]
- 26.Sekhon, A. S., J. S. K. Li, and A. K. Garg. 1982. Penicillium marneffei: serological and exoantigen studies. Mycopathologia 77:51-57. [DOI] [PubMed] [Google Scholar]
- 27.Singh, P. N., K. Ranjana, Y. I. Sing, P. Singh, S. S. Sharma, M. Kulachandra, Y. Nabakumar, A. Chakrabarti, A. A. Padhye, L. Kaufman, and L. Ajello. 1999. Indigenous disseminated Penicillium marneffei infection in the state of Manipur, India: report of four autochthonous cases. J. Clin. Microbiol. 37:2699-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sirisanthana, T., and K. Supparatpinyo. 1998. Epidemiology and management of penicilliosis in human immunodeficiency virus-infected patients. J. Infect. Dis. 3:48-53. [DOI] [PubMed] [Google Scholar]
- 29.Sukroongreung, S., C. Nilakut, S. Tantimavanich, and L. Ajello. 1991. Natural carrier of Penicillium marneffei, p. 43. In Mahidol University Annual Research Abstracts and Bibliography of Non-Formal Publications. Academic Affairs Division, Mahidol University, Bangkok, Thailand.
- 30.Trewatcharegon, S., S. Sirisinha, A. Romsai, B. Eampokalap, R. Teanpaisan, and S. C. Chaiyaroj. 2001. Molecular typing of Penicillium marneffei isolates from Thailand by NotI macrorestriction and pulsed-field gel electrophoresis. J. Clin. Microbiol. 39:4544-4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vanittanakom, N., M. Mekaprateep, P. Sriburee, P. Vanittanakom, and P. Khanjanasthiti. 1995. Efficiency of flotation method in the isolation of Penicillium marneffei from seeded soil. J. Med. Vet. Mycol. 33:271-273. [PubMed] [Google Scholar]
- 32.Vanittanakom, N., C. R. Cooper, Jr., S. Chariyalertsak, S. Yongchim, K. E. Nelson, T. Sirisanthana, and P. Vanittanakom. 1996. Restriction endonuclease analysis of Penicillium marneffei. J. Clin. Microbiol. 34:1834-1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei, X. G., Y.. Ling, C. Li, and F. S. Zhang. 1989. Study of 179 bamboo rats carrying Penicillium marneffei. Chin. J. Zoonoses 3:34-35. (In Chinese.) [Google Scholar]