Abstract
The impact of transport time and temperature on survival of group B streptococci (GBS) in Amies transport medium was evaluated. Viability of 10 or more CFU of GBS was maintained for 4 days at 24 or 3°C. However, there was a significant decrease in viability for GBS held at 30°C for 4 days.
In the 1970s, group B streptococci (GBS) were identified as the leading cause of infection and mortality among neonates in the United States (3, 6). Early-onset group B streptococcal disease occurs in ∼1% or fewer newborns who are born to women colonized with GBS (6). Prior to the widespread use of antibiotics for the prevention of GBS, the incidence of GBS sepsis was 1.8/1,000 live births (12), but this number has decreased to 0.32/1,000 live births with the implementation of GBS prevention efforts in the United States (4). However, GBS morbidity is still significant. A recent study of 108 neonatal infections due to GBS reported a case fatality rate of 13% (5).
In 2002, the Centers for Disease Control and Prevention (CDC) issued recommendations that all pregnant women in the United States be screened for vaginal and/or rectal colonization by GBS at 35 to 37 weeks' gestation by a broth enrichment culture method (3). Women identified as GBS colonized are offered intrapartum antibiotic prophylaxis in order to interrupt transmission of GBS from the mother to the neonate. Studies have shown that the frequency of GBS colonization in women of reproductive age ranges from 10 to 34% (2, 3). However, in one recent study of GBS colonization in 1,248 nonpregnant women, 40% were found to test positive either vaginally or rectally for this organism (7). Because GBS colonization is dynamic, the CDC recommends that pregnant women be screened vaginally and rectally for GBS near the time of delivery since doing so at that time should better predict GBS colonization status at delivery (3, 11).
The CDC guidelines recommend that vaginal and rectal swabs be transported in a nonnutritive medium, such as Amies or Stuart's medium, at room or refrigeration temperature (3). Many large healthcare systems are consolidating laboratories and thus require clinics and satellite hospitals to transport specimens to a central laboratory. There are also large independent laboratories that provide service to clinics from surrounding states. These situations can result in specimens being exposed to various temperatures and storage times during transport. The CDC guidelines state specifically that the viability of GBS can be maintained for up to 4 days in appropriate transport media (3). There are few published data which support this recommendation, although one study demonstrated preservation of viability for up to 4 days when an inoculum of 6 × 105 organisms per swab was used (8). A second published study evaluated preservation of GBS at 101 organisms per swab, but only up to 24 h (10). A study of GBS colonization in 1,248 nonpregnant women (7) has been published. In that population, 144 (29%) of the 500 GBS-positive cultures were detected only after broth enrichment, suggesting low-density colonization (S. L. Hillier, unpublished data). Since nearly one-third of women are colonized with GBS at lower densities, we chose to evaluate the viability of GBS in Amies transport medium at densities of 1 to 1,000 CFU per swab. The impacts of temperature and length of storage time (up to 8 days) on the recovery of GBS were also evaluated.
(A portion of this work was presented at the 103rd General Meeting of the American Society for Microbiology, Washington, D.C., 18 to 22 May 2003.)
A total of 50 isolates of group B Streptococcus initially recovered from vaginal swab specimens were evaluated in this study. The strains were initially isolated and subcultured for purity on Columbia sheep blood agar (PML Microbiologicals, Portland, Oreg.) and identified according to standard procedures (9) by using a rapid antigen detection method (Pathodx; Diagnostic Products Corporation, Los Angeles, Calif.). The isolates were identified also by capsular polysaccharide serotype by using both HCl extract and mutanolysin extract as previously described (1). To assess whether there were differences in viability observed among individual serotypes, 10 strains of each of the five major serotypes (Ia, Ib, II, III, and V) were evaluated.
Each of the 50 isolates was initially suspended in phosphate-buffered saline at a concentration equivalent to a 0.5 McFarland standard. The suspensions were then serially diluted 1:10 in sterile phosphate-buffered saline and inoculated onto blood agar plates to determine the concentration of GBS in the suspensions. Dilutions of the suspensions (10−1, 10−2, 10−3, and 10−4) were used to evaluate the detection of low numbers of GBS over time and at different storage temperatures. For each dilution, 100 μl was inoculated onto 12 sterile Dacron swabs (Fisher Scientific, Pittsburgh, Pa.) and placed in separate Amies transport tubes (MML Diagnostics, Troutdale, Oreg.) Four swabs of each inoculum of GBS were stored at 3, 24, and 30°C for up to 8 days. At days 2, 4, 6, and 8, one swab from each storage temperature and dilution was used to inoculate a group B streptococcus broth (LIM broth) (PML Microbiologicals), one of the selective broth media for GBS recommended by the CDC (3). The inoculated broth was incubated at 37°C and 6% CO2 for 24 h. The broth was subcultured after 24 h onto a Columbia sheep blood agar plate and incubated at 37°C and 6% CO2 for 24 h. The results were recorded as either positive or negative for growth of GBS. Two-tailed Fisher's exact test was used to evaluate statistically significant differences in GBS survival compared to the GBS stored at 24°C by using Epi Info (version 6.04B; CDC).
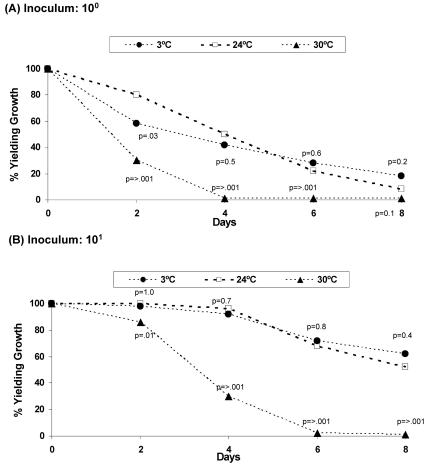
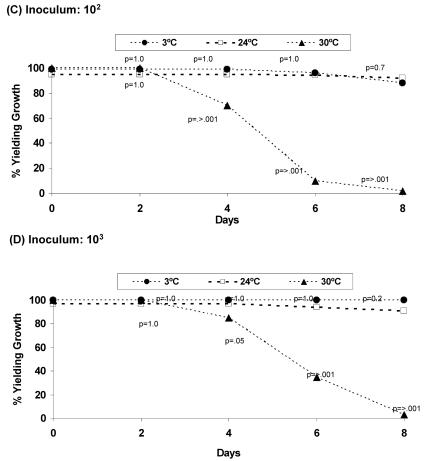
Storage time and temperature had significant impacts on the recovery of GBS (Fig. 1). GBS was recovered from 92 to 100% of swabs containing 10 or more organisms when stored at 3 or 24°C for 4 days. However, GBS recovery decreased significantly when the swabs were stored at 30°C. After 6 days, adequate sensitivity (96 to 100%) was observed only for the swabs held at 4 and 24°C and containing 100 or more CFU. Swabs that were held at 30°C had the lowest recovery of GBS, ranging from 0 to 35% after 6 days, depending on the numbers of CFU present.
FIG. 1.
Survival of 50 strains of GBS in Amies transport medium for up to 8 days at 3, 24, or 30°C. All 50 strains were evaluated once at each time and temperature point. Swabs were inoculated with <10 (A), 101 (B), 102 (C), and 103 (D) CFU of GBS. Growth was detected by using the swabs to inoculate the enrichment broth medium. The proportion yielding growth is defined as the number of swab samples yielding growth/50 isolates tested. P values were calculated based on Fisher's exact test with the samples stored at 24°C as the reference group. A P value of ≤0.05 was considered statistically significant.
At the lowest GBS concentration that was evaluated (<10 CFU per swab), there were greater effects of time and temperature on the rate of detection than were seen at the higher concentrations. After 48 h at 30°C, only 30% of the swabs containing <10 CFU yielded GBS, compared with 86% of the swabs inoculated with 100 CFU (P < 0.001). At 24°C, 80% of swabs containing <10 CFU, versus 100% of swabs containing 100 CFU (P = 0.001), yielded viable GBS. All of the capsular polysaccharide types survived equally well at each of the four concentrations and at each of the three temperatures (data not shown).
The results of this study support the CDC guidelines with respect to the survival of GBS in transport for up to 4 days at room or refrigeration temperature. However, there are a few caveats. At the lowest vaginal colonization densities, which could yield fewer than 10 GBS per swab, GBS viability after 2 days on swab specimens was better at room temperature than at refrigeration temperature. Acceptable viability was not sustained over 4 days at any of the temperatures tested for swabs having the lowest densities of GBS. A second caveat relates to the definition of room temperature. When GBS transport specimens were held at 30°C (86°F), there was a significant reduction in the viability of GBS over 4 days (P < 0.05 for all groups). In the United States, most hospital and laboratory settings are air-conditioned during the warmer months, especially in those areas of the country where summer temperatures are uncomfortably warm. However, specimens may be exposed to higher temperatures during holding or transport in non-air-conditioned conditions. If a woman has low-density GBS colonization, extended transport times of swab specimens at room temperatures of 30°C (86°F) or higher could reduce culture sensitivity. Therefore, care should be taken when transport media will be held for an extended period to ensure that exposure to higher temperatures is minimized. Finally, it is recognized that application of purified isolates of GBS onto transport swabs may not reflect GBS survival on swabs containing vaginal and/or rectal microflora. Nonetheless, the findings of the present study support the CDC guidelines (3) with respect to expected survival of GBS in transport media.
Acknowledgments
This work was supported by Public Health Service contract N01-AI 75326 from the National Institutes of Allergy and Infectious Disease.
REFERENCES
- 1.Benson, J. A., A. E. Flores, C. J. Baker, S. L. Hillier, and P. Ferrieri. 2002. Improved methods for typing nontypeable isolates of group B streptococci. Int. J. Med. Microbiol. 292:37-42. [DOI] [PubMed] [Google Scholar]
- 2.Bliss, S. J., S. D. Manning, P. Tallman, C. J. Baker, M. D. Pearlman, C. F. Marrs, and B. Foxman. 2002. Group B Streptococcus colonization in male and nonpregnant female university students: a cross-sectional prevalence study. Clin. Infect. Dis. 34:184-190. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2002. Prevention of perinatal group B streptococcal disease. Morb. Mortal. Wkly. Rep. 51:1-6. [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2004. Diminishing racial disparities in early-onset neonatal group B streptococcal disease—United States, 2000-2003. Morb. Mortal. Wkly. Rep. 53:502-505. [PubMed] [Google Scholar]
- 5.Dahl, M. S., I. Tessin, and B. Trollfors. 2003. Invasive group B streptococcal infections in Sweden: incidence, predisposing factors and prognosis. Int. J. Infect. Dis. 7:113-119. [DOI] [PubMed] [Google Scholar]
- 6.Hickman, M. E., M. A. Rench, P. Ferrieri, and C. J. Baker. 1999. Changing epidemiology of group B streptococcal colonization. Pediatrics 104:203-209. [DOI] [PubMed] [Google Scholar]
- 7.Meyn, L. A., D. M. Moore, S. L. Hillier, and M. A. Krohn. 2002. Association of sexual activity with colonization and vaginal acquisition of group B Streptococcus in nonpregnant women. Am. J. Epidemiol. 155:949-957. [DOI] [PubMed] [Google Scholar]
- 8.Ostroff, R. M., and J. W. Steaffens. 1995. Effect of specimen storage, antibiotics, and feminine hygiene products on the detection of group B Streptococcus by culture and the STREP B OIA test. Diagn. Microbiol. Infect. Dis. 22:253-259. [DOI] [PubMed] [Google Scholar]
- 9.Ruoff, K. L., R. A. Whiley, and D. Beighton. 2003. Streptococcus, p. 403-421. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. A. Pfaller, and R. H. Yolken (ed.), Manual of clinical microbiology, 8th ed. ASM Press, Washington, D.C.
- 10.Teese, N., D. Henessey, C. Pearce, N. Kelly, and S. Garland. 2003. Screening protocols for group B streptococcus: are transport media appropriate? Infect. Dis. Obstet. Gynecol. 11:199-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yancey, M. K., A. Schuchat, L. K. Brown, V. L. Venutra, and G. R. Markenson. 1996. The accuracy of late antenatal screening cultures in predicting genital group B streptococcal colonization at delivery. Obstet. Gynecol. 88:811-815. [DOI] [PubMed] [Google Scholar]
- 12.Zangwill, K. M., A. Schuchat, and J. D. Wenger. 1992. Group B streptococcal disease in the United States. Morb. Mortal. Wkly. Rep. 41:25-32. [PubMed] [Google Scholar]