Abstract
This article reports on high-rate nitrification at low pH in biofilm and suspended-biomass reactors by known chemolithotrophic bacteria. In the biofilm reactor, at low pH (4.3 ± 0.1) and low bulk ammonium concentrations (9.3 ± 3.3 mg · liter−1), a very high nitrification rate of 5.6 g of N oxidized · liter−1 · day−1 was achieved. The specific nitrification rate (0.55 g of N · g of biomass−1 · day−1) was similar to values reported for nitrifying reactors at optimal pH. In the suspended-biomass reactor, the average pH was significantly lower than that in the biofilm reactor (pH 3.8 ± 0.3), and values as low as pH 3.2 were found. In addition, measurements in the suspended-biomass reactor, using isotope-labeled ammonium (15N), showed that in spite of the very low pH, biomass growth occurred with a yield of 0.1 g of biomass · g of N oxidized−1. Fluorescence in situ hybridization using existing rRNA-targeted oligonucleotide probes showed that the nitrifying bacteria were from the monophyletic genus Nitrosomonas, suggesting that autotrophic nitrification at low pH is more widespread than previously thought. The results presented in this paper clearly show that autotrophic nitrifying bacteria have the ability to nitrify at a high rate at low pH and in the presence of only a negligible free ammonia concentration, suggesting the presence of an efficient ammonium uptake system and the means to cope with low pH.
Nitrification is carried out mainly by aerobic chemolithotrophic bacteria and consists of oxidation of ammonia to nitrite by ammonia-oxidizing bacteria (AOB), followed by oxidation of nitrite to nitrate by nitrite-oxidizing bacteria (NOB). Ammonium (the dominant ion under neutral and acidic conditions) and nitrite are oxidized according to the following stoichiometry (neglecting biomass growth) (31):
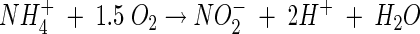 |
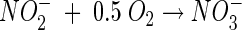 |
As shown in the above equation, the oxidation of every mole of ammonium produces 2 mol of acidity, which can result in a pH decrease in poorly buffered environments. Autotrophic microbial nitrification is known to be highly sensitive to pH, and optimal conditions have been found to be within the narrow pH range of 7 to 8. Generally, below pH values of 6.5, growth of pure cultures of autotrophic ammonia-oxidizing bacteria in liquid culture does not occur (5, 24). The high sensitivity of nitrification to acidic conditions has been attributed mainly to the exponential decrease in free ammonia (NH3) with decreasing pH (NH3 + H+ ↔ NH4+; pKa = 9.25) (8). Free ammonia is considered to be the substrate for the primary enzyme ammonia monooxygenase (34), and the transport of free ammonia into the cells, unlike ammonium ions, is by passive diffusion (41).
Low rates of nitrification and the presence of autotrophic nitrifying bacteria in acid soils have been reported by many researchers (5, 8, 13) with a pH as low as 3.3 but could not be replicated in dedicated liquid batch culture systems. Possible mechanisms for NH3 supply to ammonia monooxygenase under negligible concentrations in acid soils are currently ascribed to the availability of other sources of nonionic ammonia-like urea (5, 8) or to the presence of alkaline microsites where a favorable pH exists (8). There are also indications that acid-tolerant AOB of the genus Nitrosospira isolated from acid soils can be active at a low pH without the need for special NH3-generating mechanisms (8). Other studies have shown the necessity for high cell density, either biofilm or aggregates, for nitrification at a low pH, but only at a very low rate (1, 7).
Although the general protective nature of biofilms and aggregates against environmental extremes is well documented (6), a specific protective mechanism for autotrophic nitrifiers enabling local neutralization under bulk acidic conditions is debatable. Autotrophic nitrifying biofilms intrinsically produce acidity, and the diffusional resistance to the transport of protons and inorganic carbon species in the biofilm will further decrease pH in the interior of the biofilm (35). Moreover, evidence from biofilm and aggregate engineered systems for wastewater treatment has demonstrated repeatedly that autotrophic nitrifying bacteria are inhibited at low pH (24, 31, 40). In contrast, the present paper shows that autotrophic nitrifying bacteria originating from a municipal wastewater treatment plant have the ability to nitrify at low pH and at a high rate even with a negligible free ammonia concentration without any special NH3-generating mechanisms. The phenomenon of high-rate nitrification at low pH is demonstrated for both biofilm and suspended-biomass reactor systems.
MATERIALS AND METHODS
Attached biomass (biofilm) reactor.
The attached-biomass reactor consisted of a double-column fluidized bed made of transparent 40-mm-diameter polyvinyl chloride tubes. The main nitrifying column was 110 cm long (1 liter) and was filled with 350 g (700 ml) of 1- to 2-mm sintered glass particles used as the biomass carrier (Schott AG, Mainz, Germany). The main column was interconnected to a 50-cm-long aeration column. Pure oxygen was bubbled at a constant flow rate through the aeration column, and the oxygen-saturated solution was recirculated via a pump back to the main column. The oxygen flow was adjusted so that the dissolved-oxygen concentration at the outlet of the main nitrifying column was greater than 8 mg · liter−1. The reactor operated at a high recirculation ratio to provide completely mixed conditions. The upflow velocity in the column was between 50 and 60 m · h−1, and the bed expansion was 30%. All of the experiments were carried out at 30°C. The reactor was operated with continuous flow, using an Iwaki diaphragm pump. Depending on the nitrification rate, the influent flow was varied between 7 and 15 ml · min−1. The influent solution was made from tap water spiked only with various concentrations of ammonium chloride (200 to 1,000 mg · liter−1), monobasic potassium phosphate (2 to 5 mg · liter−1), and sodium bicarbonate buffer (600 to 3,000 mg · liter−1).
Reactor start-up was at a pH of >7 (excess buffer addition) with bacterial inoculum originating from the Haifa municipal wastewater treatment plant. The reactor working procedure at low pH was based on three main operational conditions: (i) the reactor feeding solution was preloaded with a buffer content slightly lower than the amount required to neutralize the protons released from complete nitrification of the influent ammonium concentration, (ii) the reactor biomass concentration was high enough to change the influent water chemistry, and (iii) the loading rate was attuned to the activity of the bacteria so that the influent alkalinity (the proton-accepting capacity) was completely destroyed.
Samples of biofilm-covered sintered glass particles were removed weekly from the reactor by creating a slight vacuum in a flexible 10-mm-diameter tube inserted into the middle of the fluidized bed (no stratification of bioparticles was observed). Duplicate samples were analyzed for dry biomass content using the volatile-suspended-solids (VSS) method (2).
Suspended-biomass reactor.
The suspended-biomass reactor consisted of a 10-liter glass flask equipped with a magnetic stirrer and aerated by pure oxygen (dissolved oxygen, >8 mg · liter−1). Biomass taken from a nitrifying fixed-bed reactor already adapted to work at low pH (pH 5) was used as the inoculum. The same working procedure and inorganic influent solution used in the attached-biomass reactor were used for the suspended-biomass reactor. The concentrations of ammonium, bicarbonate buffer, and phosphate were adjusted according to the nitrification rate. The reactor was operated with a continuous influent flow of 3.8 ml · min−1, using a Cole Parmer peristaltic pump. Effluent was discharged from the suspended-biomass reactor only once a day, resulting in a gradual accumulation of liquid in the reactor of from 1 to 6.4 liters in a 24 h-period. After each 24-h period of operation, the reactor was allowed to stand for 15 min with no mixing, aeration, and influent flow to allow for biomass settling. A 5.4-liter volume of supernatant was then drained from the reactor. Mixing, aeration, and influent flow were restarted immediately after drainage. The supernatant was filtered using Whatman GFA filter paper (1.6-μm pore size) in order to avoid bacterial loss and maintain high reactor biomass concentrations. The small amount of filtered biomass (100 to 200 mg) collected on the filter paper was gently rinsed and returned to the reactor. Daily cleaning of the reactor walls prevented any formation of biofilm. Due to the specific operating conditions, the overall reaction rate in the reactor (rate per unit volume × reactor volume) was constant throughout the day. The reactor was monitored a number of times throughout the 24-h period, and ammonium, nitrite, and nitrate concentrations were found to be constant. The pH was slightly lower (about 0.2 pH units) immediately following settling and slightly higher (about 0.3 pH units) during the first hour following the restart of influent flow. During the rest of the day, the pH was constant. Nitrogen and pH results given in this paper are the measurements recorded at the end of each 24-h period, i.e., when the volume of the reactor was 6.4 liters. Every week, a 25-ml sample for dry biomass and protein content analysis was removed from the suspended-biomass reactor. Protein was measured on triplicate 1.5-ml samples by using the Bradford method (4), while VSS analysis (2) was performed on duplicate 10-ml samples.
Chemical analysis.
Duplicate samples from the reactors were analyzed for ammonium, nitrate, nitrite, and phosphate concentrations. Nitrate, nitrite, and phosphate concentrations were determined by using a Metrohm 761 ion chromatograph equipped with a Metrosep Dual 1 anion separating column and suppressor using a carbonate (1.8 mM)-bicarbonate (1.7 mM) eluent. Ammonium concentrations were determined using a second Metrohm 761 ion chromatograph equipped with a Metrosep C2 cation separating column, using a tartaric acid (4 mM)-dipicolonic acid (1 mM) eluent. The error between the duplicate samples was never greater than 2%. Duplicate pH measurements were taken from the reactor systems at intervals of 10 min using a daily calibrated portable Eutech pH meter equipped with a standard combined electrode and temperature probe. The difference between successive measurements was never greater than 0.05 pH units. Alkalinity was measured using the Gran titration procedure (11). Oxygen was measured using a Eutech oxygen meter equipped with a galvanic electrode.
15N isotope measurements.
In addition to measuring protein and VSS content in the suspended-biomass reactor, biomass growth was determined using 15N isotope measurements. Five percent of the ammonium content in the feeding solution was replaced with the isotope 15NH4+ for 26 days (from day 7 to day 33). The percent of 15N that accumulated in the biomass was measured with an elemental analyzer (ANCA-SL; PDZ Europa, Crewe, United Kingdom) connected to an isotope ratio mass spectrometer (20-20; PDZ Europa). Based on the measured ratio of 15N to 14N and the amount of elemental nitrogen present in the biomass sample, the change in 15N content in the biomass over time was determined. The yield coefficient was then calculated from the amount of ammonium oxidized together with the change in 15N biomass content.
Analysis of nitrifying population in the biofilm and suspended-biomass reactors.
After extended operation at about pH 4 (250 days), a sample from the biofilm reactor was fixed 1:1 with ethanol (96%) and analyzed for bacterial populations by Vermicon AG (Munich, Germany). The biofilm sample was tested by using fluorescently labeled oligonucleotide probes for eubacteria, β-proteobacterial ammonia-oxidizing bacteria, ammonia-oxidizing bacteria from the Nitrosomonas europaea/eutropha, Nitrosococcus mobilis, Nitrosomonas oligotropha, and Nitrosospira groups and nitrite-oxidizing bacteria of the genus Nitrospira (Table 1). The hybridization was preformed as described previously (21). Slides were dual stained with 4′,6′-diamidino-2-phenylindole (DAPI), and biomass cell viability was indicated by the percentage of DAPI-stained cells hybridized to the eubacteria probe. To determine the share of each bacterial group relative to eubacteria, 20 different microscopic fields were evaluated and the average fraction was calculated. The error was 5 to 10% of the values given in Tables 1 and 2.
TABLE 1.
Analysis of bacterial population from laboratory biofilm reactora
| Target organism(s) | Probe (reference) | % of total eubacterial population |
|---|---|---|
| β-Proteobacterial ammonia oxidizers | BET42a (20) | 65 |
| Nitrosomonas europaea/eutropha | Nse1472 (20) | ND |
| Nitrosococcus mobilis | NmV (20) | ND |
| Nitrosomonas oligotropha lineage | Nmo218 + Noli191 (10) | 65 |
| Nitrosospira genus | Nsv443 (20) | ND |
| Nitrospira genus | Ntspa662 (20) | 25 |
Fluorescently labeled oligonucleotide probes (Vermicon AG) were used. ND, not detected.
TABLE 2.
Analysis of bacterial population from suspended-biomass laboratory reactor at end of experimental perioda
| Target organism(s) | Probe(s) (reference) | % of total eubacterial population |
|---|---|---|
| α-Proteobacteria | ALF968 (20) | 5 |
| β-Proteobacteria | BET42a (20) | 4 |
| β-Proteobacterial ammonia oxidizers | Nso1225 (20) and NEU+CTE(20) | 2 |
| Nitrosomonas europaea/eutropha | Nse1472 (20) | ND |
| Nitrosococcus mobilis | NmV (20) | 0.8 |
| Nitrosomonas marina lineage | NSMR76 (20) | ND |
| Nitrosomonas oligotropha lineage | Nmo218 (10) and Noli191 (10) | 0.8 |
| Nitrosomonas communis lineage | NmII (25) | ND |
| Nitrosomonas cryotolerans lineage | NmIV (25) | ND |
| Nitrosospira genus | Nsv443 (20) | ND |
| Nitrosospira tenuis lineage | NSMR34 (20) | ND |
| γ-Proteobacteria | GAM42a (20) | <1 |
| Gram-positive with high G+C content | HGC69A (20) | 3 |
| Cytophaga-Flexibacter subphylum | CF319a (20) | ND |
| Nitrospira genus | Ntspa662 + CompNtspa66 2 (20) | 80 |
| Nitrobacter | NIT3 + CompNIT3 (20) | ND |
| Major heterotrophic nitrifiers | ||
| Paracoccus denitrificans | Par651 (20) | ND |
| Alcaligenes faecalis | Alf6a_new (22) | ND |
| Pseudomonas putida | Ppu1437 + 2 (22) | ND |
Fluorescently labeled oligonucleotide probes (Vermicon AG) were used. ND, not detected.
In a similar manner, after 100 days of low-pH operation, a sample of settled biomass from the suspended-biomass reactor was analyzed for bacterial populations by Vermicon AG (Munich, Germany). A more extensive hierarchical set of fluorescently labeled oligonucleotide probes targeting the α-, β-, and γ-proteobacterium groups, ammonia-oxidizing bacteria, and the major subgroups of ammonia- and nitrite-oxidizing bacteria was used (Table 2).
Tests were also carried out to determine if any significant heterotrophic bacterial or fungal nitrification occurred in the biofilm and suspended-biomass reactor. Batch experiments with allylthiourea (1 μg · ml−1) and nitrapyrin at low concentrations (0.5 μg · ml−1) showed significant inhibition, indicating autotrophic nitrification (26, 39). Batch tests with increasing concentrations of acetate (up to 20 mg · liter−1) showed similar or even lower nitrification rates, further indicating no significant heterotrophic nitrification (37). In addition, batch cultures with streptomycin and tetracycline at bacteriocide concentrations almost completely inhibited nitrifying activity, ruling out the presence of fungal nitrification (19). Furthermore, using appropriate fluorescent oligonucleotide probes on a biomass sample from the suspended-biomass reactor, the important heterotrophic nitrifier groups Paracoccus denitrificans, Alcaligenes faecalis, and Pseudomonas putida were found to be absent (Table 2).
RESULTS AND DISCUSSION
Attached biomass (biofilm) reactor.
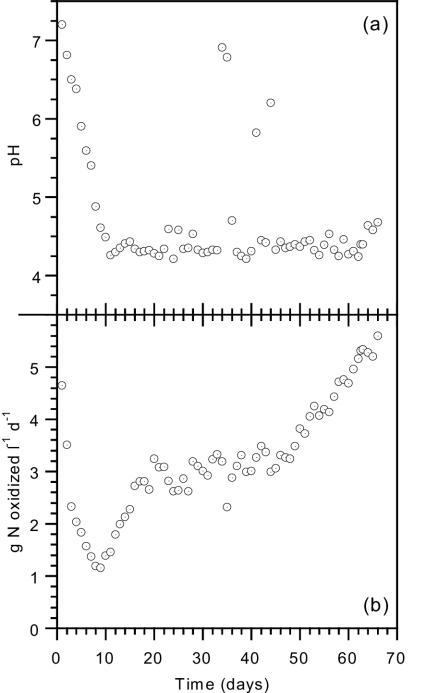
An attached-biomass reactor with sintered glass as the biofilm carrier was first operated at pH 7 (Fig. 1a) until a high nitrification rate of 4.2 g of N oxidized · liter of reactor volume−1 · day−1 and biomass content of 6.6 ± 0.2 g of VSS · liter−1 were achieved with a specific nitrification rate of 0.64 g of N oxidized · g of biomass−1 · d−1. The transition from neutral to acidic conditions was carried out by gradually reducing the ammonium load to the reactor over a period of 8 days, lowering the nitrification rate from 4.6 to 1.1 g of N oxidized · liter of reactor volume−1 · day−1, and by changing the feeding solution to contain insufficient buffer for complete nitrification of the influent ammonium concentration (Fig. 1b). In the same period, the pH dropped from 7.2 to 4.6, and the carbonate alkalinity fell to just below the H2CO3* (H2CO3 = H2CO3 + CO2 [aqueous]) equivalence point (−0.04 meq · liter−1), indicating that all of the influent buffer was destroyed and only acidity species were present. At this point, the nitrification rate was 24% of the original rate. The nitrogen load was increased for 10 days, and the nitrification rate recovered to 3.2 g of N oxidized · liter of reactor volume−1 · day−1 in spite of the low average pH (4.3 ± 0.1) and alkalinity (−0.04 ± 0.03 meq · liter−1). The reactor was maintained at a nitrification rate of about 3.0 g of N oxidized · liter of reactor volume−1 · day−1 and recorded the same average pH and negative alkalinity values for a further 30 days except for a few transient pH peaks observed during this period (days 34, 35, 40, and 43) caused by malfunctions in the oxygen supply system. From day 48 to day 66, the influent load was increased, and a very high nitrification rate of 5.6 g of N oxidized · liter of reactor volume−1 · day−1 was achieved. The average pH during this period was only slightly higher at 4.4 ± 0.1. The biomass concentration in the reactor increased by about 50% to 10.1 ± 0.4 g of VSS · liter−1, with a specific nitrification rate of 0.55 g of N · g of biomass−1 · day−1, similar to the value obtained at the end of reactor start-up at pH 7 and with other high-rate nitrification reactors operating at pH 7 reported in the literature (3, 9).
FIG. 1.
(a) Nitrifying biofilm reactor pH. (b) Performance of a nitrifying biofilm reactor at low pH. l, liter.
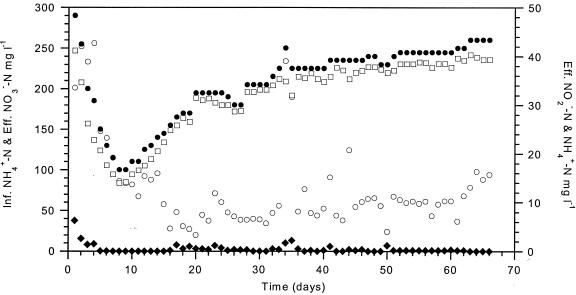
Approximately 94% of the influent ammonium was converted to nitrate, while the average nitrite concentration was 0.2 ± 0.3 mg of N · liter−1 and rarely higher than 1.0 mg of N · liter−1 (Fig. 2). The average ammonium concentration in the reactor was low, 9.3 ± 3.3 mg of N · liter−1, and the corresponding free ammonia concentration at low pH was negligible, 0.01 ± 0.006 μM NH3. Daily nitrogen mass balance based on ammonium fed, nitrate and nitrite produced, and alkalinity balance (where for every mole of NO3− produced, 2 mol of alkalinity was destroyed) showed no apparent nitrogen loss due to denitrification. This is to be expected from reactor systems operating at high concentrations of oxygen (>8 mg · liter−1 in the effluent) and a feeding solution consisting only of inorganic salts.
FIG. 2.
Ammonium, nitrite, and nitrate concentrations in the nitrifying biofilm reactor (influent [Inf.] NH4+, •; effluent [Eff.] NH4+, ○; effluent NO2−, ♦; effluent NO3−, □). l, liter.
After continuous operation of the reactor for more than 250 days at low pH and nitrification rates greater than 2 g of N oxidized · liter of reactor volume−1 · day−1, the microbial composition of the biofilm reactor was characterized by using fluorescent rRNA targeted probes (Table 1). The bacterial cells showed high viability, since more than 95% of the DAPI-stained cells were positive for the eubacterial probe. Sixty-five percent of the eubacterial population was identified as AOB, while 25% was from the genus Nitrospira, a NOB. These values are higher than those with a similar control pH 7 nitrifying biofilm reactor showing 40% AOB and 20% NOB and reported values of dedicated nitrifying reactors using fluorescently labeled oligonucleotide probes (10, 15, 17, 23, 29). The low-pH reactor also presented a more uniform morphology of detected nitrifiers, with only smaller AOB cell clusters (about 10 μm), while a more diverse colony structure with various bigger AOB cell clusters (from 10 to 25 μm) could be observed in the pH 7 reactor. Coccoid Nitrospira cells were grouped in very tight and mostly small clusters. In the biofilm of the nitrifying reactors, AOB and Nitrospira clusters formed coaggregated microcolonies. With use of more-specific oligonucleotide probes, all the AOB were revealed to be from the N. oligotropha lineage. These known groups of nitrifiers are not considered acid-tolerant bacteria, although N. oligotropha is reported to have very low Ks values of 1.9 to 4.2 μM NH3 (18). With use of selective inhibitors (19, 26, 37, 39), no signs of either bacterial or fungal heterotrophic nitrification were found. The exclusive presence of Nitrosomonas-type bacteria after long-term reactor operation is noteworthy, because tolerance to low pH has been previously attributed mostly to the monophyletic genus Nitrosospira (8, 32). This suggests that autotrophic nitrification at low pH is more widespread than previously thought.
If indeed the bacteria identified in the low-pH reactor are not acid tolerant and the biofilm's glass carrier provides no alkaline or ion exchange properties, it still could be conjectured that somehow the biofilm provides a favorable pH microenvironment. Previous microelectrode measurements profiling pH and oxygen concentrations in a nitrifying biofilm grown at low pH revealed an active biofilm with a pH similar to the bulk pH and even lower (36). Furthermore, modeling nitrifying biofilm based on known kinetic parameters and diffusion constants supported the microsensor's measurements (35). Summarizing, a proton-producing biofilm cannot possibly have a favorable pH microenvironment, with the corresponding increase in NH3 concentration strongly suggesting that autotrophic nitrifying bacteria have an efficient uptake mechanism for the ammonium cation. The phenomenon of high-rate nitrification at low pH was repeated in several other biofilm reactors with inocula from different sources (36), and pH values as low as 3.75 were observed (results not shown).
Suspended-biomass reactor.
To further prove the abilities of known chemolithotrophic bacteria to nitrify at a high rate and a low pH, the research study was extended to include a suspended-biomass reactor. Comparison between biofilm and suspended-biomass reactors is important, since biofilms and aggregates were reported to facilitate nitrification at low pH (1, 7).
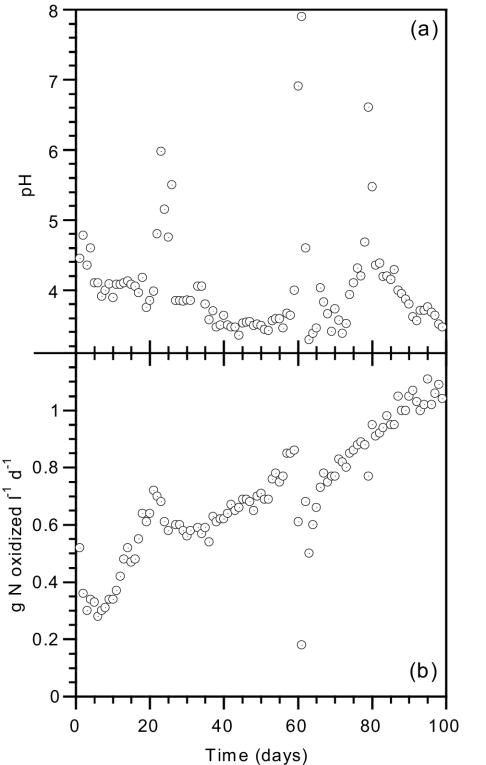
The low-pH nitrifying suspended-biomass reactor was inoculated with biomass taken from a fixed-bed reactor already acclimated to low pH (pH 5). Protons released by nitrification in the continuously fed suspended-biomass reactor destroyed all of the influent alkalinity (10 to 28 meq · liter−1 with a pH of 7.9 to 8.5) during the 100-day experimental period and resulted in a very low reactor pH, varying between 3.2 and 4.5, with an average pH of 3.8 ± 0.3 (Fig. 3a). Interestingly, the average pH in the suspended-biomass reactor was significantly lower by a half a unit than the average pH in the biofilm reactor. Although the bacteria could have operated at a higher pH simply by oxidizing a smaller amount of the influent ammonium concentration (resulting in the formation of less acidity), the bacteria seemed to be indifferent to the bulk pH as long as it was higher than 3.2. The measured alkalinity was significantly lower than that in the biofilm reactor and well below the H2CO3* equivalence point (−0.26 ± 0.16 meq · liter−1), i.e., no carbonate species with proton-accepting capacity were present in the suspended-biomass reactor, and the phosphate species concentration was low (1 to 2 mg · liter−1 as PO4−). A temporary increase in the pH was observed on days 23, 59 and 78 (Fig. 3a) due to a malfunction in oxygen supply (days 59 and 78) and a too-fast increase in the alkalinity and ammonium loading relative to the nitrification rate (day 23). During the entire experimental period, the nitrite concentration was always close to zero. As in the case of the nitrifying biofilm reactor, mass balance on both nitrogen and alkalinity showed that no denitrification occurred in the suspended-biomass reactor.
FIG. 3.
(a) Bulk pH in nitrifying suspended-biomass reactor. (b) Performance of a suspended biomass nitrifying reactor at low pH. l, liter.
The working procedure ensured that the ammonium concentration in the reactor was always low, the average concentration being 6.2 ± 3 mg · liter−1 for N-NH4+. The corresponding free ammonia concentration was negligible, with an average concentration of 0.002 ± 0.0015 μM NH3. Concentrations as low as 0.0002 μM NH3 were observed, which is four orders of magnitude lower than the lowest reported Ks for nitrifying bacteria (18).
The loading rate was gradually increased over the 100-day period. Increasing the loading rate was carried out only when lower values of reactor pH were observed, which indicated an increase in nitrification activity. At the end of the experimental period, a volumetric nitrification rate of 1.1 g of N oxidized−1 · liter of reactor volume−1 · day−1 (Fig. 3b) and a specific nitrification rate of 0.24 g if N oxidized · g of biomass−1 · day−1 was attained based on nitrate produced. The gradual increase in the nitrification rate was probably due to continued cell growth (see below) and not to adaptation, since the bacteria were already acclimated to a low pH from the beginning of the experiment.
Using fluorescently labeled oligonucleotide probes, the microbial composition of the suspended-biomass reactor was characterized (Table 2). As in the biofilm reactor, the bacterial cells showed high viability, since more than 95% of the DAPI-stained cells were positive for the eubacterial probe. Remarkably, 80% of the total eubacterial population was the nitrite oxidizer Nitrospira, while only 2% was identified as AOB. Using more-specific DNA probes, 1.6% of the AOB were identified as N. oligotropha- and N. mobilis-related groups in equal concentrations. The remaining 0.4% of AOB was undetected by any of the more specific probes used. The Nitrospira cells showed a uniform shape, coccoid cells, which were observed in small as well as large clusters. The identified ammonia oxidizers showed a diverse morphology. Dense cell clusters not greater than 10 μm in size belonging to the N. mobilis group and loosely packed cell “swarms” belonging to the N. oligotropha lineage were observed attached to larger Nitrospira clusters, forming large aggregates. Dense N. mobilis clusters were also observed separate from the large aggregates. Single AOB cells were hardly detected. No signs of either bacterial or fungal heterotrophic nitrification were found using selective inhibitors (19, 26, 37, 39) or specific molecular probes for the major heterotrophic nitrifying groups.
Based on total biomass concentration, a constant specific nitrification rate of 0.23 g of N oxidized · g of dry biomass−1 · day−1 was observed from day 25. This value is about one order of magnitude lower than reported maximal nitrification rates at pH values of between 7 and 8 (27, 30, 38) (values based on total biomass concentrations). Although this value is remarkably high for such a low pH, it is actually even much higher when based on AOB instead of the total biomass concentration because of the extremely small fraction of these bacteria.
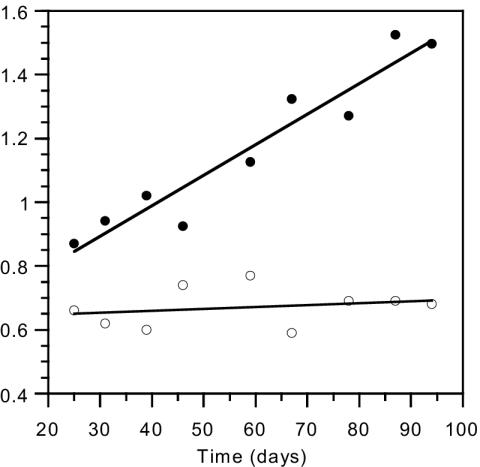
Growth in the suspended-biomass reactor was shown by an increase in the 15N isotope content of the biomass with use of a feeding solution enriched with 15N-labeled ammonium chloride in the early part of the experiment, from day 7 to day 33. Growth of bacteria was also confirmed as an increase in the reactor dry biomass concentration (from 2.7 to 4.1 g · liter−1) and protein concentration (from 0.9 to 1.5 g · liter−1) from day 25 to day 95, in spite of biomass losses caused by weekly sampling and daily filtration (Fig. 4). Although these methods do not differentiate between autotrophic and heterotrophic growth, there are several points which support the assumption that the increase in reactor protein, dry biomass, and 15N assimilation is mainly due to autotrophic growth: (i) the feeding solution contained only inorganic salts and no direct organic substrate to support substantial heterotrophic growth; (ii) a highly active nitrifying population was maintained for the whole experimental period, lasting 100 days, and the reactor nitrification rate even increased; (iii) the gradual increase in biomass concentration throughout the experiment was accompanied by a proportional rise in the nitrification rate in the reactor; and (iv) the incremental increase in biomass concentration was much greater than the measured share of the heterotrophs in the reactor. As for possible heterotrophic growth of the NOB population, this has been shown only for Nitrobacter, which was absent in the reactor, and not for Nitrospira, the only NOB found in the suspended-biomass reactor.
FIG. 4.
Biomass concentration (g of protein · liter−1; •) and specific ammonium oxidation rate (g of N oxidized · g of protein−1; ○) in the suspended-biomass reactor.
The 15N results showed a yield of 0.1 g of dry biomass · g of N oxidized · day−1. The yield coefficient observed in the suspended-biomass reactor is in the lower range of reported cell yield coefficients for nitrifying bacteria at optimal pH (0.05 to 0.33 g of dry biomass · g of N-NH4+ oxidized−1) (14, 27, 28, 38). Typical kinetic constants of nitrifying bacteria indicate that the AOB concentration should be higher than that of NOB, as was the case in the control pH 7 and low-pH biofilm reactors mentioned above. The abundance of NOB in the suspended-biomass reactor was found to be 40 times higher than that of AOB, and this suggests that the observed yield is mainly that of NOB alone.
To create such a large difference in population sizes, the yield of AOB may have been much lower due to the suspended-biomass reactor's lower pH (at least half a pH unit of difference between the biofilm and the suspended-biomass reactors) or the result of the filtration technique used daily to prevent biomass washout. In the first case, energy-consuming mechanisms for maintaining homeostatic pH and for ammonium uptake to overcome the problem of a negligible free ammonia concentration at low pH may have been responsible for the disproportionate populations. However, a normal population ratio of AOB to NOB under negligible free ammonia concentrations and low pH was already observed in the biofilm reactor. It can be speculated that at the very low pH conditions prevailing in the suspended-biomass reactor, the enzymatic system for AOB may require more energy to maintain homeostasis because of its more elaborate nature. In the second case, glass microfiber filter paper with 1.6-μm pores (Whatman GFA) used in filtration may have selectively retained the much larger NOB aggregates while smaller embryonic AOB clusters or free cells passed the filter barrier and washed out of the system. However, results from a number of filtrate samples showed that the protein and biomass concentration in the effluent was negligible. Regardless of the reasons for the disproportionately low AOB-to-NOB ratio, the fact that a high ammonium oxidation rate was accomplished by only 2% of the bacterial population is further evidence for the existence of an efficient ammonium uptake mechanism and the means to cope with low pH in autotrophic nitrifying bacteria.
In this paper, successful operation of high-rate nitrification at low pH in both attached (biofilm) and suspended-biomass reactors by chemolithotrophic bacteria is reported under conditions of no special NH3-generating mechanisms. The results from this research clearly contradict those in many other previously published studies on high-rate nitrification in reactor systems. In spite of the unique and carefully designed reactor operation procedures used here to overcome the specific sensitivities of the chemolithotrophic nitrifying bacteria, it is still perplexing why the phenomenon of high-rate nitrification at low pH was not reported before in engineered and laboratory systems. In engineered systems, CO2 limitation due to excessive degassing by conventional air aeration is probably the main reason why high-rate nitrification at low pH has not been observed (12). With regard to microbial work in this field, it is usually carried out on pure cultures in batch reactor systems equipped with vigorous air aeration, using the recommended American Type Culture Collection medium containing a high ammonium concentration of 48 mM (33). We can only surmise that using a heterogeneous nitrifying population in a continuous-flow reactor aerated with pure oxygen contributed to the successful results by preventing the accumulation of toxic HNO2 and decreasing degassing of essential CO2 occurring at low pH. In addition, the working procedure minimized bacterial loss that is vital to prevent washout of the extremely slow-growing ammonia-oxidizing bacteria. Finally, it is possible that growing the nitrifying population at low ammonium concentrations may have induced an efficient ammonium uptake mechanism, as opposed to cultivation in high-ammonium concentrations, which may repress it (16).
REFERENCES
- 1.Allison, S. M., and J. I. Prosser. 1993. Ammonia oxidation at low pH by attached populations of nitrifying bacteria. Soil Biol. Biochem. 25:935-941. [Google Scholar]
- 2.American Public Health Association (ed.). 1995. Standard methods for the examination of water and wastewater, 19th ed. American Public Health Association, Washington, D.C.
- 3.Bonomo, L., G. Pastorelli, E. Quinto, and G. Rinaldi. 2000. Tertiary nitrification in pure oxygen moving bed biofilm reactors. Water Sci. Technol. 41:361-368. [Google Scholar]
- 4.Bradford, M. 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 5.Burton, S. A. Q., and J. I. Prosser. 2001. Autotrophic ammonia oxidation at low pH through urea hydrolysis. Appl. Environ. Microbiol. 67:2952-2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Characklis, W. G., and K. C. Marshall (ed.). 1990. Biofilms. John Wiley & Sons, Inc., New York, N.Y.
- 7.De Boer, W., P. J. A. Klein Gunnewiek, M. Veenhuis, E. Bock, and H. J. Laanbroek. 1991. Nitrification at low pH by aggregated chemolithotrophic bacteria. Appl. Environ. Microbiol. 57:3600-3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Boer, W., and G. A. Kowalchuk. 2001. Nitrification in acid soils: micro-organisms and mechanisms. Soil Biol. Biochem. 33:853-866. [Google Scholar]
- 9.Doyle, J., S. Watts, D. Solley, and J. Keller. 2001. Exceptionally high-rate nitrification in sequencing batch reactors treating high ammonia landfill leachate. Water Sci. Technol. 42:315-322. [PubMed] [Google Scholar]
- 10.Gieseke, A., L. Bjerrum, M. Wagner, and R. Amann. 2003. Structure and activity of multiple nitrifying bacterial populations co-existing in a biofilm. Environ. Microbiol. 5:355-369. [DOI] [PubMed] [Google Scholar]
- 11.Gran, G. 1952. Determination of the equivalence point in potentiometric titrations, Part II. Analyst 77:661-671. [Google Scholar]
- 12.Green, M., Y. Ruskol, A. Shaviv, and S. Tarre. 2002. The effect of CO2 concentration on a nitrifying chalk reactor. Water Res. 36:2147-2151. [DOI] [PubMed] [Google Scholar]
- 13.Hayatsu, M., and N. Kosuge. 1993. Autotrophic nitrification in acid tea soils. Soil Sci. Plant Nutr. 39:209-217. [Google Scholar]
- 14.Henze, M., P. Harremoës, J. Jansen, and E. Arvin. 1995. In U. Förstner, R. J. Murphy, and W. H. Rulkens (ed.), Wastewater treatment biological and chemical processes. Springer-Verlag, Berlin, Germany.
- 15.Juretschko, S., G. Timmermann, M. Scchmid, K.-H. Schleifer, A. Pommerening-Röser, H.-P. Koops, and M. Wagner. 1998. Combined molecular and conventional analysis of nitrifying diversity in activated sludge: Nitrosococcus mobilis and Nitrospira bacteria as dominant populations. Appl. Environ. Microbiol. 64:3042-3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kleiner, D. 1981. The transport of NH3 and NH4+ across biological membranes. Biochim. Biophys. Acta 639:42-52. [DOI] [PubMed] [Google Scholar]
- 17.Kloep, F., I. Röske, and T. R. Neu. 2000. Performance and microbial structure of a nitrifying fluidized-bed reactor. Water Res. 34:311-319. [Google Scholar]
- 18.Koops, H.-P., and A. Pommerening-Roser. 2001. Distribution and ecophysiology of the nitrifying bacteria emphasizing cultured species. FEMS Microbiol. Ecol. 39:1-9. [Google Scholar]
- 19.Landi, L., L. Badalucco, F. Pomarě, and P. Nannipieri. 1993. Effectiveness of antibiotics to distinguish the contributions of fungi and bacteria to net nitrogen mineralization, nitrification and respiration. Soil Biol. Biochem. 25:1771-1778. [Google Scholar]
- 20.Loy, A., M. Horn, and M. Wagner. 2003. probeBase—an online resource for rRNA-targeted oligonucleotide probes. Nucleic Acids Res. 31:514-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manz, W., R. Amann, W. Ludwig, M. Wagner, and K.-H. Schleifer. 1992. Phylogenetic oligodeoxynucleotide probes for the major subclasses of proteobacteria: problems and solutions. Syst. Appl. Microbiol. 15:593-600. [Google Scholar]
- 22.Neef, A. 1997. Anwendung der in situ-einzelzell-identifizierung von bakterien zur populations analyse in komplexen mikrobiellen biozönosen. Ph.D. thesis. Technische Universität München, Munich, Germany.
- 23.Nogueira, R., L. F. Melo, U. Purkhold, S. Wuertz, and M. Wagner. 2000. Nitrifying and heterotrophic population dynamics in biofilm reactors: effects of hydraulic retention time and the presence of organic carbon. Water Res. 36:469-481. [DOI] [PubMed] [Google Scholar]
- 24.Painter, H. A. 1986. Nitrification in the treatment of sewage and wastewaters, p. 185-211. In J. I. Prosser (ed.), Nitrification. IRL Press, Oxford, United Kingdom.
- 25.Pommerening-Röser, A., G. Rath, and H.-P. Koops. 1996. Phylogenetic diversity within the genus Nitrosomonas. System. Appl. Microbiol. 19:344-351. [Google Scholar]
- 26.Powell, S. J., and J. I. Prosser. 1986. Inhibition of ammonium oxidation by nitrapyrin in soil and liquid culture. Appl. Environ. Microbiol. 52:782-787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Randall, C. W., J. L. Barnard, and H. D. Stensel (ed.). 1992. Design and retrofit of wastewater treatment plant for biological nutrient removal. Water quality management library series, vol. 5. Technomic, Lancaster, Pa.
- 28.Rittman, B. E., and P. L. McCarty (ed.). 2001. Environmental biotechnology: principles and applications. McGraw-Hill, Boston, Mass.
- 29.Schramm, A., D. de Beer, A. Gieseke, and R. Amann. 2000. Microenvironments and distribution of nitrifying bacteria in a membrane-bound biofilm. Environ. Microbiol. 2:680-686. [DOI] [PubMed] [Google Scholar]
- 30.Schroeder, E. D. 1977. Activated sludge and other suspended culture processes, p. 236-287. In B. J. Clark and M. Eichberg (ed.), Water and wastewater treatment. McGraw-Hill, New York, N.Y.
- 31.Siegrist, H., and W. Gujer. 1987. Demonstration of mass transfer and pH effects in a nitrifying biofilm. Water Res. 21:1481-1487. [Google Scholar]
- 32.Stephen, J. G. A. Kowalchuk, M. V. Bruns, A. E. McCaig, C. J. Phillips, T. M. Embley, and J. I. Prosser. 1998. Analysis of β-subgroup proteobacterial ammonia oxidizer populations in soil by denaturing gradient gel electrophoresis analysis and hierarchical phylogenetic probing. Appl. Environ. Microbiol. 64:2958-2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suzuki, I., and S. C. Kwok. 1969. Oxidation of ammonia by spheroplasts of Nitrosomonas europaea. J. Bacteriol. 99:897-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suzuki, I., U. Dular, and S. C. Kwok. 1974. Ammonia or ammonium ion as substrate for oxidation by Nitrosomonas europaea cells and extracts. J. Bacteriol. 120:556-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szwerinski, H., E. Arvin, and P. Harremoës. 1986. pH-decrease in nitrifying biofilms. Water Res. 20:971-976. [Google Scholar]
- 36.Tarre, S., M. Beliavski, N. Denekamp, A. Gieseke, D. de Beer, and M. Green. 2004. High nitrification rate at low pH in a fluidized bed reactor with chalk as the biofilm carrier. Water Sci. Technol. 49:95-105. [PubMed] [Google Scholar]
- 37.Tate, R. L. 1977. Nitrification in histosols: a potential role for the heterotrophic nitrifier. Appl. Environ. Microbiol. 33:911-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tchobanoglous, G., and F. L. Burton. 1991. Conversion of ammonia by biological nitrification, p. 694-711. In B. J. Clark and J. M. Morris (ed.), Metcalf and Eddy, Inc., wastewater engineering: treatment, disposal, and reuse, 3rd ed. McGraw-Hill, New York, N.Y.
- 39.Verstraete, W., and M. Alexander. 1972. Heterotrophic nitrification by Arthrobacter sp. J. Bacteriol. 110:955-961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Villaverde, S., P. A. Garcia-Encina, and F. Fdz-Polanco. 1997. Influence of pH over nitrifying biofilm activity in submerged biofilters. Water Res. 31:1180-1186. [Google Scholar]
- 41.Woods, P. M. 1986. Nitrification as a bacterial energy source. p. 39-62. In J. I. Prosser (ed.), Nitrification. IRL Press, Oxford, United Kingdom.