Abstract
Curvularia infections in humans are relatively uncommon despite the ubiquitous presence of this soil-dwelling dematiaceous fungus in the environment. Originally thought to be solely a pathogen of plants, Curvularia has been described as a pathogen of humans and animals in the last half-century, causing respiratory tract, cutaneous, and corneal infections. Only three previous cases of central nervous system involvement by Curvularia have been documented in the medical literature. We report a fatal case of cerebral Curvularia infection in which there was no known history of immunocompromise or prior respiratory tract or sinus infection in the patient.
CASE REPORT
A 21-year-old African-American male with a past medical history significant only for asthma presented to our medical center complaining of headache for the previous 2 months. The headache was located in the parietal and occipital regions and was constant with increasing severity over the past month. He obtained no relief with the use of over-the-counter medications. The headache was associated with double vision, dizziness, unsteady gait, anorexia with a 10-pound weight loss, low-grade fever, chills, and night sweats. His social history included smoking one pack of cigarettes per day and occasional cocaine and marijuana use. He denied intravenous drug use and was taking no medications at the time of admission. Physical examination findings included a temperature of 100.4°F; blood pressure of 101/67 mm Hg; neurologic status, alert and oriented to person, place, and time; no nuchal rigidity; no neurologic deficits; and diffuse inspiratory and expiratory wheezes bilaterally. Laboratory findings included a normal complete blood count, a urine drug screen positive for barbiturates and cannabinoids, and a slightly decreased serum sodium level. A computed tomography (CT) scan of the brain with and without contrast revealed a 4.8- by 3.8-cm area of intraparenchymal hypodensity on the right side of the brain involving the right basal ganglia and anterior horn adjacent to the right lateral ventricle with a density suggestive of hemorrhage. Apparent cerebral edema with a 4.0-mm left midline shift was also noted.
The patient was admitted to the intensive care unit and begun on mannitol and dexamethasone to reduce intracranial swelling. A stereotactic needle aspiration and biopsy of the cerebral mass was performed 2 days later. Cytologic analysis of the aspirated material showed rare pleomorphic septate fungal elements in a background of neutrophils, lymphocytes, multinucleated histiocytes, and necrotic debris (Fig. 1). Histologic sections of the stereotactic brain biopsy tissue demonstrated brain parenchyma with granulomatous inflammation and necrosis associated with scattered light-brown fungal hyphae (Fig. 2). The fungal hyphae in the biopsy specimen were much more uniform in diameter than those seen in the fluid sample. Only rare septa were identified. Treatment of the patient with amphotericin B, vancomycin, and ceftriaxone was begun. Culture of the biopsy tissue submitted to the microbiology laboratory showed Curvularia lunata. The patient's vancomycin and ceftriaxone were discontinued, and flucytosine was begun. Clindamycin was added when the patient's temperature rose continually during his hospital course. Conventional amphotericin B therapy was discontinued, and liposomal amphotericin B was begun. Serial CT scans performed over the next several weeks showed involvement of the right lateral ventricle and evidence of surrounding edema, bilateral meningeal enhancement, a midline shift, and ring enhancement of the mass as well as progressive ventricular dilatation and necrosis. A four-vessel cerebral arteriogram showed that the lesion had spread to the left cerebral hemisphere with increasing intracranial pressure. A CT scan of the sinuses showed only mild thickening of the right ethmoid sinus mucosa. The patient developed bilateral deep venous thrombosis of his upper extremities and was started on low-molecular-weight heparin. Seizure activity was documented, and an electroencephalogram was consistent with diffuse encephalopathy. Phenytoin sodium was begun, and the patient remained febrile in the intensive care unit. Blood and urine cultures were negative. A chest radiograph was negative for signs of aspiration pneumonia. The patient's neurologic status continued to decline throughout his hospital course, and he developed respiratory distress and died 1 month after admission. Permission for an autopsy limited to the cranial cavity was granted by the patient's family. At autopsy, the 1,285-g brain showed marked cerebral edema with cerebellar tonsil grooving indicative of herniation. The inferior surfaces of the right frontal and temporal lobes showed marked vascular congestion and hemorrhage. Yellow-green exudate overlay the pons, the brain stem, and the inferior aspect of the cerebellum. Serial coronal sections of the brain (Fig. 3) revealed that the right cerebral hemisphere, right temporal lobe, and right basal ganglia were larger than the left ones. A left-sided shift of the right cingulate gyrus and right basal ganglia was seen. The right thalamus and the right body of the caudate nucleus were obliterated by a 4.5- by 4.0-cm yellow-tan irregular firm lesion with a green-yellow rim surrounded by soft edematous brain parenchyma. The right lentiform nucleus and internal capsule were replaced by a 2.3- by 1.7-cm brown-black soft lesion surrounded by soft green brain parenchyma. The choroid plexus was undermined by yellow-green exudate. Multiple histologic sections from the brain at autopsy showed extensive necrosis and granulomatous inflammation associated with fungal elements. The fungal elements demonstrated by periodic acid-Schiff and silver stains consisted primarily of pleomorphic branched hyphae with prominent septations. Bulbous hyphal projections were readily apparent. Numerous intracerebral and meningeal arteries showed vasculitis and invasion of their walls by fungal elements. Multiple vessels showed thrombosis, and generalized cerebral edema was present.
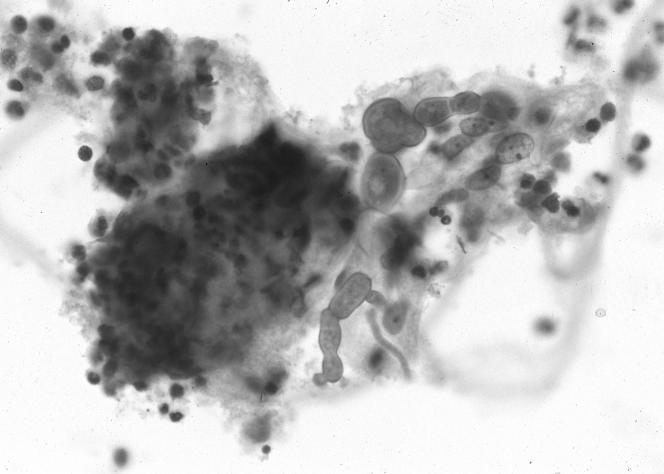
FIG. 1.
Fine-needle aspirate of cerebral mass showing septate fungal hyphae in a background of mixed inflammation.
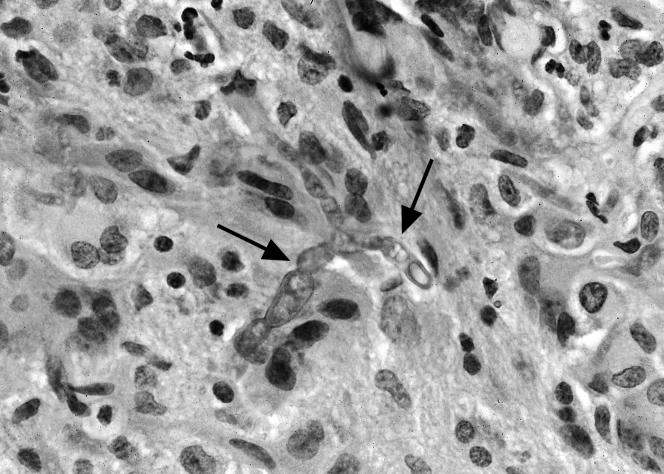
FIG. 2.
Stereotactic brain biopsy specimen showing invasive septate fungal hyphae (arrows).
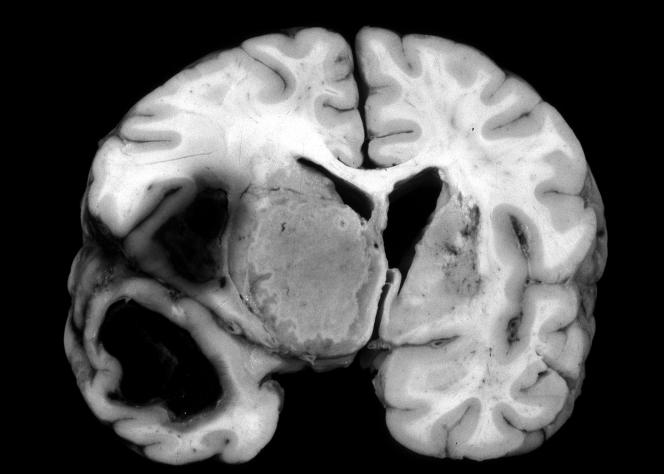
FIG. 3.
Coronal section of brain showing a fungal lesion in the region of the right basal ganglia.
Curvularia is a dematiaceous fungus found ubiquitously in soil. First described as Acrothecium lunata by Wakker and Went (12) in 1898, Curvularia was not documented as a human pathogen until 1959, when it was isolated from a mycetoma (2). Since that time, Curvularia has been reported as a cause of disease in the upper respiratory tract (3, 9, 15), lower respiratory tract (11, 16), skin (10), endocardium (7), and cornea (1). Only three previous cases involving the central nervous system have been described (4, 6, 8, 13, 14, 16).
Lampert et al. (8) reported the first case of cerebral infection by Curvularia in 1977. In this sentinel case, a 13-year-old mentally retarded male developed cough, fever, and hemiparesis. Imaging studies revealed a right lung infiltrate as well as a 5.0- by 3.0-cm mass in the right frontoparietal region of the brain. Histologic examination of a lung biopsy specimen and the excised brain lesion showed granulomatous inflammation, necrosis, and numerous fungal elements. Cultures of the tissue specimens yielded Curvularia pallescens. The patient was treated with antifungal agents and improved clinically. He was discharged and showed no evidence of fungal infection until 3 years later, when Friedman et al. (6) reported recurrence of the patient's pulmonary and cerebral fungal infection. Despite intrathecal antifungal therapy, the patient died approximately 2 weeks after hospitalization.
Rohwedder et al. (16) reported a second case of cerebral infection by a Curvularia species in 1979. In this case, a 25-year-old male with a prolonged history of chronic leg ulcers, which developed after abrasion of his lower extremity during a football game, presented with cervical lymphadenopathy, a pleural effusion, and a paraspinous abscess. Cultures of a cervical lymph node, pleural fluid, and the leg ulcers revealed C. lunata. The patient was treated unsuccessfully with antifungal agents, and approximately 6 months after presentation he developed a cerebral abscess in addition to his existing chronic fungal lesions. The cerebral lesion was located near the motor area of the brain, and operation was deferred. No lesional tissue from the central nervous system was obtained for histology and culture, but the authors attributed the cerebral lesion to dissemination of the patient's Curvularia infection. While the patient showed improvement from a neurologic standpoint following aggressive antifungal therapy, eradication of the fungal infection was never documented, and the last clinical information available on the patient reported that he was bedridden due to back pain caused by recurrent disease.
In the case reported by de la Monte and Hutchins (4) and further described by Pierce et al. (13, 14), a 41-year-old male presented with seizures and blurred vision. Imaging studies revealed a large lung lesion in the left upper lobe as well as multiple cerebral lesions. The clinical impression was that of lung carcinoma with cerebral metastasis. Resection of the lung lesion revealed granulomatous inflammation with multiple fungal organisms present; culture of the lung tissue revealed C. lunata. The patient was successfully treated with a 30-month course of amphotericin B; his cerebral lesions regressed, and he remained disease-free 1 year after discontinuation of therapy.
Interestingly, infections with Curvularia do not appear to require an immunosuppressed host. In a review of human Curvularia infections, Rinaldi et al. (15) found that of 24 patients, only 2 were systemically immunosuppressed. Of the three previously reported cases of Curvularia infection of the central nervous system, investigations of immunocompetence were reported for only two of the cases, and in only one of these cases were minor functional abnormalities of cell-mediated immunity detected. These functional abnormalities were detected after the onset of the fungal infection and improved slightly following antifungal therapy. In the present case, no evidence of immunosuppression was established.
Reports of Curvularia sinusitis have increased dramatically over the last 2 decades. These infections can produce extensive compression and destruction of the bony walls of the sinuses as well as adjacent cranial bone (5, 18). In none of the previously reported cases of central nervous system involvement by Curvularia was sinusitis documented, nor was Curvularia sinusitis demonstrated in the present case.
For the anatomic pathologist, identification of an invasive fungal infection in tissue sections is possible; definitive identification of species of a fungal organism based on morphology in tissue, however, is not always possible. Definitive identification of species relies upon fungal culture results, but certain morphological characteristics may suggest a category of fungal organism. Curvularia and other dematiaceous fungi are seen in tissue sections as 2- to 6-μm-wide hyphal forms which differ greatly in length and may be fragmented. Vesicular swellings of hyphae producing bizarre hyphal outlines are common (17). Pigmentation of these classically brown hyphae may be variable in tissue sections. The fungal organisms may be associated with abscess cavities or with intense granulomatous inflammation.
The increased detection of Curvularia as a cause of human disease widens the differential diagnosis of fungal infections seen by the anatomic pathologist. The morphological similarities of many of the pathogenic and opportunistic fungi require correlation between clinical and anatomic pathologists for definitive classification of these organisms. The rare occurrence of cerebral involvement by Curvularia exemplified by the present case underscores the significant morbidity and possible mortality associated with this pathogen.
REFERENCES
- 1.Anderson, B., S. S. Roberts, Jr., C. Gonzalez, and E. W. Chick. 1959. Mycotic ulcerative keratitis. Arch. Opthalmol. 62:169-197. [DOI] [PubMed] [Google Scholar]
- 2.Baylet, J., R. Camain, and G. Segretain. 1959. Identification of the agents of maduromycoses of Senegal and Mauritania. Description of a new species. Bull. Soc. Pathol. Exot. Fil. 52:448-477. (In French.) [PubMed] [Google Scholar]
- 3.Berry, A. J., T. M. Kerkering, A. M. Giordano, and J. Chiancone. 1984. Phaeohyphomycotic sinusitis. Pediatr. Infect. Dis. J. 3:150-152. [DOI] [PubMed] [Google Scholar]
- 4.de la Monte, S. M., and G. M. Hutchins. 1985. Disseminated Curvularia infection. Arch. Pathol. Lab. Med. 109:872-874. [PubMed] [Google Scholar]
- 5.Ebright, J. R., P. H. Chandrasekar, S. Marks, M. R. Fairfax, A. Aneziokoro, and M. R. McGinnis. 1999. Invasive sinusitis and cerebritis due to Curvularia clavata in an immunocompetent adult. Clin. Infect. Dis. 28:687-689. [DOI] [PubMed] [Google Scholar]
- 6.Friedman, A. D., J. M. Campos, L. B. Rorke, D. A. Bruce, and A. M. Arbeter. 1981. Fatal recurrent Curvularia brain abscess. J. Pediatr. 99:413-415. [DOI] [PubMed] [Google Scholar]
- 7.Kaufman, S. M. 1971. Curvularia endocarditis following cardiac surgery. J. Clin. Pathol. 56:466-470. [DOI] [PubMed] [Google Scholar]
- 8.Lampert, H. B., J. H. Hutto, W. H. Donnelly, and S. T. Shulman. 1977. Pulmonary and cerebral mycetoma caused by Curvularia pallescens. J. Pediatr. 91:603-605. [DOI] [PubMed] [Google Scholar]
- 9.Loveless, M. O., R. E. Winn, M. Campbell, et al. 1981. Mixed invasive infection with Alternaria species and Curvularia species. Am. J. Clin. Pathol. 76:491-493. [DOI] [PubMed] [Google Scholar]
- 10.Maghoub, E. S. 1973. Mycetomas caused by Curvularia lunata, Madurella grisea, Aspergillus nidulans, and Nocardia brasiliensis in Sudan. Sabouraudia 11:179-182. [PubMed] [Google Scholar]
- 11.McAleer, R., D. B. Kroenert, J. L. Elder, and J. H. Froudist. 1981. Allergic bronchopulmonary disease caused by Curvularia lunata and Drechslera hawaiiensis. Thorax 26:338-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parmalee, J. A. 1956. The identification of the Curvularia parasite of gladiolus. Mycologia 48:558. [Google Scholar]
- 13.Pierce, N. F., J. C. Millan, B. S. Bender, and J. L. Curtis. 1986. Disseminated Curvularia infection. Arch. Pathol. Lab. Med. 110:871. [PubMed] [Google Scholar]
- 14.Pierce, N. F., J. C. Millan, B. S. Bender, and J. L. Curtis. 1986. Disseminated Curvularia infection. Additional therapeutic and clinical considerations with evidence of medical cure. Arch. Pathol. Lab. Med. 110:959-961. [PubMed] [Google Scholar]
- 15.Rinaldi, M. G., P. Phillips, J. G. Schwartz, R. E. Winn, G. R. Holt, F. W. Shagets, J. Elrod, G. Nishioka, and T. B. Aufdemorte. 1987. Human Curvularia infections. Report of five cases and review of the literature. Diagn. Microbiol. Infect. Dis. 6:27-39. [DOI] [PubMed] [Google Scholar]
- 16.Rohwedder, J. J., J. L. Simmons, H. Colfer, and B. Gatmaitan. 1979. Disseminated Curvularia lunata infection in a football player. Arch. Intern. Med. 139:940-941. [PubMed] [Google Scholar]
- 17.Salfelder, K. 1990. Atlas of fungal pathology, p. 56. Kluwer Academic Publishers, Hingham, Mass.
- 18.Schroeder, Jr., W. A., D. G. Yingling, P. C. Horn, and W. D. Stahr. 2002. Frontal sinus destruction from allergic eosinophilic fungal rhinosinusitis. Mo. Med. 99:197-199. [PubMed] [Google Scholar]