Abstract
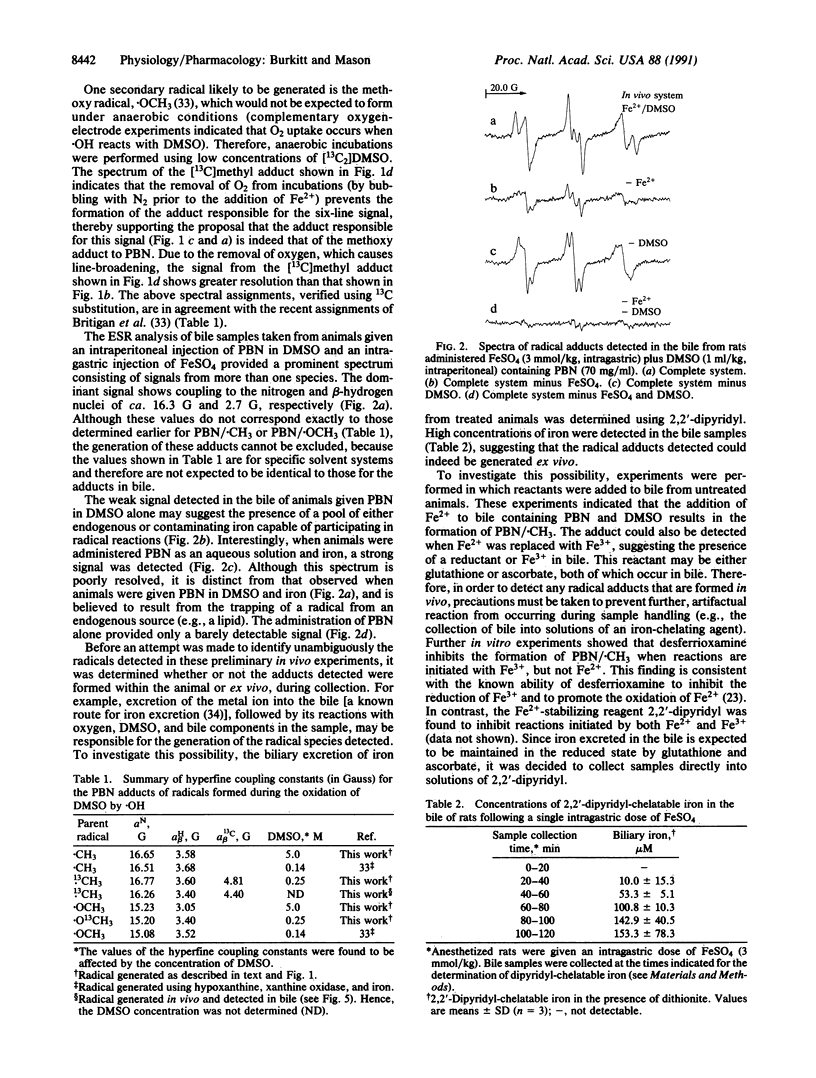
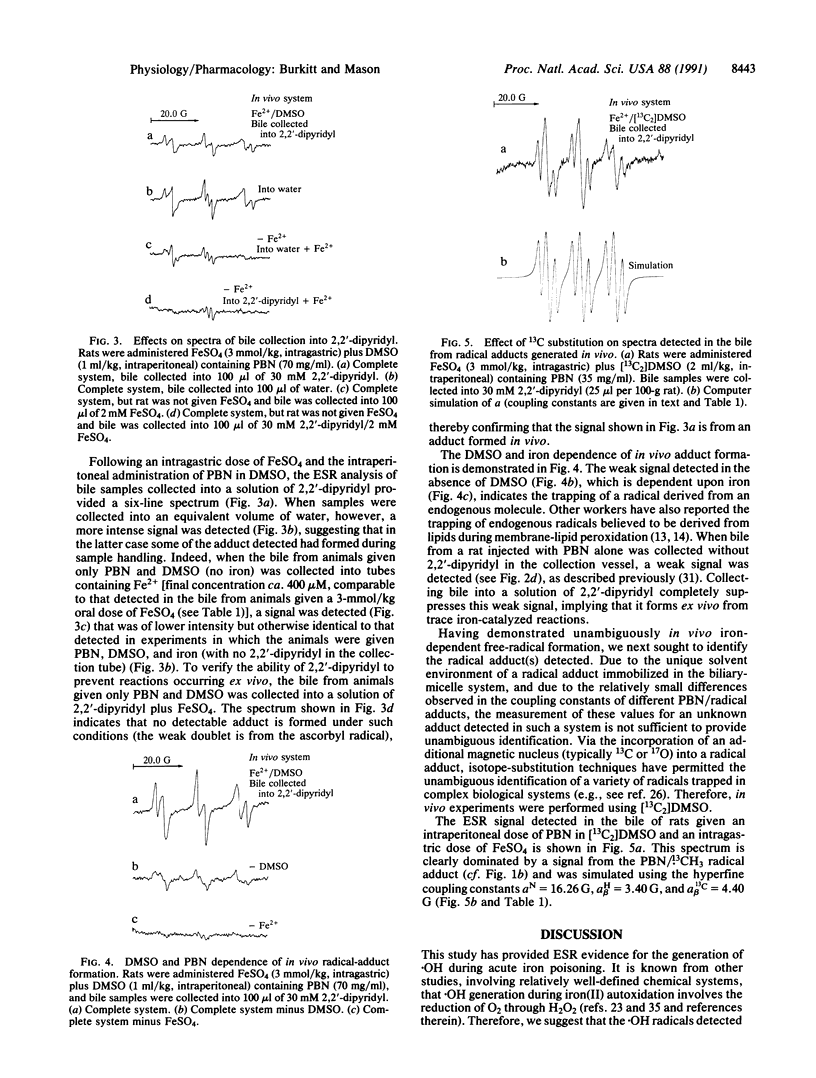
Although the hydroxyl radical is often implicated as the species responsible for the initiation of oxidative damage in iron-overload conditions, no ESR evidence for the formation of the radical in vivo has been reported. We have employed a secondary radical-trapping technique in which the hydroxyl radical reacts with dimethyl sulfoxide to form the methyl radical, which is then detected as its adduct of the spin trap N-t-butyl-alpha-phenylnitrone in the bile of animals given an intragastric dose of ferrous sulfate. The identity of this adduct was verified by isotope-substitution techniques. We show that unless measures are taken to inactivate the iron excreted in the bile of treated animals, reactions between iron, oxygen, dimethyl sulfoxide, N-t-butyl-alpha-phenylnitrone, and bile components lead to the formation of artifacts during sample collection.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisen P., Listowsky I. Iron transport and storage proteins. Annu Rev Biochem. 1980;49:357–393. doi: 10.1146/annurev.bi.49.070180.002041. [DOI] [PubMed] [Google Scholar]
- Arthur J. R., McPhail D. B., Goodman B. A. Spin trapping of free radicals in homogenates of heart from selenium and vitamin E deficient rats. Free Radic Res Commun. 1988;4(5):311–315. doi: 10.3109/10715768809066896. [DOI] [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978 Mar 23;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Bacon B. R., Britton R. S. The pathology of hepatic iron overload: a free radical--mediated process? Hepatology. 1990 Jan;11(1):127–137. doi: 10.1002/hep.1840110122. [DOI] [PubMed] [Google Scholar]
- Bacon B. R., Park C. H., Brittenham G. M., O'Neill R., Tavill A. S. Hepatic mitochondrial oxidative metabolism in rats with chronic dietary iron overload. Hepatology. 1985 Sep-Oct;5(5):789–797. doi: 10.1002/hep.1840050514. [DOI] [PubMed] [Google Scholar]
- Bacon B. R., Tavill A. S., Brittenham G. M., Park C. H., Recknagel R. O. Hepatic lipid peroxidation in vivo in rats with chronic iron overload. J Clin Invest. 1983 Mar;71(3):429–439. doi: 10.1172/JCI110787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britigan B. E., Coffman T. J., Buettner G. R. Spin trapping evidence for the lack of significant hydroxyl radical production during the respiration burst of human phagocytes using a spin adduct resistant to superoxide-mediated destruction. J Biol Chem. 1990 Feb 15;265(5):2650–2656. [PubMed] [Google Scholar]
- Burkitt M. J., Gilbert B. C. The autoxidation of iron(II) in aqueous systems: the effects of iron chelation by physiological, non-physiological and therapeutic chelators on the generation of reactive oxygen species and the inducement of biomolecular damage. Free Radic Res Commun. 1991;14(2):107–123. doi: 10.3109/10715769109094123. [DOI] [PubMed] [Google Scholar]
- Burkitt M. J., Gilbert B. C. The control of iron-induced oxidative damage in isolated rat-liver mitochondria by respiration state and ascorbate. Free Radic Res Commun. 1989;5(6):333–344. doi: 10.3109/10715768909073416. [DOI] [PubMed] [Google Scholar]
- Caujolle F. M., Caujolle D. H., Cros S. B., Calvet M. M. Limits of toxic and teratogenic tolerance of dimethyl sulfoxide. Ann N Y Acad Sci. 1967 Mar 15;141(1):110–126. doi: 10.1111/j.1749-6632.1967.tb34871.x. [DOI] [PubMed] [Google Scholar]
- Crotty J. J. Acute iron poisoning in children. Clin Toxicol. 1971 Dec;4(4):615–619. doi: 10.3109/15563657108990984. [DOI] [PubMed] [Google Scholar]
- Edwards C. Q., Griffen L. M., Goldgar D., Drummond C., Skolnick M. H., Kushner J. P. Prevalence of hemochromatosis among 11,065 presumably healthy blood donors. N Engl J Med. 1988 May 26;318(21):1355–1362. doi: 10.1056/NEJM198805263182103. [DOI] [PubMed] [Google Scholar]
- HOPPE J. O., MARCELLI G. M. A., TAINTER M. L. An experimental study of the toxicity of ferrous gluconate. Am J Med Sci. 1955 Nov;230(5):491–498. doi: 10.1097/00000441-195523050-00003. [DOI] [PubMed] [Google Scholar]
- Jacobs A. Low molecular weight intracellular iron transport compounds. Blood. 1977 Sep;50(3):433–439. [PubMed] [Google Scholar]
- Kang J. O., Slivka A., Slater G., Cohen G. In vivo formation of hydroxyl radicals following intragastric administration of ferrous salt in rats. J Inorg Biochem. 1989 Jan;35(1):55–69. doi: 10.1016/0162-0134(89)84005-x. [DOI] [PubMed] [Google Scholar]
- Knecht K. T., Mason R. P. In vivo radical trapping and biliary secretion of radical adducts of carbon tetrachloride-derived free radical metabolites. Drug Metab Dispos. 1988 Nov-Dec;16(6):813–817. [PubMed] [Google Scholar]
- LeSage G. D., Kost L. J., Barham S. S., LaRusso N. F. Biliary excretion of iron from hepatocyte lysosomes in the rat. A major excretory pathway in experimental iron overload. J Clin Invest. 1986 Jan;77(1):90–97. doi: 10.1172/JCI112307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd R. V., Mason R. P. Evidence against transition metal-independent hydroxyl radical generation by xanthine oxidase. J Biol Chem. 1990 Oct 5;265(28):16733–16736. [PubMed] [Google Scholar]
- Miller D. M., Buettner G. R., Aust S. D. Transition metals as catalysts of "autoxidation" reactions. Free Radic Biol Med. 1990;8(1):95–108. doi: 10.1016/0891-5849(90)90148-c. [DOI] [PubMed] [Google Scholar]
- Mulligan M., Althaus B., Linder M. C. Non-ferritin, non-heme iron pools in rat tissues. Int J Biochem. 1986;18(9):791–798. doi: 10.1016/0020-711x(86)90055-8. [DOI] [PubMed] [Google Scholar]
- Poli G., Albano E., Tomasi A., Cheeseman K. H., Chiarpotto E., Parola M., Biocca M. E., Slater T. F., Dianzani M. U. Electron spin resonance studies on isolated hepatocytes treated with ferrous or ferric iron. Free Radic Res Commun. 1987;3(1-5):251–255. doi: 10.3109/10715768709069790. [DOI] [PubMed] [Google Scholar]
- Rice-Evans C., McCarthy P., Hallinan T., Green N. A., Gor J., Diplock A. T. Iron overload and the predisposition of cells to antioxidant consumption and peroxidative damage. Free Radic Res Commun. 1989;7(3-6):307–313. doi: 10.3109/10715768909087956. [DOI] [PubMed] [Google Scholar]
- Sevanian A., Nordenbrand K., Kim E., Ernster L., Hochstein P. Microsomal lipid peroxidation: the role of NADPH--cytochrome P450 reductase and cytochrome P450. Free Radic Biol Med. 1990;8(2):145–152. doi: 10.1016/0891-5849(90)90087-y. [DOI] [PubMed] [Google Scholar]
- Turrens J. F., Boveris A. Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria. Biochem J. 1980 Nov 1;191(2):421–427. doi: 10.1042/bj1910421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver J., Pollack S. Low-Mr iron isolated from guinea pig reticulocytes as AMP-Fe and ATP-Fe complexes. Biochem J. 1989 Aug 1;261(3):787–792. doi: 10.1042/bj2610787. [DOI] [PMC free article] [PubMed] [Google Scholar]



