Abstract
Aeromonas popoffii is a recently described species isolated mainly from freshwater. An isolate of Aeromonas popoffii was found to be responsible for a urinary tract infection in a 13-year-old boy suffering from spina bifida with enterocystoplasty. This is the first reported case of human infection attributed to this species.
CASE REPORT
A 13-year-old boy was hospitalized in April 2003 for replacement of a urethral catheter. He suffered from congenital spina bifida and myelomeningocele complicated by a neurogenic bladder treated by anticholinergic drugs and intermittent bladder catheterization for 5 years. Imaging explorations did not reveal vesico-ureteral reflux. In 2001, the patient unsuccessfully underwent two transurethral injections of macroplastic substance on the vesical neck in order to palliate increasing urinary incontinence. He underwent an enterocystoplasty, using the sigmoid colon with surgical correction of the vesical neck (Young Dees type) and bilateral ureteral reimplantation with an antireflux valve (Cohen type) in April 2003. On the 10th postoperatory day, he returned home and was again treated by intermittent bladder catheterization (about four times a day). On day 15, the patient presented a fever of 38°C associated with left flank pain, and he was hospitalized. The urine analysis showed 105 polymorphonuclear leukocytes and 106 bacteria per ml. Blood leukocytes rose from 5,700 to 8,500/μl, platelet count rose from 321,000 to 488,000/μl, and the sedimentation rate was 28 mm for the first hour. Preventive antibiotic treatment had been continued postoperatively with intravenously administered ceftriaxone (2 g/day) and metronidazole (1,500 mg/day) in the hospital, which was changed to amoxiclav given orally (1,500 mg/day) when he went home.
The microbiological result confirmed a urinary tract infection due to Aeromonas sp., with intermediate susceptibility to amoxicillin and amoxicillin-clavulanate and susceptibility to ceftriaxone, ciprofloxacin, gentamicin, and cotrimoxazole. Treatment was changed to orally administered cotrimoxazole (800/160 mg twice per day), with a favorable clinical evolution later.
Urine was cultured aerobically on Trypticase soy agar (bio-Mérieux, Marcy l'Etoile, France) at 37°C. After 48 h, 3-mm whitish colonies with irregular edges were observed. Gram staining revealed gram-negative, straight motile rods. Catalase and oxidase were positive, with resistance to vibriostatic agent O/129. The API-20 NE (bioMérieux) profile was 7 477 745, corresponding to a low level of discrimination between Aeromonas hydrophila or Aeromonas caviae (percentage of identification, 91.5%; T index, 0.76) and Vibrio parahaemolyticus (8.3% identical; T index, 0.58) (profile book, 6th ed., 1997).
The antibiotic susceptibility profile was studied on sheep blood Mueller-Hinton agar plates by the disk diffusion method according to NCCLS recommendations (10). The MICs were 2 μg/ml for amoxicillin, 0.25 μg/ml for ceftriaxone, 4 μg/ml for imipenem, 0.5 μg/ml for amikacin, 0.75 μg/ml for ciprofloxacin, and 0.25 μg/ml for cotrimoxazole.
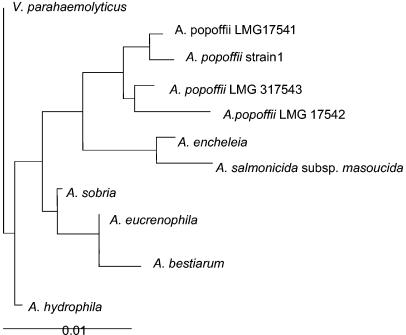
Further identification of the isolate was done by 16S rRNA gene sequence analysis. DNA of a single colony was extracted by using the Fast-prep DNA extraction kit and the Fast-prep DNA device as described by the supplier (Bio 101 Inc., La Jolla, Calif.) (5). The 16S rRNA gene was amplified by using the primers FD1 (5′AGAGTTTGATCCTGGCTGAG 3′) and RP2 (5′ACGGCTACCTTGTTACGACTT 3′). The PCRs were performed with a Perkin-Elmer 9600 thermocycler under the following conditions: after an initial denaturation step (95°C for 2 min), a three-step cycle of 94°C for 30 s, 50°C for 30 s, and 72°C for 1 min was repeated 35 times. The sequence determination was performed as previously described (3, 4). The partial 1,377-bp sequence was aligned and compared with all eubacterial 16S ribosomal DNA (rDNA) gene sequences available in the GenBank and EMBL databases by using the multisequence advanced BLAST National Center for Biotechnology Information comparison software (2). This sequence shared 99.9% similarity with that of Aeromonas popoffii LMG 317541 (GenBank accession number AJ224308) (6, 7). After initial alignment with CLUSTAL W (14), neighbor-joining analysis was performed by using PAUP 4.0b1 software (Sinauer, Sunderland, Mass.). Figure 1 shows the dendrogram we obtained. V. parahaemolyticus was the most closely related Vibrio species and was used as an outgroup. Our isolate, strain 1, is close to A. popoffii strain LMG 17543 and belongs to a cluster encompassing A. popoffii strains LMG 317543 and LMG 17542. The most closely related species is Aeromonas encheleia, clustering with Aeromonas salmonicida subsp. masoucida (99% similarity). The third cluster is formed by Aeromonas veronii biotype sobria, Aeromonas eucrenophila, and Aeromonas bestiarum. The farthest Aeromonas species is A. hydrophila.
FIG. 1.
The dendrogram was obtained with 16S rDNA sequences. V. parahaemolyticus was used as an outgroup. Strain 1 is the strain from our patient.
The patient presented clinical symptoms (fever and left flank pain) and biological signs (leucocyturia and inflammatory stigmas) of a urinary tract infection with a high risk factor (repetitive indwelling of urethral catheter). At the same time, an A. popoffii strain was isolated from urine. The isolate grew readily in pure culture, and no other A. popoffii strain was isolated in the same laboratory. The patient was started on cotrimoxazole therapy. Two days later, the urine sample was sterile. These data confirmed that this patient suffered from an A. popoffii urinary tract infection.
Classification of the genus Aeromonas has changed continuously over the last decade, with the description of new species and the reclassification of known species. Aeromonas spp. are not frequently involved in urinary tract infections. However, in 1998, Hsueh et al. reported isolation of A. veronii biotype veronii, responsible for urinary tract infection in a 69-year-old male patient suffering from diabetes mellitus and chronic hepatitis. The urinary tract infection was also related to an indwelling device (8). The Aeromonas species mostly implicated in human infections are A. hydrophila, A. caviae, and A. veronii biotype sobria (1).
In 1997, Huys and colleagues described a new species, A. popoffii, isolated from Flemish drinking water production plants and Scottish drinking water supplies (9). Although the clinical origin of some A. popoffii isolates was noted by Huys et al. in 1997, there has been no clinical description of human A. popoffii infection.
The niche of A. popoffii is probably freshwater. However, this bacterium is able to produce some virulence factors, conferring a pathogenic role. These genes were present in all A. popoffii strains studied by Soler et al. (11, 12). For the isolate described here, we demonstrated the presence of genes coding for aerolysin/hemolysin and serine protease. These facts suggest that this isolate was neither a contaminant nor a simple colonization. This description could be relevant for infectious disease consulting.
The 16S rDNA sequences offer a reliable and straightforward tool for identification of A. popoffii strains (13), and routine use of this method should increase our knowledge regarding the clinical spectrum of A. popoffii infections in humans.
Nucleotide sequence accession number.
The partial 1,377-bp sequence of the A. popoffii strain isolated in this study has been submitted to GenBank under accession number AY534350.
Acknowledgments
Thanks are due to C. Bibard for excellent technical help.
REFERENCES
- 1.Abbott, S. L., W. K. Cheung, and J. M. Janda. 2003. The genus Aeromonas: biochemical characteristics, atypical reactions, and phenotypic identification schemes. J. Clin. Microbiol. 41:2348-2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul, X., F. Stephen, L. Thomas, X. Madden, A. Alejandro, X. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auzias, A., C. Bollet, R. Ayari, M. Drancourt, and D. Raoult. 2003. Corynebacterium freneyi bacteremia. J. Clin. Microbiol. 41:62777-62778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beau, F., C. Bollet, T. Coton, E. Garnotel, and M. Drancourt. 1999. Molecular identification of a Nocardiopsis dassonvillei blood isolate. J. Clin. Microbiol. 37:3366-3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheung, A. L., K. J. Eberhardt, and V. A Fischetti. 1994. A method to isolate RNA from Gram-positive bacteria and mycobacteria. Anal. Biochem. 222:511-514. [DOI] [PubMed] [Google Scholar]
- 6.Demarta, A., M. Tonolla, A. P. Caminada, N. Ruggeri, and R. Peduzzi. 1999. Signature region within the 16S rDNA sequences of Aeromonas popoffii. FEMS Microbiol. Lett. 172:239-246. [DOI] [PubMed] [Google Scholar]
- 7.Demarta, A., M. Tonolla, A. Caminada, M. Beretta, and R. Peduzzi. 2000. Epidemiological relationships between Aeromonas strains isolated from symptomatic children and household environments as determined by ribotyping. Eur. J. Epidemiol. 16:447-453. [DOI] [PubMed] [Google Scholar]
- 8.Hsueh, P. R., L. J. Teng, L. N. Lee, P. C. Yang, Y. C. Chen, S. W. Ho, and K. T. Luh. 1998. Indwelling device-related and recurrent infections due to Aeromonas species. Clin. Infect. Dis. 26:651-658. [DOI] [PubMed] [Google Scholar]
- 9.Huys, G., P. Kampfer, M. Altwegg, I. Kersters, A. Lamb, R. Coopman, J. Luthy-Hottenstein, M. Vancanneyt, P. Janssen, and K. Kersters. 1997. Aeromonas popoffii sp. nov., a mesophilic bacterium isolated from drinking water production plants and reservoirs. Int. J. Syst. Bacteriol. 47:1165-1171. [DOI] [PubMed] [Google Scholar]
- 10.National Committee for Clinical Laboratory Standards. 2003. Performance standards for antimicrobial disk susceptibility tests. Approved standard M2-A8. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 11.Soler, L., M. J. Figueras, M. R. Chacon, J. Vila, F. Marco, A. J. Martinez-Murcia, and J. Guarro. 2002. Potential virulence and antimicrobial susceptibility of Aeromonas popoffii recovered from freshwater and seawater. FEMS Immunol. Med. Microbiol. 32:243-247. [DOI] [PubMed] [Google Scholar]
- 12.Soler, L., M. J. Figueras, M. R. Chacon, J. Guarro, and A. J. Martinez-Murcia. 2003. Comparison of three molecular methods for typing Aeromonas popoffii isolates. Antonie Leeuwenhoek 83:341-349. [DOI] [PubMed] [Google Scholar]
- 13.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weisburg, W. G., S. M. Barns, D. A. Pelletier, and D. J. Lane. 1991. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 173:697-703. [DOI] [PMC free article] [PubMed] [Google Scholar]