Abstract
Biomaterial-centered infections of orthopedic percutaneous implants are serious complications which can ultimately lead to osteomyelitis, with devastating effects on bone and surrounding tissues, especially since the biofilm mode of growth offers protection against antibiotics and since removal frequently is the only ultimate solution. Recently, it was demonstrated that as a possible pathway to prevent infections of percutaneous stainless steel implants, electric currents of 60 to 100 μA were effective at stimulating the detachment of initially adhering staphylococci from surgical stainless steel. However, initially adhering bacteria are known to adhere more reversibly than bacteria growing in the later stages of biofilm formation. Hence, the aim of this study was to examine whether a growing Staphylococcus epidermidis biofilm can be stimulated to detach from surgical stainless steel by the use of electric currents. In separate experiments, four currents, i.e., 60 and 100 μA of direct current (DC) and 60 and 100 μA of block current (50% duty cycle, 1 Hz), were applied for 360 min to stimulate the detachment of an S. epidermidis biofilm that had grown for 200 min. A 100-μA DC yielded 78% detachment, whereas a 100-μA block current under the same experimental conditions yielded only 31% detachment. The same trend was found for 60 μA, with 37% detachment for a DC and 24% for a block current. Bacteria remaining on the surface after the current application were less viable than they were prior to the current application, as demonstrated by confocal laser scanning microscopy. In conclusion, these results suggest that DCs are preferred for curing infections.
Biomaterial-centered infections are serious complications associated with the use of biomaterial implants and devices. A special class of biomaterials that are prone to infection are the percutaneous pins used in orthopedic fixation frames. Infections of these pins can ultimately lead to osteomyelitis, with devastating effects on bone and surrounding tissues (11). These so-called pin tract infections have been shown to have a huge incidence of up to 71% (16, 18) caused by breaches in the skin. Due to the biofilm mode of growth, microorganisms are shielded from the host defense mechanism and are also difficult to eradicate with antibiotics. The literature indicates that 500 to 5,000 times higher levels of antibiotics are needed to achieve the same antimicrobial effects on biofilm organisms as on planktonic microorganisms (1, 4, 5, 15). For attempts to cure or prevent biofilm formation by pin tract infections, multiple techniques are being employed, but there is no consensus on the best technique (8). There is, however, general agreement that the site should be inspected for infection and that a cleansing agent (hydrogen peroxide or povidone) should be used (2), but these agents have negative effects on the surrounding tissues (3, 14). Furthermore, proper pin care can also be difficult and time-consuming for patients and their families (8).
Biofilm formation commences with the adhesion of microorganisms to an implant surface. This initial microbial adhesion has been extensively studied and is generally believed to depend on the physicochemical properties of the microbial and biomaterial surfaces, such as hydrophobicity and charge (10). Previously, it was demonstrated that >75% of initially adhering staphylococci could be stimulated to detach from surgical stainless steel by the application of an electric current of <100 μA, either a direct current (DC) or block current, while yielding a 2-log reduction in the viability of the remaining organisms (20). It was suggested that the reason for the higher detachment percentage was caused by electroosmotic fluid flow directed to and from the surface (17). The alternating field causes the hydrated ions to move along the applied field, dragging water with them. This fluid flow causes an extra force that stimulates detachment.
The influence of electric currents on the detachment of microorganisms from conducting surfaces during the growth stage of biofilm formation has not yet been studied, but it could be entirely different from the effects seen on initially adhering organisms. During growth, not only do microorganisms multiply in large numbers, but adhesion also proceeds from a more reversible to a more irreversible state through the excretion of extracellular polymeric substances (EPS), which act as a glue between the biofilm and the substratum surface, by the adhering organisms.
Therefore, the aim of this study was to determine whether it is possible to stimulate bacterial detachment of a growing Staphylococcus epidermidis biofilm from surgical stainless steel by using DCs and block currents (50% duty cycle, 1 Hz) of 60 and 100 μA.
MATERIALS AND METHODS
Bacterial strains.
Experiments were conducted with S. epidermidis HBH276. This strain was previously shown to detach during initial adherence from stainless steel when exposed to DCs and block currents (20). The strain was cultured in tryptic soy broth (Oxoid, Basingstoke, United Kingdom) at 37°C in ambient air. For each experiment, the strain was inoculated from blood agar into a batch culture and grown for 24 h. This culture was used to inoculate a second culture that was grown for 16 h prior to harvesting. Bacteria were harvested by centrifugation (5 min at 6,000 × g at 10°C), washed twice with demineralized water, and suspended to a concentration of 3 × 108 per ml in phosphate-buffered saline (PBS; 10 mM potassium phosphate buffer and 0.15 M NaCl, pH 7). Before they were suspended in PBS, the bacteria were sonicated at 30 W for 10 s while cooling in an ice-water bath to obtain single cells.
Stainless steel.
AISI 316 LVM stainless steel (Stryker Corp., Kiel, Germany) was ground down to grit number 1200 and subsequently polished with 6- and 3-μm-diameter water-based diamond suspensions (Metadi 3- and 6-μm-diameter diamond suspension and Trident polishing cloth; Buehler, Lake Bluff, Ill.) for 3 and 1.5 min, respectively. Grinding and polishing were done with a polishing machine with a 30-N load and oppositely rotating axes (Phoenix Beta and Vector grinder-polisher; Buehler). The polished steel was cleaned by a 5-min sonication in 2% alkaline cleaning agent (RBS35 in water; Omniclean) followed by a thorough rinsing with tap water, sonication in ethanol, and rinsing in Millipore-filtered demineralized water. After being cleaned, the steel was passivated according to American Society for Testing and Materials standard F86-91, thoroughly rinsed with Millipore-filtered demineralized water, and dried in an oven at 80°C prior to being used as an electrode surface.
Parallel plate flow chamber and surface growth and detachment experiments.
Bacterial adhesion and biofilm growth as well as subsequent detachment were studied in a parallel plate flow chamber with a distance of 0.6 mm between the top and bottom plates (19). The bottom plate consisted of the surgical stainless steel electrode (area, 21 cm2), while the top plate, employed as a counterelectrode, was made of an indium tin oxide (ITO), DC-sputtered glass (Philips Natlab, Eindhoven, The Netherlands). The ITO-coated glass plates were cleaned in the same way as the stainless steel, and an electrical wire was glued to the surface with silver dag (Electrodag 1415; Acheson, Port Huron, Mich.). Before the flow chamber and electrodes were assembled, each part was sterilized by being submerged in 70% (vol/vol) ethanol. All other parts requiring sterility were autoclaved prior to assembly.
The flow chamber was equipped with a heating element attached to a nickel-coated brass block placed directly in contact with the stainless steel bottom plate. The temperature of the block was kept constant at 36.5°C throughout the entire experiment. Adhering bacteria were observed with a CCD-MXR camera mounted on a metallurgical microscope equipped with a 40× ultra-long-working-distance objective. All fluids used were circulated through the camber by use of a peristaltic pump at a flow rate of 0.012 ml/s (shear rate, 10 s−1). PBS was first flowed through the chamber for 40 min, followed by the bacterial suspension, until 106 bacteria cm−2 were found adhering to the stainless steel bottom plate, which took approximately 25 min under the experimental conditions applied. Subsequently, the chamber was perfused for 40 min with cell-free PBS to remove planktonic bacteria from the system, followed by the perfusion of growth medium (tryptic soy broth) for 200 min. After perfusion of the growth medium, 10 mM potassium phosphate buffer (pH 7) was directed through the system for 400 min, during the course of which a specific current was applied through the anode and the cathode. The electric current was kept constant during the final 360 min of the experiment with the aid of an LM334Z semiconductor (National Semiconductor Corp., Santa Clara, Calif.) and was driven by a voltage that varied in both frequency and duty cycle, with the stainless steel bottom plate acting as a cathode. The voltage and current applied to the electrodes were both observed with conventional multimeters. The LM334Z output potential was adapted continuously to meet the required and adjusted current, irrespective of the applied voltage to the integrated circuit (IC). The following currents were tested: a 100-μA DC, a 60-μA DC, a 100-μA (50% duty cycle, 1 Hz) block current, and a 60-μA (50% duty cycle, 1 Hz) block current. The limited space in the parallel plate flow chamber did not allow the use of a reference electrode to measure the surface potential. Additionally, a control experiment was done in which the electric current was omitted.
During each experiment, images were taken of bacteria adhering to the stainless steel bottom plate and were stored in the computer to obtain the number of adhering bacteria per unit area versus time during the application of the electric current. All experiments were done in triplicate with separately cultured bacteria and freshly prepared top and bottom plates.
Viability staining.
In order to determine possible effects of the electric current on the viability of the staphylococci that remained adherent after the current application, we operated two parallel plate flow chambers simultaneously with bacterial suspensions taken from the same culture. One flow chamber was used as a control in the absence of an electric current, while the second flow chamber was connected to the current source. During the time of current application, the control flow chamber was perfused with 10 mM potassium phosphate buffer. After the experiment, both chambers were disconnected, filled with staining fluid (Live/Dead Baclight bacterial viability kit; Molecular Probes, Leiden, The Netherlands), and incubated for 15 min in the dark. On different, randomly chosen locations on each surface, micrographs were taken with a confocal laser scanning microscope (CLSM) with a 40× ultra-long-working-distance objective, with the microscope set for fluorescein isothiocyanate (excitation at 488 nm and emission at 500 to 600 nm) and tetramethylrhodamine isocyanate (excitation at 543 nm and emission at 560 to 700 nm) to show dead and live bacteria, respectively. Results were expressed as percentages of dead bacteria.
RESULTS
Figure 1 illustrates the numbers of adhering and growing S. epidermidis HBH276 cells during the course of an experiment with two different currents. As shown in the figure, bacteria adhered during the first 25 min, after which the increases in the numbers of adhering staphylococci were entirely due to growth up to 260 min. At the onset of the electric current after 300 min, the staphylococci started to detach under the influence of the electric current.
FIG. 1.
Examples of time series involved in the adhesion and growth of S. epidermidis HBH276 biofilms and their detachment from stainless steel by the use of two different currents (□, 100-μA DC; ▴, 100-μA [50% duty cycle, 1 Hz] block current) in 10 mM potassium phosphate buffer. The four dashed lines indicate the four consecutive phases of the experiments. (A) Bacterial deposition (25 min); (B) perfusion with buffer (40 min); (C) growth medium (200 min); (D) perfusion with buffer (40 min); and (E) application of electric current during perfusion with buffer (360 min).
The time dependence of the detachment process allowed us to calculate so-called initial detachment rates (jdet,0 = |dn/dt| [cm−2 s−1]) and total detachment percentages [(1 − Nafter/Nprior) × 100], for which Nprior and Nafter are the numbers of adhering bacteria prior to and after the application of the electric current, respectively. The total detachment percentages and detachment rates, as well as the actually measured average voltage differences between the cathode and the anode, are given in Table 1 for the different electric currents. All currents applied induced significant staphylococcal detachment, albeit with different total detachment percentages. The total detachment percentages for both 60 and 100 μA were significant higher (P = 0.05) for the DCs than for the corresponding block currents. The total detachment percentage was also higher for a 100-μA DC than for a 60-μA DC, whereas the difference between 100- and 60-μA block currents was not significant. Consequently, only the 100-μA DC had a total detachment percentage that was significantly different from all others after 360 min of application (P < 0.05). The detachment rate, on the other hand, showed no correlation with the voltage difference or the electric current applied. Note that the 100-μA DC had a significantly different detachment rate than those observed under other conditions.
TABLE 1.
Voltage differences between the cathode and the anode and total detachment percentages and detachment rates of S. epidermidis HBH276 biofilm upon the application of different electric currents in a 10 mM potassium phosphate buffer after 200 min of growth
| Current strength (μA) and type | Vavg (V) | Total detachment (%)a | Detachment rate (cm−2 s−1)a |
|---|---|---|---|
| No current | NAb | 3 ± 1 | NAb |
| 100 (DC) | 2.10 | 78 ± 5 | 842 ± 455 |
| 100 (50%, 1 Hz) | 1.75 | 31 ± 6 | 226 ± 62 |
| 60 (DC) | 1.80 | 37 ± 6 | 237 ± 62 |
| 60 (50%, 1 Hz) | 1.55 | 24 ± 2 | 275 ± 38 |
Results are presented as means ± standard errors obtained from triplicate experiments with separate cultured organisms and new stainless steel surfaces.
NA, not applicable.
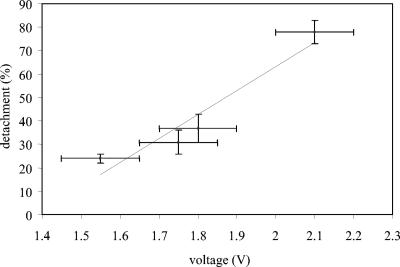
The potential needed for the application of a specific current varied with the current applied. In Fig. 2, the voltage differences between the electrodes corresponding to the various currents applied are plotted versus the total detachment rates. As shown in the figure, there is an almost linear increase in total detachment with increasing applied potentials.
FIG. 2.
Total detachment versus voltage difference between the cathode (stainless steel) and the anode (ITO-coated glass) during electric current-stimulated detachment of S. epidermidis HBH276 biofilms. Error bars denote the standard errors for three experiments with separately cultured bacteria and freshly prepared plates.
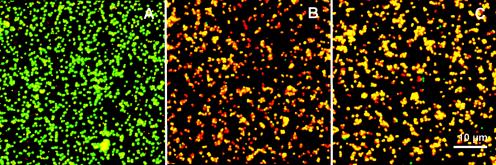
Figure 3 shows CLSM micrographs after dead-live staining of bacteria remaining on the surfaces after application of the electric current. The percentage of viable bacteria was about 97% in the absence of an electric current, while only 3% of the bacteria were viable after the application of a 100-μA DC and 2% were viable after a 100-μA block current.
FIG. 3.
CLSM micrographs of bacteria adhering to stainless steel and stained with a live-dead stain after experiments with and without the application of an electric field. Green, live cells; red, dead cells. (A) Control experiment with no electric current; (B) 100-μA block current; and (C) 100-μA DC.
DISCUSSION
In this paper, we demonstrated that a growing S. epidermidis HBH276 biofilm can be stimulated to detach from surgical stainless steel with the aid of DC and block electric currents, whereas control experiments showed that detachment was virtually absent under the applied shear conditions. The remaining bacteria after the application of an electric field had a viability of only 2 to 3%. Therefore, although this study was initially aimed toward a biomedical application, the use of electric currents to stimulate bacterial detachment may also find applications in other fields in which biofilms form under shear on conducting surfaces, such as applications for industry or water pipelines.
Previously, it was shown that initially adhering bacteria, which interact solely through Lifshitz-Van der Waals, electrostatic, and acid-base interactions, could be stimulated to detach by the use of DCs and block currents. The block currents yielded higher detachment percentages than DCs due to the electroosmotic fluid flow (17). However, when biofilm bacteria are concerned, DCs cause higher detachment percentages than their counterpart block currents. This suggests that the influence of electroosmotic fluid flow on biofilm bacteria is absent. When adhering bacteria start to grow and excrete EPS, adhering bacteria become trapped in a more-or-less gel-like structure (6, 9, 12). The EPS matrix prevents water and entrapped particles, such as hydrated ions, to move freely. Because the ions are limited in their movement, the electroosmotic fluid flow is small or even absent, despite the applied electric field, and thus will not cause additional detachment. However, the absence of electroosmotic fluid flow cannot explain the fact that the detachment stimulated by block currents is less than that stimulated by DCs. It is likely that the decrease in detachment percentage with decreasing potentials (Fig. 2) is a direct consequence of a decrease in the Stern potential and therefore in the Gibbs interaction energy, as outlined in the DLVO (Derjaguin, Landau, Verseug, and Overbeek) theory (13).
The potential varied linearly with the applied current, yielding an electric resistance of the biofilm of 7,700 ± 500 Ω. This resistance was constant throughout each experiment, regardless of subsequent bacterial detachment, suggesting that the electric resistance is mainly caused by EPS remaining on the substratum surface after detachment of the adhering bacteria (7).
To summarize, we have described a method by which bacterial biofilms can be stimulated to detach from surgical stainless steel by use of a small electric current to disconnect the link between the biofilm and the conducting substratum (9, 10). This method may have an application in the medical field, as it can be used in combination with conventional pin site care to prevent or cure infections by applying the current between a circular electrode placed around the pin and the pin itself. The conventional cleaning technique with water and soap can provide the flow needed for bacterial transportation, and between cleanings the current can kill the adhering bacteria.
REFERENCES
- 1.Anwar, H., M. K. Dasgupta, and J. W. Costerton. 1990. Testing the susceptibility of bacteria in biofilms to antibacterial agents. Antimicrob. Agents Chemother. 34:2043-2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernardo, L. M. 2001. Evidence-based practice for pin site care in injured children. Orthop. Nurs. 20:29-34. [DOI] [PubMed] [Google Scholar]
- 3.Bulstra, S. K., R. Kuijer, P. Eerdmans, and A. J. van der Linden. 1994. The effect in vitro of irrigating solutions on intact rat articular cartilage. J. Bone Joint Surg. Br. 76:468-470. [PubMed] [Google Scholar]
- 4.Costerton, J. W., K. J. Cheng, G. G. Geesey, T. I. Ladd, J. C. Nickel, M. Dasgupta, and T. J. Marrie. 1987. Bacterial biofilms in nature and disease. Annu. Rev. Microbiol. 41:435-464. [DOI] [PubMed] [Google Scholar]
- 5.Costerton, J. W., B. Ellis, K. Lam, F. Johnson, and A. E. Khoury. 1994. Mechanism of electrical enhancement of efficacy of antibiotics in killing biofilm bacteria. Antimicrob. Agents Chemother. 38:2803-2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donlan, R. M. 2002. Biofilms: microbial life on surfaces. Emerg. Infect. Dis. 8:881-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomez-Suarez, C., J. Pasma, A. J. van der Borden, J. Wingender, H. C. Flemming, H. J. Busscher, and H. C. van der Mei. 2002. Influence of extracellular polymeric substances on deposition and redeposition of Pseudomonas aeruginosa to surfaces. Microbiology 148:1161-1169. [DOI] [PubMed] [Google Scholar]
- 8.Gordon, J. E., J. Kelly-Hahn, C. J. Carpenter, and P. L. Schoenecker. 2000. Pin site care during external fixation in children: results of a nihilistic approach. J. Pediatr. Orthop. 20:163-165. [PubMed] [Google Scholar]
- 9.Gottenbos, B., H. C. van der Mei, and H. J. Busscher. 1999. Models for studying initial adhesion and surface growth in biofilm formation on surfaces. Methods Enzymol. 310:523-534. [DOI] [PubMed] [Google Scholar]
- 10.Gottenbos, B., H. C. van der Mei, and H. J. Busscher. 2000. Initial adhesion and surface growth of Staphylococcus epidermidis and Pseudomonas aeruginosa on biomedical polymers. J. Biomed. Mater. Res. 50:208-214. [DOI] [PubMed] [Google Scholar]
- 11.Gracia, E., A. Lacleriga, M. Monzon, J. Leiva, C. Oteiza, and B. Amorena. 1998. Application of a rat osteomyelitis model to compare in vivo and in vitro the antibiotic efficacy against bacteria with high capacity to form biofilms. J. Surg. Res. 79:146-153. [DOI] [PubMed] [Google Scholar]
- 12.Gristina, A. G. 1994. Implant failure and the immuno-incompetent fibro-inflammatory zone. Clin. Orthop. 298:106-118. [PubMed] [Google Scholar]
- 13.Hermansson, M. 1999. The DLVO theory in microbial adhesion. Colloids Surf. B Biointerfaces 14:105-119. [Google Scholar]
- 14.Kaysinger, K. K., N. C. Nicholson, W. K. Ramp, and J. F. Kellam. 1995. Toxic effects of wound irrigation solutions on cultured tibiae and osteoblasts. J. Orthop. Trauma 9:303-311. [DOI] [PubMed] [Google Scholar]
- 15.Khoury, A. E., K. Lam, B. Ellis, and J. W. Costerton. 1992. Prevention and control of bacterial infections associated with medical devices. ASAIO J. 38:M174-M178. [DOI] [PubMed] [Google Scholar]
- 16.Mostafavi, H. R., and P. Tornetta III. 1997. Open fractures of the humerus treated with external fixation. Clin. Orthop. 337:187-197. [DOI] [PubMed] [Google Scholar]
- 17.Poortinga, A. T., J. Smit, H. C. van der Mei, and H. J. Busscher. 2001. Electric field induced desorption of bacteria from a conditioning film covered substratum. Biotechnol. Bioeng. 76:395-399. [DOI] [PubMed] [Google Scholar]
- 18.Sims, M., and M. Saleh. 2000. External fixation—the incidence of pin site infection: a prospective audit. J. Orthop. Nurs. 4:59. [Google Scholar]
- 19.Sjollema, J., H. J. Busscher, and A. H. Weerkamp. 1989. Real-time enumeration of adhering microorganisms in a parallel plate cell using automated image analysis. J. Microbiol. Methods 9:73-78. [Google Scholar]
- 20.van der Borden, A. J., H. C. van der Mei, and H. J. Busscher. 2004. Electric-current-induced detachment of Staphylococcus epidermidis strains from surgical stainless steel. J. Biomed. Mater. Res. 68B:160-164. [DOI] [PubMed] [Google Scholar]